In response to DNA damage or replication stress, DNA damage and replication checkpoints are activated and play critical roles in preventing entry into mitosis. The checkpoint-mediated G2 delay is orchestrated by the apical kinases ATR and ATM, and their critical effector kinases, Chk1 and Chk2, respectively.1 The ATR-Chk1 branch is predominantly activated by high levels of RPA-coated single stranded DNA (ssDNA), and the ATM-Chk2 branch by double strand breaks (DSBs). The main underlying mechanism of the damage checkpoint-mediated G2 arrest in response to DNA damage cues involves suppression of mitotic cyclin-dependent kinase (CDK1), which is critical for driving cells into and through mitosis. Downregulation of mitotic CDK1 by the damage checkpoint is compounded by inhibitory phosphorylation of CDK1 mediated by WEE1 and MYT1 kinases, and downregulation of the phosphatase CDC25, which can remove the above-mentioned inhibitory phosphorylation and thereby reactivate CDK1.1
In the CDK-centric view of mitosis, the above-mentioned mechanisms are sufficient to explain why and how G2 checkpoint activation by DNA damage incurred during DNA replication prevents the onset of mitosis, and particularly chromosome condensation, to allow for DNA damage repair. However, the situation may be more complex than that. The checkpoint may additionally down-regulate effectors with roles in the onset of mitosis and/or factors that interfere with DNA repair processes scheduled to take place in the prolonged G2 phase. This question has been recently investigated by the Surana and Hudson laboratories who reported that in response to DNA damage, the Chk2 kinase is critical to prevent recruitment of condensin complexes to chromatin and, subsequently, chromosome condensation, independently from CDK1 inhibition (Fig. 1).2
Figure 1.
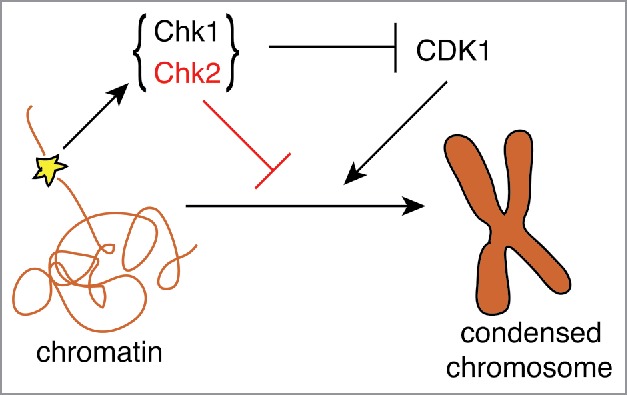
DNA damage (yellow star) activates the DNA damage checkpoint, which negatively regulates DNA condensation both directly, via CDK1 inhibition, and indirectly, predominantly through Chk2.
To observe a direct effect of DNA damage on chromosome condensation, Zhang et al. forced cells with activated G2 damage checkpoint to enter mitosis by expressing a constitutively active form of CDK1 (CDK1AF) — containing mutations at Thr14 and Tyr15 that prevent inhibitory phosphorylation by WEE1 and MYT1 — from a conditional Tet-ON promoter.2 They inflicted DNA damage using a Topoisomerase II inhibitor, Adryamycin, which causes formation of DSBs. As predicted, the authors found that CDK1AF drives entry into mitosis also when cells have an activated G2 checkpoint, but interestingly, those cells display abnormal mitoses with largely decondensed chromosomes. Inhibiting the G2 checkpoint with caffeine or using individual or combinatorial inhibition of the checkpoint kinases with small inhibitors could largely alleviate the condensation defect,2 suggesting a direct link between G2 damage checkpoint activation and suppression of chromosome condensation.
How does G2 checkpoint activation suppress condensation? Zhang et al. find that checkpoint activation triggered by topoisomerase II inhibition correlates with impaired recruitment of condensin complexes to chromosomes, in spite of unrestrained CDK1 activity.2 The condensation defect could be largely alleviated by siRNA against Chk2, but much less so using siRNA against Chk1 or ATR. Moreover, the authors found that Chk2 alters the status of phosphorylation of 2 subunits of the condensin complexes.2 As CDK1-mediates phosphorylation of condensins in mitosis and this is essential for chromosome condensation,3,4 one possibility is that activation of the Chk2 damage checkpoint axis counteracts CDK1-mediated phosphorylation/activation of condensins. It is also possible, however, that Chk2 targets condensins and this affects their phosphorylation by CDK1. Regardless of the exact mechanism involved, which at the moment remains unknown, this recent study highlights that independently of promoting CDK1 inactivation in response to DNA damage, the G2 damage checkpoint prevents the recruitment of condensins to chromosomes and thus, actively prevents chromosome condensation.2
The above-mentioned findings expand on the multipronged effects of the G2 damage checkpoint activation in halting the onset of mitosis and highlight novel, physiologically important targets of the G2 checkpoint. The work also opens new lines for future research with potential to distinguish how different types of DNA lesions causing ATR/Chk1 or ATM/Chk2 checkpoint activation affect the function of condensins in mitosis. Notably, Topoisomerase II, inhibited by Adryamycin, is required for chromosome condensation in vivo and mitotic chromatids reconstitution in vitro.5,6 It remains formally possible that the effects of Adryamycin observed in this recent study are different from the effects of other drugs, endogenous types of lesions, or replication problems that also trigger G2 delay. These alternative situations may also be easier to study in molecular detail with respect to condensin function, as Topoisomerase II is itself a critical player in mitotic chromosome condensation.5 Interestingly, earlier studies in budding yeast indicate that Topoisomerase II and condensin are responsible for fragility of late replicating regions when the function of the ATR checkpoint is impaired.7
In conclusion, the recent study of Zhang et al 2 opens the door toward understanding how different branches of the replication and damage checkpoint may be critical in preventing the action of condensins early in mitosis, to prevent replication-associated fragility or to ensure efficient repair of genomic damage.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Branzei D, Foiani M. Regulation of DNA repair throughout the cell cycle. Na Rev Mol Cell Biol 2008; 9:297-308; PMID:18285803; http://dx.doi.org/ 10.1038/nrm2351 [DOI] [PubMed] [Google Scholar]
- [2].Zhang T, Si-Hoe SL, Hudson DF, Surana U. Condensin recruitment to chromatin is inhibited by Chk2 kinase in response to DNA damage. Cell Cycle 2016; 15(24):3454-3470; PMID:27792460; http://dx.doi.org/9774278 10.1080/15384101.2016.1249075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kimura K, Hirano M, Kobayashi R, Hirano T. Phosphorylation and activation of 13S condensin by Cdc2 in vitro. Science 1998; 282:487-90; PMID:9774278; http://dx.doi.org/ 10.1126/science.282.5388.487 [DOI] [PubMed] [Google Scholar]
- [4].Abe S, Nagasaka K, Hirayama Y, Kozuka-Hata H, Oyama M, Aoyagi Y, Obuse C, Hirota T. The initial phase of chromosome condensation requires Cdk1-mediated phosphorylation of the CAP-D3 subunit of condensin II. Genes Dev 2011; 25:863-74; PMID:21498573; http://dx.doi.org/ 10.1101/gad.2016411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shintomi K, Takahashi TS, Hirano T. Reconstitution of mitotic chromatids with a minimum set of purified factors. Nat Cell Biol 2015; 17:1014-23; PMID:26075356; http://dx.doi.org/ 10.1038/ncb3187 [DOI] [PubMed] [Google Scholar]
- [6].Samejima K, Samejima I, Vagnarelli P, Ogawa H, Vargiu G, Kelly DA, de Lima Alves F, Kerr A, Green LC, Hudson DF, et al.. Mitotic chromosomes are compacted laterally by KIF4 and condensin and axially by topoisomerase IIalpha. J Cell Biol 2012; 199:755-70; PMID:23166350; http://dx.doi.org/ 10.1083/jcb.201202155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hashash N, Johnson AL, Cha RS. Topoisomerase II- and condensin-dependent breakage of MEC1ATR-sensitive fragile sites occurs independently of spindle tension, anaphase, or cytokinesis. PLoS Genet 2012; 8:e1002978; PMID:23133392; http://dx.doi.org/ 10.1371/journal.pgen.1002978 [DOI] [PMC free article] [PubMed] [Google Scholar]


