Abstract
The bifunctional wax ester synthase/acyl-coenzyme A:diacylglycerol acyltransferase (WS/DGAT) is the key enzyme in storage lipid accumulation in the gram-negative bacterium Acinetobacter calcoaceticus ADP1, mediating wax ester, and to a lesser extent, triacylglycerol (TAG) biosynthesis. Saccharomyces cerevisiae accumulates TAGs and steryl esters as storage lipids. Four genes encoding a DGAT (Dga1p), a phospholipid:diacylglycerol acyltransferase (Lro1p) and two acyl-coenzyme A:sterol acyltransferases (ASATs) (Are1p and Are2p) are involved in the final esterification steps in TAG and steryl ester biosynthesis in this yeast. In the quadruple mutant strain S. cerevisiae H1246, the disruption of DGA1, LRO1, ARE1, and ARE2 leads to an inability to synthesize storage lipids. Heterologous expression of WS/DGAT from A. calcoaceticus ADP1 in S. cerevisiae H1246 restored TAG but not steryl ester biosynthesis, although high levels of ASAT activity could be demonstrated for WS/DGAT expressed in Escherichia coli XL1-Blue in radiometric in vitro assays with cholesterol and ergosterol as substrates. In addition to TAG synthesis, heterologous expression of WS/DGAT in S. cerevisiae H1246 resulted also in the accumulation of fatty acid ethyl esters as well as fatty acid isoamyl esters. In vitro studies confirmed that WS/DGAT is capable of utilizing a broad range of alcohols as substrates comprising long-chain fatty alcohols like hexadecanol as well as short-chain alcohols like ethanol or isoamyl alcohol. This study demonstrated the highly unspecific acyltransferase activity of WS/DGAT from A. calcoaceticus ADP1, indicating the broad biocatalytic potential of this enzyme for biotechnological production of a large variety of lipids in vivo in prokaryotic as well as eukaryotic expression hosts.
Neutral storage lipids like triacylglycerols (TAGs), wax esters, and steryl esters are frequently present in plants, animals, fungi, and yeast as well as in bacteria (2, 20). Due to their influence on various serious human diseases like obesity, diabetes, and arteriosclerosis (8, 17, 28, 29, 34), the biosynthesis of storage lipids in different organisms has in recent years become an important focus of research, which has yielded significant new insights into the molecular biological processes involved.
The yeast Saccharomyces cerevisiae accumulates TAGs and steryl esters as cytosolic lipid particles, which consist of 51% TAGs and 44% steryl esters and contribute up to 70% of the total lipid content (19). Depending on culture conditions, the total lipid content of baker's yeast can vary between 3.5 and 10.7% of the cellular dry weight (11). Steryl esters predominantly consisting of ergosteryl esters are synthesized in S. cerevisiae by two acyl-coenzyme A:sterol acyltransferase (ASAT) isoenzymes encoded by ARE1 and ARE2 (37, 38, 40). Biosynthesis of TAGs in yeast is more complex and proceeds via two independent pathways (reviewed in reference 31). Acyl-coenzyme A (acyl-CoA)-dependent TAG biosynthesis is catalyzed by an acyl-CoA:diacylglycerol acyltransferase (DGAT) belonging to the DGAT2 family encoded by DGA1 (21, 27, 30). In addition, a phospholipid:diacylglycerol acyltransferase encoded by LRO1 synthesizes TAGs in an acyl-CoA-independent reaction by using the sn-2 acyl group of phosphatidylcholine as the acyl donor (6, 22). Dga1p and Lro1p synthesize the majority of TAGs in S. cerevisiae; however, there are highly divergent reports on the contribution of the two pathways to TAG synthesis depending on the wild-type strain background and growth phases (21, 27, 30). Additionally, the ASAT isoenzymes Are1p and Are2p exhibit a low level of DGAT side activity and contribute only slightly to TAG synthesis. However, again there are contradictory data on whether only Are1p (26) or only Are2p (21) or both (27) are responsible for residual TAG production. In a quadruple mutant strain of S. cerevisiae in which DGA1, LRO1, ARE1 and ARE2 were disrupted, no accumulation of TAGs and steryl esters occurred, indicating that these four genes code for the only enzymes catalyzing the final steps in neutral lipid synthesis in yeast (21, 27).
In bacteria, the most abundant class of storage lipids are polyhydroxyalkanoic acids (33); less frequently, TAGs (2) and wax esters (9) have also been detected as storage lipids. Substantial TAG accumulation seems to be widely distributed in species belonging to the actinomycetes group (1, 4, 23), whereas the biosynthesis of wax esters (oxoesters of primary long-chain fatty alcohols and long-chain fatty acids) has been frequently reported for Acinetobacter species (7). Acinetobacter calcoaceticus ADP1 accumulates wax esters and, to a lesser extent, TAGs under growth-limiting conditions and deposits these storage lipids as insoluble inclusions in the cytoplasm (24). Recently, we identified a bifunctional enzyme from this strain which exhibits simultaneously both acyl-CoA:fatty alcohol acyltransferase (wax ester synthase [WS]) and DGAT activity. It was shown that this bifunctional WS/DGAT is the only enzyme catalyzing the final reaction steps in wax ester and is the major enzyme for TAG biosynthesis in A. calcoaceticus ADP1 (12). The WS/DGAT enzyme represents a novel class of acyltransferases that share no homologies to known enzymes involved in storage lipid or phospholipid synthesis in prokaryotic or eukaryotic organisms. WS/DGAT was characterized as a rather unspecific enzyme that accepts a broad range of various-chain-length saturated and unsaturated fatty alcohols and acyl-CoAs as substrates (12). Furthermore, WS/DGAT was shown to be capable of synthesizing wax diesters in vitro as well as in vivo with α,Ω-alkanediols as substrates and even of using monoacylglycerols as acyl acceptors to some extent (13).
The present study aimed at the heterologous expression of functional WS/DGAT in S. cerevisiae since this attempt had failed for the WS from jojoba (Simmondsia chinensis), which was the first enzyme exhibiting WS activity characterized at a molecular level (18). The influence of WS/DGAT expression on the synthesis of TAGs, steryl esters, and other fatty acid esters in yeast was analyzed.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
The strains and plasmids used in this study are listed in Table 1. Strains of S. cerevisiae were cultivated at 28°C in synthetic minimal dropout medium lacking uracil and containing 0.6% (wt/vol) yeast nitrogen basis medium (Difco, Detroit, Mich.), 0.13% (wt/vol) yeast synthetic dropout supplement without uracil (Sigma, Deisenhofen, Germany), 0.0075% (wt/vol) adenine hemisulfate, and 2% (wt/vol) glucose or galactose. Recombinant strains of Escherichia coli were cultivated in Luria-Bertani (LB) medium (25) at 37°C in the presence of ampicillin (75 mg/liter) and tetracycline (12.5 mg/liter). Cells of S. cerevisiae and E. coli were grown aerobically in 300-ml baffled Erlenmeyer flasks containing 50 ml of medium on an orbital shaker (130 rpm).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or referenceb |
|---|---|---|
| Strains | ||
| E. coli XL1-Blue | recA1 endA gyrA96 thi1 hsdR17 (rk− mk+) supE44 relA1 λ lac[F′ proAB laclqZΔM15 Tn10(Tcr)] | 5 |
| S. cerevisiae G175 | MATα ADE2 MET his3 leu2 ura3 trp1 TAG+ SE+; parental strain of H1246 | ScanBi |
| S. cerevisiae H1246 | MATα are1-Δ::HIS3 are2-Δ::LEU2 dga1-Δ::KanMX4 Iro1-Δ::TRP1 ADE2 ura3 TAG− SE− | ScanBi, 27 |
| Plasmids | ||
| pBluescript KS | AprlacPOZ′ | Stratageneb |
| pKS::atfA | atfA (formerly designated as wax/dgat) from A. calcoaceticus ADP1 with artificial Shine-Dalgarno sequence for E. coli cloned as 1.4-kbp EcoRI-BamHI fragment into pBluescript KS− colinear to lacZ′ | 12 |
| pESC-URA | E. coli-S. cerevisiae shuttle vector; URA3 GAL1 GAL10 Apr | Stratageneb |
| pESC-URA::atfA | atfA (formerly designated as wax/dgat) from A. calcoaceticus ADP1 with artificial Kozak initiation sequence cloned as 1.4-kbp BamHI-SalI fragment into pESC-URA colinear to the GAL1 promoter | This study |
TAG, TAG accumulation; SE, sterol ester accumulation; Apr, ampicillin resistance.
ScanBi, Scandinavian Biotechnology Research (Alnarp, Sweden). Stratagene is located in La Jolla, Calif.
General molecular biological techniques.
Standard molecular biological techniques were applied (25). For polyethylene glycol-mediated transformation of yeast cells, the lithium acetate method was used (10).
Heterologous expression of WS/DGAT in yeast and E. coli.
For expression in yeast, the atfA gene (for acyltransferase; formerly designated the wax/dgat gene [12]) encoding WS/DGAT was amplified from pKS::atfA as the template by tailored PCR by using the oligonucleotides 5′-AAAGGATCCACTATGCGCCCATTACATCCGATT-3′ (5′ primer with the ATG start codon shown in bold) introducing a BamHI restriction site (underlined) and a Kozak translation initiation sequence (14, 15, 16) and 5′-TTTGTCGACTTAATTGGCTGTTTTAATATCTT-3′ (3′ primer) introducing a SalI restriction site (underlined). The PCR product was cloned into the BamHI-SalI-restricted vector pESC-URA colinear to the GAL1 promoter inducible by galactose. Recombinant yeast strains were cultivated in synthetic minimal dropout medium lacking uracil and containing 2% (wt/vol) galactose for 24 h at 28°C. Cells were then harvested, washed, and resuspended in 125 mM sodium phosphate buffer (pH 7.4), and crude extracts were obtained by a twofold French press passage. Recombinant E. coli strains were cultivated in LB medium for 6 h at 37°C in the presence of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Cells were then harvested, washed, resuspended in 125 mM sodium phosphate buffer (pH 7.4), and disrupted by ultrasonification.
Determination of enzyme activities.
WS activity was measured in a total volume of 250 μl of 125 mM sodium phosphate buffer (pH 7.4) containing 3.75 mM 1-hexadecanol, 4.63 mg of bovine serum albumin (BSA) per ml, and 4.72 μM [1 -14C]palmitoyl-CoA (specific activity, 1.961 Bq pmol−1). 1-Hexadecanol and BSA were applied as a double-concentrated stock solution emulsified by ultrasonification. The reaction was done with crude extracts of recombinant E. coli or S. cerevisiae strains, and 100 μg of protein was used in each assay. The assay was incubated at 35°C for 30 min, and the reaction was stopped by extraction with 500 μl of chloroform-methanol (1:1, vol/vol). After centrifugation, the chloroform phase was withdrawn, and the extracted lipids were separated by thin-layer chromatography (TLC) as described below. The radiolabeled reaction products on the TLC plate were detected by autoradiography, and radioactivity was measured by scintillation counting by using palmityl palmitate as a nonlabeled reference substance.
For determination of DGAT activity, the same assay was applied; however, 3.75 mM 1,2-dipalmitoylglycerol instead of 1-hexadecanol was used as a substrate, and triolein was used as a reference substance. For determination of ASAT activity, 3.75 mM cholesterol or ergosterol was used as a substrate, and cholesteryl palmitate was used as a reference substance.
Lipid analysis.
TLC was done as described previously (12) by using the solvent system hexane-diethylether-acetic acid (90:7.5:1, vol/vol/vol) for TAG, wax ester, and steryl ester analysis. Triolein, palmityl palmitate, and cholesteryl palmitate were purchased from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany) and used as reference substances for TAGs, wax esters, and steryl esters, respectively.
Fatty acid analysis of whole cells was done by gas chromatography (GC) as previously described (35). For this, 5 to 7.5 mg of lyophilized cells was subjected for 4 h to methanolysis at 100°C in the presence of 15% (vol/vol) sulfuric acid suspended in methanol. The resulting fatty acid methyl esters were analyzed by GC on an Agilent 6850 GC (Waldbronn, Germany) equipped with a BP21 capillary column (50 m by 0.22 mm; film thickness of 250 nm) (SGE, Darmstadt, Germany) and a flame ionization detector (Agilent Technologies). A 2-μl portion of the organic phase was analyzed after split injection (1:20); hydrogen (constant flow of 0.6 ml min−1) was used as a carrier gas. The temperatures of the injector and detector were 250 and 275°C, respectively. The following temperature program was applied: 120°C for 5 min, increase of 3°C min−1 to 180°C, increase of 10°C min−1 to 220°C, and 220°C for 31 min. Substances were identified by comparison of their retention times with those of standard fatty acid methyl esters.
The putative TAGs, fatty acid ethyl esters, and fatty acid isoamyl esters were purified by preparative TLC. For the direct recovery from the chromatogram, a ChromeXtract (ChromAn, Leipzig, Germany) was used. Lipid structures were determined by electrospray ionization mass spectrometry (ESI-MS) in positive ion mode on a QUATTRO LCZ (Waters-Micromass, Manchester, United Kingdom) with nanospray inlet or by coupled GC/MS.
GC/MS analyses of isolated lipids or total lipid extracts of whole cells dissolved in chloroform were done on a series 6890 GC system equipped with a series 5973 electron ionization mass selective detector (Hewlett Packard, Waldbronn, Germany). A 3-μl portion of the organic phase was analyzed after splitless injection by employing a BP21 capillary column (50 m by 0.22 mm; film thickness of 250 nm) (SGE). Helium (constant flow of 0.6 ml min−1) was used as a carrier gas. The temperatures of the injector and detector were 250 and 240°C, respectively. The same temperature program as described for GC analysis was applied. Data were evaluated by using the National Institute of Standards and Technology Mass Spectral Search program (32).
RESULTS
Functional heterologous expression of WS/DGAT from A. calcoaceticus ADP1 in yeast. In the wild-type strain S. cerevisiae G175, the activities of DGAT as well as of ASAT were detected as expected (Table 2). The low ASAT activity levels with cholesterol or ergosterol as a substrate were due to the use of [1-14C]palmitoyl-CoA as a substrate in the radiometric enzyme assays in this study. Are1p and Are2p are specific for unsaturated C16 and C18 acyl-CoAs and are only barely active with palmitoyl-CoA (37, 40). Furthermore, sterols were applied to the assays as emulsions with BSA, whereas sterols are usually applied to ASAT assays emulsified with phospholipids (38) or nonionic detergents (37, 39). Surprisingly, even low WS activity could be detected in wild-type S. cerevisiae G175. WS activity has never previously been described for yeast. WS activity was almost absent in the quadruple mutant strain S. cerevisiae H1246 (Table 2).
TABLE 2.
WS, DGAT, and ASAT activities in crude extracts of different recombinant S. cerevisiae and E. coli strainsa
| Strain | Enzyme sp act (pmol [mg of protein · min]−1)
|
|||
|---|---|---|---|---|
| WSb | DGAT | ASAT
|
||
| With cholesterol | With ergosterol | |||
| S. cerevisiae G175(pESC-URA) | 1.12 ± 0.03 | 4.45 ± 0.08 | 1.73 ± 0.11 | 0.67 ± 0.06 |
| S. cerevisiae H1246(pESC-URA) | 0.12 ± 0.02 | 0.12 ± 0.01 | 0.13 ± 0.03 | 0.16 ± 0.01 |
| S. cerevisiae H1246(pESC-URA::atfA) | 55.40 ± 3.22 | 44.37 ± 2.11 | 5.87 ± 0.23 | 19.62 ± 0.18 |
| E. coli XL1-Blue(pBluescript KS) | 0.18 ± 0.03 | 0.12 ± 0.02 | 0.15 ± 0.04 | 0.17 ± 0.02 |
| E. coli XL1-Blue(pKS::atfA) | 74.41 ± 3.53 | 19.00 ± 0.82 | 48.57 ± 5.12 | 70.94 ± 2.40 |
Yeast cells were cultivated for 24 h at 28°C in synthetic minimal dropout medium without uracil and containing 2% (wt/vol) galactose. Cells of recombinant E. coli strains were cultivated for 6 h at 37 °C in LB medium containing 1 mM IPTG. Enzyme activities were measured as described in Materials and Methods by using 1-hexadecanol, 1,2-dipalmitoylglycerol, and cholesterol or ergosterol for measuring WS, DGAT, and ASAT activities, respectively. Data are mean values of two independent experiments ± SD.
Wax esters and steryl esters almost comigrated under the applied TLC conditions. Therefore, the production of radiolabeled wax esters and sterol esters was virtually indistinguishable, and WS activities had to be corrected for the formation of radiolabeled sterol esters formed by ASAT activity by utilizing endogenous sterols occurring in yeast, which was measured in control assays with [1-14C]palmitoyl-CoA, but lacking any external acyl acceptor.
WS/DGAT from A. calcoaceticus ADP1 was successfully expressed as a functionally active enzyme in S. cerevisiae H1246. The recombinant mutant showed high levels of WS and DGAT activity; therefore, this protein exhibited its bifunctional character also in the yeast background. However, the ratio of WS to DGAT activity in the recombinant yeast was about 1.2 (Table 2), which was significantly lower in comparison to the ratios of the activity levels of both enzymes measured in A. calcoaceticus ADP1 (11.1) or in recombinant E. coli (3.9) expressing WS/DGAT (reference 12) (Table 2).
WS/DGAT expression leads to storage lipid accumulation in S. cerevisiae H1246.
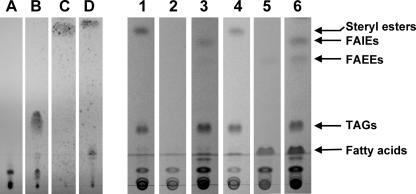
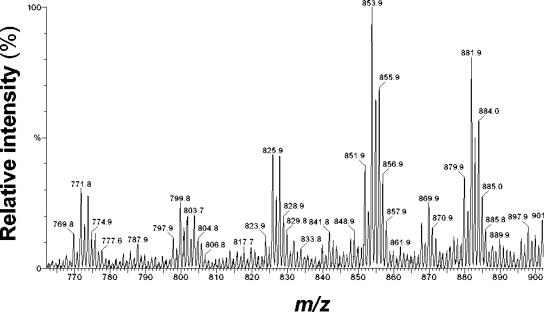
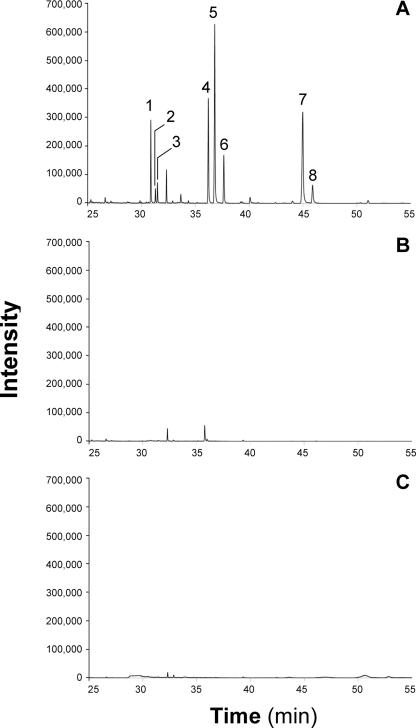
Heterologous expression of WS/DGAT from A. calcoaceticus ADP1 not only conferred high DGAT activity to S. cerevisiae H1246 in vitro but also restored TAG biosynthesis in this storage lipid-deficient mutant in vivo, whereas steryl esters or wax esters were not accumulated in detectable amounts as revealed by TLC analysis (Fig. 1). Chemical analysis of TAGs purified from S. cerevisiae H1246 harboring pESC-URA::atfA by ESI-MS revealed a high molecular weight, ranging from m/z = 771.7 [C14:0/C14:0/C16:1 + Na]+ to m/z = 884.0, corresponding to [C16:0/C18:0/C18:1 + Na]+ and [C16:1/C18:0/C18:0 + Na]+ (Fig. 2) and indicating a similar fatty acid composition as revealed for total cellular lipids (Table 3). In addition to TAGs, two other substances were accumulated in S. cerevisiae H1246 harboring pESC-URA::atfA (Fig. 1); these were purified by preparative TLC and identified by means of ESI-MS and GC/MS as a mixture of various long-chain-length fatty acid isoamyl esters (FAIEs) and fatty acid ethyl esters (FAEEs), respectively (data not shown). GC/MS analysis of total lipid extracts from recombinant yeast strains proved that these FAIEs and FAEEs were produced exclusively in the mutant strain S. cerevisiae H1246 harboring pESC-URA::atfA but were absent in the wild-type S. cerevisiae G175 and the mutant S. cerevisiae H1246 harboring only the vector control (Fig. 3). Previous studies had already demonstrated that WS/DGAT is capable of utilizing a broad range of linear, saturated and unsaturated, and medium- and long-chain-length fatty alcohols ranging from C12 to C20 (12). Furthermore, in this study radiometric in vitro assays with crude extract of a recombinant E. coli XL1-Blue strain expressing WS/DGAT revealed that this acyltransferase can also utilize the shorter-chain-length alcohols highly efficiently as substrates, and even the branched-chain alcohol isopentanol (isoamyl alcohol) and the water-soluble alcohol ethanol are accepted as substrates with relatively high specificity (Table 4). Experiments with a purified WS/DGAT obtained by chromatographic enrichment from the soluble protein fraction of a recombinant E. coli strain confirmed this broad range of alcohol utilization (T. Stöveken, R. Kalscheuer, and A. Steinbüchel, unpublished results). Thus, these results clearly indicated that FAIEs and FAEEs were synthesized in S. cerevisiae H1246(pESC-URA::atfA) by WS/DGAT activity. In general, the accumulation of TAGs, FAIEs, and FAEEs in the mutant S. cerevisiae H1246 expressing WS/DGAT was reflected by an increased total fatty acid content, whereas recombinant storage lipid synthesis had no significant influence on fatty acid composition (Table 3).
FIG. 1.
Storage lipid biosynthesis in recombinant S. cerevisiae. Cells were cultivated for 24 h at 28°C in synthetic minimal dropout medium without uracil and with 2% (wt/vol) galactose (samples 1 to 3) or 2% (wt/vol) galactose plus 0.1% (wt/vol) oleic acid (samples 4 to 6) and analyzed by TLC. Lane A, ergosterol; lane B, triolein; lane C, cholesteryl pamitate; lane D, palmityl palmitate; lanes 1 and 4, S. cerevisiae G175(pESC-URA); lanes 2 and 5, S. cerevisiae H1246(pESC-URA); lanes 3 and 6, S. cerevisiae H1246(pESC-URA::atfA). Total lipid extracts obtained from 1.5 mg of lyophilized cells (each) were applied to lanes 1 to 6.
FIG. 2.
ESI-MS analysis of TAGs purified from S. cerevisiae H1246(pESC-URA::atfA). Cells were cultivated for 24 h at 28°C in synthetic minimal dropout medium without uracil and with 2% (wt/vol) galactose. TAGs were purified from total lipid extracts of lyophilized cells by preparative TLC and then subjected to ESI-MS analysis. All pseudomolecular ions correspond to [M + Na]+.
TABLE 3.
Total lipid content and fatty acid composition of recombinant yeast strainsa
| S. cerevisiae strain | Total lipids (nmol of acyl groups/mg of CDW) | Acyl composition (mol %)
|
||||
|---|---|---|---|---|---|---|
| C14:0 | C16:0 | C16:1 | C18:0 | C18:1 | ||
| G175(pESC-URA) | 194.9 | TR | 12.3 | 38.6 | 7.3 | 41.8 |
| H1246(pESC-URA) | 108.2 | TR | 5.3 | 38.0 | 8.8 | 47.9 |
| H1246(pESC-URA::atfA) | 138.5 | TR | 11.0 | 42.2 | 12.8 | 34.0 |
Cells were cultivated for 24 h at 28°C in synthetic minimal dropout medium without uracil and with 2% (wt/vol) galactose. Lyophilized cells were analyzed by GC. TR, trace (<1 mol%); CDW, cell dry weight.
FIG. 3.
GC/MS analysis of total lipid extracts from recombinant yeast strains. Cells were cultivated for 24 h at 28°C in synthetic minimal dropout medium without uracil and with 2% (wt/vol) galactose. Total lipid extracts obtained from 2.5 mg of lyophilized cells were applied. The following strains were used: S. cerevisiae H1246(pESC-URA::atfA) (A), S. cerevisiae H1246(pESC-URA) (B), and S. cerevisiae G175(pESC-URA) (C). Identified substances: 1, ethyl palmitate (m/z = 284 [C18H36O2]+); 2, isoamyl myristate (m/z = 298 [C19H38O2]+); 3, ethyl palmitoleate (m/z = 282 [C18H34O2]+); 4, ethyl stearate (m/z = 312 [C20H40O2]+); 5, isoamyl palmitate (m/z = 326 [C21H42O2]+); 6, isoamyl palmitoleate (m/z = 324 [C21H40O2]+); 7, isoamyl stearate (m/z = 354 [C23H46O2]+); 8, isoamyl oleate (m/z = 352 [C23H44O2]+).
TABLE 4.
Substrate specificities of WS/DGAT with various-chain-length alcoholsa
| Alcohol | Enzyme sp act (pmol [mg of protein · min]−1) |
|---|---|
| Ethanol | 30.0 ± 2.3 |
| Butanol | 62.8 ± 5.8 |
| Isoamyl alcohol | 72.1 ± 2.2 |
| Hexanol | 110.2 ± 1.0 |
| Octanol | 99.5 ± 11.1 |
| Decanol | 126.1 ± 5.5 |
| Hexadecanol | 99.7 ± 6.1 |
Enzyme activities were measured in radiometric assays employing crude extract of E. coli XL1-Blue(pKS::atfA) as described in Materials and Methods. Data are mean values of three independent measurements ± SD.
ASAT activity of WS/DGAT.
The inability of WS/DGAT to restore steryl ester biosynthesis in S. cerevisiae H1246 indicated that this enzyme might not possess ASAT activity. This possibility, however, conflicts with the detection of significant ASAT activity in S. cerevisiae H1246 harboring pESC-URA::atfA (Table 2). Therefore, radiometric in vitro ASAT assays with cholesterol or ergosterol as a substrate were also performed by employing crude extract of a recombinant E. coli XL1-Blue strain expressing WS/DGAT. These assays revealed the efficient utilization of both sterols as substrates by WS/DGAT and indicated that this enzyme therefore definitely possesses ASAT activity as well. The ASAT activity level with ergosterol as a substrate was higher than with cholesterol and almost as high as the WS activity (Table 2). The A. calcoaceticus WS/DGAT heterologously expressed in recombinant S. cerevisiae H1246 also exhibited ASAT activity; however, this activity was significantly lower than in E. coli (Table 2). In contrast, the level of DGAT activity of WS/DGAT was twice as high in yeast as in E. coli. Thus, the enzyme showed different substrate specificities depending on whether E. coli or yeast was used as host for heterologous expression.
DISCUSSION
In this report it was demonstrated that the bifunctional WS/DGAT from the bacterium A. calcoaceticus ADP1, which constitutes a novel type of acyltransferase, was heterologously expressed in baker's yeast in a functionally active form conferring high WS as well as DGAT activity to S. cerevisiae. This is the first report of the high-level expression of an enzyme with WS activity in yeast. In contrast, the WS from the jojoba plant could not be functionally expressed in S. cerevisiae (18). However, low WS activity could also be detected in wild-type S. cerevisiae G175, whereas WS activity has never previously been described for yeast. Since WS activity was almost absent in the quadruple mutant strain S. cerevisiae H1246, this activity is probably mediated by one or more of the four genes, DGA1, LRO1, ARE1, and ARE2, which are inactivated in this mutant. Which of the corresponding acyltransferases exhibit WS activity might be determined in the future by employing triple mutants of S. cerevisiae expressing each gene separately (21, 27).
The expression of WS/DGAT in S. cerevisiae H1246 restored TAG biosynthesis in this storage lipid-deficient mutant strain, which clearly demonstrated again that TAG biosynthesis is undoubtedly one of the inherent functions of this enzyme; DGAT activity, however, was relatively low in comparison to WS activity in A. calcoaceticus ADP1 or recombinant E. coli, resulting in the accumulation of only small amounts of TAGs in the natural host, A. calcoaceticus ADP1 (12).
As expected, WS/DGAT expression did not result in wax ester synthesis and accumulation in spite of conferring a high level of WS activity to S. cerevisiae H1246, since baker's yeast is not capable of providing long-chain fatty alcohols as substrates. However, it is known that fatty alcohols occur as intermediates during the degradation of long-chain alkanes. Wax ester biosynthesis, therefore, might be achieved in one of the many yeasts known to utilize alkanes as carbon sources for growth if WS/DGAT from A. calcoaceticus ADP1 could be functionally expressed (36). This suggests a course for the biotechnological production of wax esters in recombinant yeasts.
This study demonstrated that WS/DGAT from A. calcoaceticus ADP1 can also utilize sterols as substrates and, thus, possesses ASAT activity. However, significant differences in substrate specificities were observed, depending on whether yeast or E. coli was used as the host for heterologous expression. In contrast to results in E. coli, the enzyme recombinantly expressed in S. cerevisiae exhibited a much higher level of DGAT activity but only relatively low activity levels with cholesterol and ergosterol as substrates. Slight variations between bacteria and yeast in protein folding or posttranslational modifications could be a possible explanation for this, although no experimental data are available supporting this assumption. The relatively low ASAT activity in yeast might be the reason that WS/DGAT was unable to restore steryl ester synthesis in S. cerevisiae H1246. Another reason might be a lower intracellular concentration of sterols in the mutant, since it has been shown that down-regulation of sterol biosynthesis occurred in mutants defective in ARE1 and ARE2 (3, 37). However, the sterol content in S. cerevisiae H1246 was not determined in this study.
WS/DGAT exhibits an extremely low specificity regarding the chain length of fatty alcohols that can be utilized as substrates. Long-chain fatty alcohols are among the natural substrates of this enzyme in A. calcoaceticus ADP1, resulting in the synthesis of wax esters. However, medium- and short-chain-length alcohols and even ethanol can also be accepted as substrates, as was shown in this study. This indicates the extraordinary and remarkably broad substrate range of WS/DGAT from A. calcoaceticus ADP1. Fatty alcohols and diacylglycerols are the natural substrates of this enzyme in A. calcoaceticus ADP1; however, α,Ω-alkanediols and monoacylglycerols (13) as well as sterols and short- and medium-chain-length alcohols have also been identified as suitable substrates, as shown in this study.
Thus, this enzyme represents not merely a bifunctional but rather a multifunctional acyltransferase. The type of lipid that is synthesized by WS/DGAT in vivo strongly depends on the physiological background of the expression host regarding the provision, through its metabolism, of substrates for the enzyme. In addition to CoA-activated fatty acids, fatty alcohols and diacylglycerols are the natural substrates of WS/DGAT in A. calcoaceticus ADP1, resulting in wax ester and TAG production. In contrast, heterologous expression of WS/DGAT in the alkane-degrading bacterium Pseudomonas citronellolis resulted only in recombinant wax ester synthesis (12). Another example is the mutant strain A. calcoaceticus ADP1acr1ΩKm that produced high-molecular-weight (C48 to C50) wax diesters during cultivation on 1,16-hexadecanediol (13). In S. cerevisiae H1246, however, the metabolism of the yeast cells provided, under the applied cultivation conditions, diacylglycerols, ethanol, and isoamyl alcohol, which is a typical fusel alcohol formed during anaerobic amino acid catabolism in yeast; the compounds were utilized by the recombinantly expressed WS/DGAT as alternative acyl acceptors, resulting in the formation of TAGs, FAIEs, and FAEEs. These results indicate that exploiting the unspecificity of WS/DGAT from A. calcoaceticus ADP1, which allows the use of different prokaryotic and eukaryotic expression hosts, may lead to the biosynthesis of a large variety of lipophilic compounds in vivo. Thus, WS/DGAT has a broad biocatalytic potential for the biotechnological production of novel lipids.
Acknowledgments
We gratefully thank M. Gustavsson, E. Wiberg, P. Stolt, and S. Stymne (Scandinavian Biotechnology Research, Alnarp, Sweden) for generous provision of the S. cerevisiae strains G175 and H1246.
REFERENCES
- 1.Alvarez, H. M., R. Kalscheuer, and A. Steinbüchel. 1997. Accumulation of storage lipids in species of Rhodococcus and Nocardia and effects of inhibitors and polyethylene glycol. Fett/Lipid 99:239-246. [Google Scholar]
- 2.Alvarez, H. M., and A. Steinbüchel. 2002. Triacylglycerols in prokaryotic microorganisms. Appl. Microbiol. Biotechnol. 60:367-376. [DOI] [PubMed] [Google Scholar]
- 3.Arthington-Skaggs, B. A., D. N. Crowell, H. Yang, S. L. Sturley, and M. Bard. 1996. Positive and negative regulation of a sterol biosynthetic gene (ERG3) in the post-squalene portion of the yeast ergosterol pathway. FEBS Lett. 392:161-165. [DOI] [PubMed] [Google Scholar]
- 4.Barksdale, L., and K. S. Kim. 1977. Mycobacterium. Bacteriol. Rev. 41:217-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullock, W. O., J. M. Fernandez, and J. M. Stuart. 1987. XL1-Blue: a high efficiency plasmid transforming recA Escherichia coli strain with β-galactosidase selection. BioTechniques 5:376-379. [Google Scholar]
- 6.Dahlqvist, A., U. Ståhl, M. Lenman, A. Banas, M. Lee, L. Sandager, H. Ronne, and S. Stymne. 2000. Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc. Natl. Acad. Sci. USA 97:6487-6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fixter, L. M., M. N. Nagi, J. G. McCormack, and C. A. Fewson. 1986. Structure, distribution and function of wax esters in Acinetobacter calcoaceticus. J. Gen. Microbiol. 132:3147-3157. [Google Scholar]
- 8.Gotto, A. M., Jr. 1998. Triglyceride as a risk factor for coronary artery disease. Am. J. Cardiol. 82:22Q-25Q. [DOI] [PubMed] [Google Scholar]
- 9.Ishige, T., A. Tani, Y. Sakai, and N. Kato. 2003. Wax ester production by bacteria. Curr. Opin. Microbiol. 6:244-250. [DOI] [PubMed] [Google Scholar]
- 10.Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson, B., S. J. Nelson, and C. M. Brown. 1972. Influence of glucose concentration on the physiology and lipid composition of some yeasts. Antonie Leeuwenhoek 38:129-136. [DOI] [PubMed] [Google Scholar]
- 12.Kalscheuer, R., and A. Steinbüchel. 2003. A novel bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase mediates wax ester and triacylglycerol biosynthesis in Acinetobacter calcoaceticus ADP1. J. Biol. Chem. 278:8075-8082. [DOI] [PubMed] [Google Scholar]
- 13.Kalscheuer, R., S. Uthoff, H. Luftmann, and A. Steinbüchel. 2003. In vitro and in vivo biosynthesis of wax diesters by an unspecific bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase from Acinetobacter calcoaceticus ADP1. Eur. J. Lipid Sci. Technol. 105:578-584. [Google Scholar]
- 14.Kozak, M. 1987. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 15:8125-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozak, M. 1990. Downstream secondary structure facilitates recognition of initiator codons by eukaryotic ribosomes. Proc. Natl. Acad. Sci. USA 87:8301-8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozak, M. 1991. An analysis of vertebrate mRNA sequences: intimations of translational control. J. Cell Biol. 115:887-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krauss, R. M. 1998. Triglycerides and atherogenic lipoproteins: rationale for lipid management. Am. J. Med. 105:58S-62S. [DOI] [PubMed] [Google Scholar]
- 18.Lardizabal, K. D., J. G. Metz, T. Sakamoto, W. C. Hutton, M. R. Pollard, and M. W. Lassner. 2000. Purification of a jojoba embryo wax synthase, cloning of its cDNA, and production of high levels of wax in seeds of transgenic Arabidopsis. Plant Physiol. 122:645-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leber, R., E. Zinser, G. Zellnig, F. Paltauf, and G. Daum. 1994. Characterization of lipid particles of the yeast, Saccharomyces cerevisiae. Yeast 10:1421-1428. [DOI] [PubMed] [Google Scholar]
- 20.Murphy, D. J., and J. Vance. 1999. Mechanism of lipid-body formation. Trends Biol. Sci. 24:109-115. [Google Scholar]
- 21.Oelkers, P., D. Cromley, M. Padamsee, J. T. Billheimer, and S. L. Sturley. 2002. The DGA1 gene determines a second triglyceride synthetic pathway in yeast. J. Biol. Chem. 277:8877-8881. [DOI] [PubMed] [Google Scholar]
- 22.Oelkers, P., A. Tinkelenberg, N. Erdeniz, D. Cromley, J. T. Billheimer, and S. L. Sturley. 2000. A lecithin cholesterol acyltransferase-like gene mediates diacylglycerol esterification in yeast. J. Biol. Chem. 275:15609-15612. [DOI] [PubMed] [Google Scholar]
- 23.Olukoshi, E. R., and N. M. Packter. 1994. Importance of stored triacylglycerols in Streptomyces: possible carbon source for antibiotics. Microbiology 140:931-943. [DOI] [PubMed] [Google Scholar]
- 24.Reiser, S., and C. Somerville. 1997. Isolation of mutants of Acinetobacter calcoaceticus deficient in wax ester synthesis and complementation of one mutation with a gene encoding a fatty acyl coenzyme A reductase. J. Bacteriol. 179:2969-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Sandager, L., A. Dahlqvist, A. Banas, U. Ståhl, M. Lenman, M. Gustavsson, and S. Stymne. 2000. An acyl-CoA:cholesterol acyltransferase (ACAT)-related gene is involved in the accumulation of triacylglycerols in Saccharomyces cerevisiae. Biochem. Soc. Trans. 28:700-702. [PubMed] [Google Scholar]
- 27.Sandager, L., M. H. Gustavsson, U. Ståhl, A. Dahlqvist, E. Wiberg, A. Banas, M. Lenmann, H. Ronne, and S. Stymne. 2002. Storage lipid synthesis is non-essential in yeast. J. Biol. Chem. 277:6478-6482. [DOI] [PubMed] [Google Scholar]
- 28.Smith, S. J., S. Cases, D. R. Jensen, H. C. Chen, E. Sande, B. Tow, D. A. Sanan, J. Raber, R. H. Eckel, and R. V. Farese, Jr. 2000. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat. Genet. 25:87-90. [DOI] [PubMed] [Google Scholar]
- 29.Song, S. 2002. The role of increased liver triglyceride content: a culprit of diabetic hyperglycaemia? Diabetes Metab. Res. Rev. 18:5-12. [DOI] [PubMed] [Google Scholar]
- 30.Sorger, D., and G. Daum. 2002. Synthesis of triacylglycerols by the acyl-coenzyme A:diacylglycerol acyltransferase Dga1p in lipid particles of the yeast Saccharomyces cerevisiae. J. Bacteriol. 184:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorger, D., and G. Daum. 2003. Triacylglycerol biosynthesis in yeast. Appl. Microbiol. Biotechnol. 61:289-299. [DOI] [PubMed] [Google Scholar]
- 32.Stein, S., A. Levitsky, O. Fateev, and G. Mallard. 1998. The NIST mass spectral search program, version 1.6d. National Institute of Standards and Technology, Gaithersburg, Md.
- 33.Steinbüchel, A. 1996. PHB and other polyhydroxyalkanoic acids, p. 403-464. In H. J. Rehm, G. Reed, A. Pühler, and P. Stadler (ed.), Biotechnology, 2nd ed., vol. 6. Wiley VCH, Heidelberg, Germany. [Google Scholar]
- 34.Taskinen, M. R. 1997. Triglyceride is the major atherogenic lipid in NIDDM. Diabetes Metab. Rev. 13:93-98. [DOI] [PubMed] [Google Scholar]
- 35.Timm, A., and A. Steinbüchel. 1990. Formation of polyesters consisting of medium-chain-length 3-hydroxyalkanoic acids from gluconate by Pseudomonas aeruginosa and other fluorescent pseudomonads. Appl. Environ. Microbiol. 56:3360-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Beilen, J. B., Z. Li, W. A. Duetz, T. H. M. Smits, and B. Witholt. 2003. Diversity of alkane hydroxylase systems in the environment. Oil Gas Sci. Technol. 58:427-440. [Google Scholar]
- 37.Yang, H. Y., M. Bard, D. A. Bruner, D. A. Gleeson, R. J. Deckelbaum, G. Aljinovic, T. M. Pohl, R. Rothstein, and S. L. Sturley. 1996. Sterol esterification in yeast: a two-gene process. Science 272:1353-1356. [DOI] [PubMed] [Google Scholar]
- 38.Yu, C., N. J. Kennedy, C. C. Chang, and J. A. Rothblatt. 1996. Molecular cloning and characterization of two isoforms of Saccharomyces cerevisiae acyl-CoA:sterol acyltransferase. J. Biol. Chem. 271:24157-24163. [DOI] [PubMed] [Google Scholar]
- 39.Zinser, E., F. Paltauf, and G. Daum. 1993. Sterol composition of yeast organelle membranes and subcellular distribution of enzymes involved in sterol metabolism. J. Bacteriol. 175:2853-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zweytick, D., E. Leitner, S. D. Kohlwein, C. Yu, J. Rothblatt, and G. Daum. 2000. Contribution of Are1p and Are2p to steryl ester synthesis in the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 267:1075-1082. [DOI] [PubMed] [Google Scholar]