Abstract
The nucleotide sequences, genetic organization, and distribution of plasmids pEU30 (30,314 bp) and pEL60 (60,145 bp) from the plant pathogen Erwinia amylovora are described. The newly characterized pEU30 and pEL60 plasmids inhabited strains isolated in the western United States and Lebanon, respectively. The gene content of pEU30 resembled plasmids found in plant-associated bacteria, while that of pEL60 was most similar to IncL/M plasmids inhabiting enteric bacteria.
Erwinia amylovora is the causal agent of fire blight, a serious disease of apple and pear that occurs in many locations throughout the world. The virulence plasmid pEA29 is conserved among all natural strains of E. amylovora, and several PCR assays for the identification of E. amylovora were developed on the basis of sequences within a 0.9- to 1.1-kb PstI fragment of this plasmid (2, 12). Knowledge of other less widely occurring plasmids in E. amylovora is quite limited. pCPP60, a 56-kb plasmid from E. amylovora Ea322 and Ea273, was characterized by restriction enzyme analysis and detected in 10 of 40 strains collected from different hosts and geographic regions (18), and recently, small plasmids of 1.8 and 2.8 kb from E. amylovora IH3-1 and IL-5, respectively, were characterized by sequence analysis (10). Some strains of E. amylovora with resistance to streptomycin harbor plasmid pEa34 or pEa8.7, each carrying the functional strA-strB gene pair (4, 13). Considering the limited number of plasmids found in E. amylovora, we were intrigued about the possible role of other plasmids inhabiting strains of this plant pathogen. Here, we report the occurrence of two previously unknown plasmids in E. amylovora, a 30-kb plasmid pEU30 from strain UTRJ2 and a 60-kb plasmid pEL60 from strain LebB66. We sequenced these plasmids, determined their genetic organization, and predicted their possible role in E. amylovora. We also examined the frequency and geographic distribution of pEU30 and pEL60 in a geographically diverse collection of E. amylovora strains.
Bacterial strains and plasmid selection.
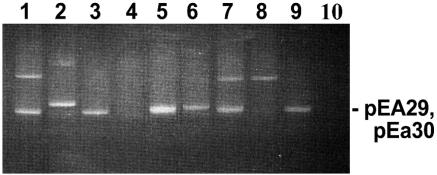
We selected 54 strains for plasmid analysis (Table 1) from a collection of 167 E. amylovora strains from the United States and Canada (8, 11), and Lebanon (17) that were previously uncharacterized for plasmid content. Plasmid isolations were done using the method of Kado and Liu (6) as modified by Sundin et al. (19). All 54 strains examined contained the ubiquitous plasmid pEA29; however, analyses of intact plasmids and plasmids digested with KpnI or PstI revealed additional plasmids or restriction fragments in some strains (data not shown). The plasmid profiles of selected new strains were compared with profiles of E. amylovora strains Ea322, CA11, and Ea88 that contain the other characterized plasmids from this species (Fig. 1). Each wild-type strain contains pEA29, and pCPP60 is present in Ea322 (18), pEa34 is present in CA11 (4), and plasmids of approximately 30 and 60 kb are present in strains UTRJ2 and LebB66, respectively (Fig. 1). The 30-kb plasmid in strain UTRJ2 appears to comigrate with pEA29 and is better visualized in the pEA29-cured strain UTRJ2− (Fig. 1, lane 6). The 60-kb plasmid in strain LebB66 clearly differed in size from pCPP60 (Fig. 1), and primers designed to detect pEL60 (see below) did not amplify DNA from pCPP60 (data not shown). As a result of this work, strains UTRJ2 and LebB66 were identified as containing novel plasmids for genetic analysis.
TABLE 1.
Distribution of pEU30 and pEL60 within a collection of 167 strains of E. amylovora
| Source of straina | Host | No. of strainsb | No. of strains containing:
|
|
|---|---|---|---|---|
| pEL60 | pEU30 | |||
| CA, USA | Apple | 4 | 0 | 0 |
| ID, USA | Apple | 3 | 0 | 0 |
| Plum | 1 | 0 | 0 | |
| LA, USA | Ind. hawth.c | 5 | 0 | 0 |
| MI, USA | Apple | 35 (19) | 0 | 0 |
| NY, USA | Apple | 1 | 0 | 0 |
| OR, USA | Pear | 8 | 0 | 4 |
| UT, USA | Apple | 10 (6) | 0 | 5 |
| WA, USA | Apple | 10 (1) | 0 | 2 |
| Pear | 11 | 0 | 5 | |
| Canada | Apple | 2 | 0 | 0 |
| France | Pear | 1 | 0 | 0 |
| Ind. hawth. | 1 | 0 | 0 | |
| Lebanon | Apple | 28 (21) | 13 | 0 |
| Pear | 12 (4) | 7 | 0 | |
| Quince | 9 (3) | 3 | 0 | |
| New Zeal. | Apple | 10 | 0 | 0 |
| IL, USA | Rubus spp. | 1 | 0 | 0 |
| MI, USA | Rubus spp. | 8 | 0 | 0 |
| Canada | Raspberry | 5 | 0 | 0 |
| Canada | Unknown | 1 | 0 | 0 |
| OK, USA | Unknown | 1 | 0 | 0 |
Abbreviations: CA, California; IL, Illinois; LA, Louisiana; MI, Michigan; NY, New York; New Zeal., New Zealand; OK, Oklahama; OR, Oregon; USA, United States of America; UT, Utah; WA, Washington.
The numbers listed indicate the total numbers of strains examined for the presence of pEU30 and pEL60 by PCR assay, and numbers in parentheses indicate the number of strains initially examined for indigenous plasmid content.
Ind. hawth., Indian hawthorn.
FIG. 1.
Plasmid profiles of selected E. amylovora strains and pEA29-cured derivatives. Lane 1, Ea322; lane 2, CA11; lane 3, Ea88; lane 4, Ea88 cured of pEA29; lane 5, UTRJ2; lane 6, UTRJ2− (cured of pEA29); lane 7, LebB66; lane 8, LebB66− (cured of pEA29); lane 9, MR1; lane 10, water control.
Plasmid sequencing and gene annotation.
We cured plasmid pEA29 from strains UTRJ2 and LebB66 via a previously described incompatibility eviction assay by use of pC9, a construct that contains the pEA29 ori region (9). pEA29-cured strains, from which pC9 was also eliminated, were designated UTRJ2− and LebB66−. Plasmids pEU30 and pEL60 were isolated from E. amylovora strains UTRJ2− and LebB66−, respectively, by use of NucleoBond Plasmid Maxi kits (Clontech Laboratories, Palo Alto, Calif.), following the manufacturer's instructions for low-copy plasmids. Each purified plasmid was partially digested with Sau3AI, yielding ca. 1- to 2.5-kb fragments that were excised and purified using a Nucleotrap gel extraction kit (Clontech Laboratories) according to the manufacturer's instructions except that the DNA was eluted with water at 80°C. Fragment ligations into pGem7zf- (Promega, Madison, Wis.) and subsequent preparative steps for high-throughput sequencing were performed as previously described (8). All sequencing reactions were run at the Michigan State University Genomics Technology Support Facility. Sequence fragments were analyzed and assembled using Lasergene software of DNASTAR Inc. (Madison, Wis.). Gaps in the sequence were closed by primer walking from existing sequences by use of purified plasmids as templates. This procedure resulted in the complete sequence of pEU30 (3.1× coverage per consensus base) and pEL60 (3.8× coverage) on both strands. Potential open reading frames (ORFs) more than 300 bp in size were identified using the online program Orfinder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html) and the program GeneJockey (Biosoft; Cambridge, United Kingdom). To assign possible functions to ORFs, searches of the nonredundant GenBank database were done using the suite of BLAST programs (1). BLASTx searches of the entire sequence were also done to identify potential ORFs smaller than 300 bp.
Sequence and analysis of pEU30.
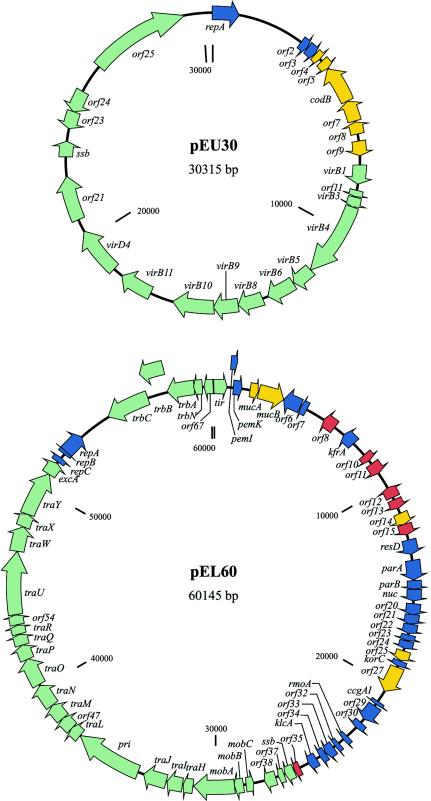
Plasmid pEU30 contained 30,314 bp with a G+C content of 48.2% (Fig. 2A); annotation of this sequence revealed 25 putative ORFs. Analyses using BLASTn and BLASTx searches of the GenBank nonredundant databases revealed that 23 of 25 (92.0%) ORFs had homology with existing gene sequences; further analyses with BLASTp enabled the assignment of putative gene functions to these ORFs (Table 2). The majority of pEU30 ORFs encoded genes involved in replication, maintenance, or conjugative transfer (Fig. 2A and Table 2). Five clustered ORFs, including three transcriptional regulators (orf5, orf7, and orf9), a cytosine permease (orf6), and an acetyltransferase (orf8) which shared homology with a gene that flanks the hrp type III secretion system genes of E. amylovora Ea321, encoded other functions (Table 2). The conjugative transfer system of pEU30 was a VirB-like system that was most similar to that of pPSR1, a plasmid from another plant pathogen, Pseudomonas syringae (20).
FIG. 2.
Circular genetic maps of the 30,314-bp plasmid pEU30 (A) and 60,145-bp plasmid pEL60 (B) and their 25 and 68 identified ORFs, respectively. repA is designated orf1 in pEU30; tir is designated orf1 of pEL60 to closely match the gene order of the highly related plasmid pCTX-M3 from C. freundii (GenBank accession number NC_0044644). Genes are color coded on the basis of function as follows: replication and stability, blue; ecological fitness, yellow; transcriptional regulators, red; and conjugation- or plasmid-specific functions, green. The number or identity of each ORF is located inside the circle. On the pEL60 genetic map, the asterisks denote locations of 27 and 2.6-kb sequence insertions downstream of repA and pemK in the closely related plasmid pCTX-M3.
TABLE 2.
Similarity of pEU30 ORFs to proteins in the GenBank nonredundant database
| pEU30 ORF | Best similarity (% amino acid identity/similarity) at the gene product level to: | Accession no. |
|---|---|---|
| repA | Yersinia enterocolitica hypothetical Orf1 (54/70) | CAA73746 |
| orf2 | Mesorhizobium loti killer protein Mlr1576 (54/71) | NP_103132 |
| orf3 | M. loti antidote protein Mlr1577 (44/67) | NP_103133 |
| orf4 | R721 hypothetical protein YgiB (41/67) | NP_065372 |
| orf5 | Yersinia pestis pYC transcriptional regulator Orf3 (35/60) | NP_053152 |
| codB | Streptomyces coelicolor cytosine permease (39/59) | NP_624885 |
| orf7 | Photorhabdus luminescens transcriptional regulator Orf66 (32/52) | AAO17220 |
| orf8 | E. amylovora acetyltransferase Orf12 (37/54) | AAB06009 |
| orf9 | R6K regulatory protein Actx (35/52) | CAC20137 |
| virB1 | P. syringae pv. syringae pPSR1 VirB1 (41/57) | NC_005205 |
| orf11 | No significant homology | |
| virB3 | P. syringae pv. syringae pPSR1 VirB3 (40/58) | NC_005205 |
| virB4 | P. syringae pv. syringae pPSR1 VirB4 (49/65) | NC_005205 |
| virB5 | P. syringae pv. syringae pPSR1 VirB5 (46/68) | NC_005205 |
| virB6 | P. syringae pv. syringae pPSR1 VirB6 (39/60) | NC_005205 |
| virB8 | P. syringae pv. syringae pPSR1 VirB8 (32/54) | NC_005205 |
| virB9 | P. syringae pv. syringae pPSR1 VirB9 (46/58) | NC_005205 |
| virB10 | P. syringae pv. syringae pPSR1 VirB10 (32/47) | NC_005205 |
| virB11 | P. syringae pv. syringae pPSR1 VirB11 (54/66) | NC_005205 |
| virD4 | P. syringae pv. syringae pPSR1 VirD4 (47/65) | NC_005205 |
| orf21 | Escherichia coli STEC autoagglutinating adhesin Saa (36/53) | AAL26283 |
| ssb | Serratia marcescens Ssb (84/89) | P25762 |
| orf23 | No significant homology | |
| orf24 | Xylella fastidiosa conserved hypothetical protein XFa0050 (43/66) | NP_061705 |
| orf25 | P. syringae pv. syringae pPSR1 relaxosome Orf53 (40/56) | NC_005205 |
Sequence and analysis of pEL60.
Plasmid pEL60 contained 60,145 bp with a G+C content of 51.5%. Annotation of this sequence revealed 68 ORFs (Fig. 2B); however, putative functions could only be assigned to 46 (68.7%) of the ORFs. As with pEU30, the genes on pEL60 with known homologs were most closely related to genes from other γ-proteobacteria. pEL60 was remarkably similar to plasmid pCTX-M3 from Citrobacter freundii (unpublished data; GenBank accession number NC004464), sharing 66 of 68 ORFs (Table 3). pCTX-M3 is approximately 29 kb larger than pEL60 due to the presence of two insertions that encode various antibiotic-resistance genes, an integron, and other putative mobile elements (Fig. 2B). Characteristics of pEL60 include an IncL/M replication region and tra-like conjugative transfer genes that are similar to genes found on the IncL/M plasmid pACM1 from Klebsiella oxytoca (15) and also to tra genes from plasmids isolated from other enteric bacteria and from the plant pathogen P. syringae pv. tomato DC3000 (Table 2). pEL60 was not related to the 56-kb plasmid (18) found in some E. amylovora strains, as a labeled pEL60 probe did not hybridize to EcoRI-digested total plasmid DNA from E. amylovora Ea273 (data not shown). pEL60 also encodes a mucAB-like mutagenic DNA repair system (Table 3) that is related to rulAB, a similar repair system encoding tolerance to UV radiation in P. syringae (21). The organization and linkage of pemIK and mucAB on pEL60 are identical to those of the IncL/M plasmid R446b from Morganella morganii (7). mucAB may be of environmental significance to E. amylovora, because mutagenic DNA repair determinants such as P. syringae rulAB confer UV radiation tolerance that may enhance ecological fitness on plant surfaces (21).
TABLE 3.
Similarity of pEL60 ORFs to proteins in the GenBank nonredundant database
| pEL60 ORF | Presence in pCTX-M3 | Best similarity (% amino acid identity/similarity) at the gene product level toa: | Accession no. |
|---|---|---|---|
| tir | + | R100 transfer inhibition protein Tir (84/89) | NP_052992 |
| pemI | + | R100 stable inheritance protein PemI (97/100) | NP_052994 |
| pemK | + | R100 stable inheritance protein PemK (94/97) | NP_052994 |
| mucA | + | R446b mutagenic DNA repair protein MucA (98/98) | AAC82517 |
| mucB | + | R446b mutagenic DNA repair protein MucB (99/99) | AAC82518 |
| orf6 | + | ColIb-P9 hypothetical protein YacC (87/91) | NP_052452 |
| orf7 | + | Proteus vulgaris hypothetical protein (41/59) | NP_640006 |
| orf8 | − | No significant homology | |
| kfrA | − | pO157 maintenance protein KfrAs (53/62) | NP_052633 |
| orf10 | + | Cf Orf10b, NSH | |
| orf11 | + | Cf Orf11; NSH | |
| orf12 | + | Cf Orf13; NSH | |
| orf13 | + | Cf Orf14; NSH | |
| orf14 | + | Salmonella enterica subsp. enterica serovar Typhi putative membrane protein STY4561 (44/65) | NP_458647 |
| orf15 | + | Cf Orf17; NSH | |
| resD | + | ColIBM-C1139 resolvase ResD (50/66) | P18021 |
| parA | + | ColIb-P9 stability protein ParA (64/78) | NP_052472 |
| parB | + | ColIb-P9 stability protein ParB (32/52) | NP_052473 |
| nuc | + | Yersinia pestis plasmid endonuclease YPTM1.73 (43/63) | NP_395419 |
| orf20 | + | Cf Orf19; NSH | |
| orf21 | + | Cf Orf20; NSH | |
| orf22 | + | R27 hypothetical protein R0167 (27/44) | NP_058380 |
| orf23 | + | Shewanella oneidensis conserved hypothetical SO0799 (29/44) | NP_716430 |
| orf24 | + | S. enterica subsp. enterica serovar Typhi putative membrane protein STY4595 (36/53) | NP_458679 |
| orf25 | + | Escherichia coli RadC-like protein YfjY (61/74) | NP_417131 |
| korC | + | RK2 transcriptional repressor KorC (44/61) | Q52331 |
| orf27 | + | S. enterica subsp. enterica serovar Typhi putative membrane protein STY1015 (42/56) | NP_455494 |
| ccgAI | + | R46 CcgAI (62/81) | NP_511209 |
| orf29 | + | ColIb-P9 conserved hypothetical YdiA (53/71) | NP_052499 |
| orf30 | + | R721 hypothetical protein YagA (49/71) | NP_065301 |
| rmoA | + | R721 RmoA-like protein YdfA (47/70) | NP_065337 |
| orf32 | + | E. coli hypothetical protein c0276 (53/60) | NP_752220 |
| orf33 | + | ColIb-P9 hypothetical protein YcgC (28/48) | NP_052486 |
| orf34 | + | Cf Orf30; NSH | |
| klcA | + | RK2 anti-restriction protein KlcA (41/51) | P52603 |
| orf36 | + | Cf Orf32; NSH | |
| ssb | + | S. oneidensis Ssb (48/68) | NP_719558 |
| orf38 | + | S. enterica subsp. enterica serovar Typhi hypothetical plasmid protein HCM2.0094c (39/58) | NP_569566 |
| orf39 | + | Cf Orf34; NSH | |
| mobC | + | Pseudomonas syringae pv. tomato MobC (34/60) | NP_808620 |
| mobB | + | P. syringae pv. tomato MobB (36/63) | NP_790826 |
| mobA | + | P. syringae pv. tomato MobA (35/51) | NP_808687 |
| traH | + | P. syringae pv. tomato TraH (41/54) | NP_808701 |
| traI | + | P. syringae pv. tomato TraI (53/68) | NP_808702 |
| traJ | + | P. syringae pv. tomato TraJ (46/62) | NP_808703 |
| pri | + | P. syringae pv. tomato primase Pri (32/48) | NP_808707 |
| traL | + | Klebsiella oxytoca TraL (100/100) | AAD33806 |
| orf48 | + | K. oxytoca hypothetical protein Orf6 (99/99) | AAD33807 |
| traM | + | P. syringae pv. tomato TraM (28/53) | NP_808709 |
| traN | + | ColIb-P9 TraN (45/59) | NP_052519 |
| traO | + | P. syringae pv. tomato TraO (31/48) | NP_808711 |
| traP | + | ColIb-P9 TraP (23/40) | NP_052517 |
| traQ | + | ColIb-P9 TraQ (35/55) | NP_052516 |
| traR | + | CfTraR; NSH | |
| orf55 | + | CfOrf38; NSH | |
| traU | + | P. syringae pv. tomato TraU (37/55) | NP_808718 |
| traW | + | P. syringae pv. tomato TraW (39/58) | NP_808720 |
| traX | + | P. syringae pv. tomato TraX (33/49) | NP_808721 |
| traY | + | ColIb-P9 TraY (37/55) | NP_052508 |
| excA | + | P. syringae pv. tomato exclusion-determining protein ExcA (28/45) | NP_808723 |
| repC | + | K. pneumoniae RepC (85/93) | U27345 |
| repB | + | K. pneumoniae RepB (93/93) | AAA87027 |
| repA | + | K. pneumoniae RepA (94/97) | AAA87028 |
| trbC | + | P. syringae pv. tomato TrbC (40/60) | NP_808661 |
| trbB | + | ColIb-P9 TrbB (37/52) | NP_052503 |
| trbA | + | P. syringae pv. tomato TrbA (32/51) | NP_808724 |
| trbN | + | E. coli putative lytic transglycosylase TrbN | NP_053074 |
| orf68 | + | CfOrf47; NSH |
Amino acid identities with corresponding ORFs from pCTX-M3 are not listed but were >90% for all ORFs denoted as present in pCTX-M3 except for Pri and ORF12, for which the amino acid identities were 72 and 76%, respectively. The protein with the next best similarity is listed; where no other significant homology (NSH) was noted, the corresponding pCTX-M3 ORF is listed.
Cf Orf refers to genes from C. freundii pCTX-M3.
Plasmid stability and distribution of pEU30 and pEL60 within an E. amylovora strain collection.
We assessed the stability of pEU30 and pEL60 in their native host strains in a 100-generation (10 generations/day) serial transfer experiment in a minimal broth medium (5). A total of 25 colonies were examined after 100 generations, and plasmid maintenance was 96 and 100% for pEU30 and pEL60, respectively. Plasmid maintenance of pEU30 was 100% after 80 and 90 generations. These experiments indicated that both plasmids are highly stable in their native E. amylovora host strains.
We used colony PCR assays to detect the presence of pEU30 and pEL60 within a collection of 167 E. amylovora strains from various hosts and regions of the world. PCR assays were performed as previously described (10), and PCR products were analyzed by electrophoresis using 1% agarose gels. We used the primer set AJ889 (5′-GCCGGGGCGTGGAACAGAAG-3′) and AJ890 (5′-TCATGCCGGAAGAGTCAAACC-3′) for the detection of pEU30 and used the primer set AJ901 (5′-GCCGGCCGCTGTTACCGGGTTTG-3′) and AJ902 (5′-TCTGTGGTGCCGCGTTGATGAAGC-3′) for the detection of pEL60. The primers amplified sequences of 483 bp (pEU30, located within the virB10 gene) and 402 bp (pEL60, comprising the 3′ end of parB [intergenic region] and the 5′ end of nuc), and primer specificity was checked by confirming that the primers did not amplify DNA from other known E. amylovora plasmids (data not shown). To check primer fidelity, we sequenced PCR fragments from five and three strains containing pEU30 and pEL60, respectively, and the correct DNA fragment was amplified in each case (data not shown). pEU30 was detected in a total of 16 (9.6%) strains from this collection and was only detected in E. amylovora strains isolated from apple or pear in the western United States; the plasmid was present in 41.0% of the strains from that region (Table 1). pEL60 was detected in a total of 23 strains (13.8%) from the collection and only in E. amylovora strains from Lebanon (Table 1). pEL60 was present in 46.9% of the strains from Lebanon which were isolated from three plant hosts (Table 1).
Significance of pEU30 and pEL60 in E. amylovora.
pEL30 was comprised mostly of genes encoding plasmid-specific functions but also encoded several putative transcriptional regulatory proteins and ORF8, which has similarity to ORF12, a GNAT family acetyltransferase encoded by a chromosomal gene located immediately adjacent to genes encoding the type III secretion system of E. amylovora Ea321 (3). The conjugal transfer genes of pEU30 were most similar to the virB system of P. syringae plasmid pPSR1 (20), which is interesting because both of these transfer systems are located on plasmids from plant pathogens. From these results, it appears that the genetic content of pEU30 is similar to that of other plasmids inhabiting plant pathogenic bacteria.
pEL60, which was present in 47% of the E. amylovora strains from Lebanon, encodes a variety of genes thought to be involved in maintenance and conjugation functions. The most notable aspects of pEL60 are its relationship with other enterobacterial IncL/M plasmids and its high similarity to the C. freundii plasmid pCTX-M3. This observation suggests that the environmental enteric plant pathogen E. amylovora can access the horizontal gene pool shared among other clinical enteric bacteria. An alternative possibility is that C. freundii obtained a pEL60-like plasmid from E. amylovora prior to the evolution of pCTX-M3. IncL/M plasmids are widespread among enteric pathogens, typically encode resistance to multiple antibiotics, and harbor mobile elements including integrons and IS elements associated with antibiotic-resistance (Abr) genes (14, 16, 22). For example, pCTX-M3 contains insertions of 27 and 2.6 kb downstream of repA and pemK, respectively. These sequence insertions include an integron, two copies of IS26, and several antibiotic resistance genes, including blaTEM1, blaCTX-M3, aac3, dhfr, sul1, and three putative macrolide-resistance genes (GenBank Accession NC_0044644). Unfortunately, most IncL/M plasmids are not well characterized beyond the replication regions and Abr regions; thus, the genetic relationships of these plasmids to pEL60 are unknown. However, the close relationship of pCTX-M3 and pEL60, aside from the differences in Abr gene content, could be indicative of additional similarities between pEL60 and other IncL/M resistance plasmids. pEL60, at 60.1 kb, could represent an IncL/M backbone plasmid that has acquired Abr genes, integrons, and IS elements during its spread among other enteric pathogens. For example, the sizes of other multiple-Abr IncL/M plasmids include 80 kb for pIBK1 and 85 kb for pCFF04 from Klebsiella pneumoniae (16), 100 kb for pSEM from Salmonella enterica serotype Typhimurium (22), and 89 kb for pCTX-M3, sizes that could allow for 20- to 40-kb insertions of Abr genes into a pEL60-like IncL/M plasmid backbone.
Unlike pEA29, the conserved plasmid that plays an important role in E. amylovora virulence (9), pEU30 and pEL60 do not appear to encode many genes that are likely to influence the fire blight disease. Instead, these plasmids may represent molecules that can spread efficiently, with limited effects on the reproductive fitness of their bacterial host. Coexistence of pEU30 or pEL60 with their E. amylovora hosts may be a first step in the future acquisition of novel genetic determinants by these plasmids that affect ecological fitness. Regardless of possible function, we conclude that some populations of E. amylovora are plasmid diverse and that this characteristic may be helpful in understanding strain biogeography.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the pEU30 and pEL60 sequences are NC_005247 and NC_005246, respectively.
Acknowledgments
We thank G. E. Halcomb, S. V. Thomson, and A. T. Saad for sending E. amylovora strains, Kim Maxson-Stein and Lisa Erickson for technical assistance, and Marlene Cameron for assistance with figure preparation.
This work was supported by grants from the USDA-CSREES and the Michigan Apple Committee and by the Michigan Agricultural Experiment Station.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A.A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bereswill, S., A. Pahl, P. Bellemann, W. Zeller, and K. Geider. 1992. Sensitive and species-specific detection of Erwinia amylovora by polymerase chain reaction analysis. Appl. Environ. Microbiol. 58:3522-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogdanove, A. J., Z.-M. Wei, L. Zhao, and S. V. Beer. 1996. Erwinia amylovora secretes harpin via a type III pathway and contains a homolog of yopN of Yersinia spp. J. Bacteriol. 178:1720-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiou, C.-S., and A. L. Jones. 1991. The analysis of plasmid-mediated streptomycin resistance in Erwinia amylovora. Phytopathology 81:710-714. [Google Scholar]
- 5.Falkenstein, H., W. Zeller, and K. Geider. 1989. The 29 kb plasmid common in strains of Erwinia amylovora modulates development of fire blight symptoms. J. Gen. Microbiol. 135:2643-2650. [Google Scholar]
- 6.Kado, C. I., and S. T. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulaeva, O. I., E. V. Koonin, J.C. Wootton, A.S. Levine, and R. Woodgate. 1998. Unusual insertion element polymorphisms in the promoter and terminator regions of the mucAB-like genes of R471a and R446b. Mutat. Res. 397:247-262. [DOI] [PubMed] [Google Scholar]
- 8.Maxson-Stein, K., G. C. McGhee, J. J. Smith, A.L. Jones, and G. W. Sundin. 2003. Genetic analysis of a pathogenic Erwinia sp. isolated from pear in Japan. Phytopathology 93:1393-1399. [DOI] [PubMed] [Google Scholar]
- 9.McGhee, G. C., and A. L. Jones. 2000. Complete nucleotide sequence of ubiquitous plasmid pEA29 from Erwinia amylovora strain Ea88: gene organization and intraspecies variation. Appl. Environ. Microbiol. 66:4897-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGhee, G. C., E. L. Schnabel, K. Maxson-Stein, B. Jones, V. K. Stromberg, G.H. Lacy, and A. L. Jones. 2002. Relatedness of chromosomal and plasmid DNAs of Erwinia pyrifoliae and Erwinia amylovora. Appl. Environ. Microbiol. 68:6182-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McManus, P. S., and A. L. Jones. 1994. Epidemiology and genetic analysis of streptomycin-resistant Erwinia amylovora from Michigan and evaluation of oxytetracycline for control. Phytopathology 84:627-633. [Google Scholar]
- 12.McManus, P. S., and A. L. Jones. 1995. Detection of Erwinia amylovora by nested PCR and PCR-dot-blot and reverse-blot hybridizations. Phytopathology 85:618-623. [Google Scholar]
- 13.Palmer, E. L., B. L. Teviotdale, and A. L. Jones. 1997. A relative of the broad-host-range plasmid RSF1010 detected in Erwinia amylovora. Appl. Environ. Microbiol. 63:4604-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petit, A., G. Gerbaud, D. Sirot, P. Courvalin, and J. Sirot. 1990. Molecular epidemiology of TEM-3 (CTX-1) β-lactamase. Antimicrob. Agents Chemother. 34:219-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preston, K. E., C. C. A. Radomski, and R. A. Venezia. 2000. Nucleotide sequence of a 7-kb fragment of pACM1 encoding an IncM DNA primase and other putative proteins associated with conjugation. Plasmid 44:12-23. [DOI] [PubMed] [Google Scholar]
- 16.Prodinger, W. M., M. Fille, A. Bauernfeind, I. Stemplinger, S. Amann, B. Pfausler, C. Lass-Flori, and M. P. Dierich. 1996. Molecular epidemiology of Klebsiella pneumoniae producing SHV-5 β-lactamase: parallel outbreaks due to multiple plasmid transfer. J. Clin. Microbiol. 34:564-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saad, A. T., L. Hanna, and E. Choueiri. 2000. Evaluation of streptomycin and oxytetracycline resistance of Erwinia amylovora populations in Lebanon. Phytopathology 90:S68. [Google Scholar]
- 18.Steinberger, E. M., G.-Y. Cheng, and S. V. Beer. 1990. Characterization of a 56-kb plasmid of Erwinia amylovora Ea322: its noninvolvement in pathogenicity. Plasmid 24:12-24. [DOI] [PubMed] [Google Scholar]
- 19.Sundin, G. W., A. L. Jones, and D. W. Fulbright. 1989. Copper resistance in Pseudomonas syringae pv. syringae and its associated transfer in vitro and in planta with a plasmid. Phytopathology 79:861-865. [Google Scholar]
- 20.Sundin, G. W., C. T. Mayfield, Y. Zhao, T.S. Gunasekera, G.C. Foster, and M. Ullrich. 2004. Complete nucleotide sequence and analysis of pPSR1 (72,601 bp), a pPT23A family plasmid from Pseudomonas syringae pv. syringae A2. Mol. Genet. Genom. 270:462-475. [DOI] [PubMed] [Google Scholar]
- 21.Sundin, G. W., and J. Murillo. 1999. Functional analysis of the Pseudomonas syringae rulAB determinant in tolerance to ultraviolet B (290-320 nm) radiation and distribution of rulAB among P. syringae pathovars. Environ. Microbiol. 1:75-87. [DOI] [PubMed] [Google Scholar]
- 22.Villa, L., C. Pezzela, F. Tosini, P. Visca, A. Petrucca, and A. Carattoli. 2000. Multiple-antibiotic resistance mediated by structurally related IncL/M plasmids carrying an extended-spectrum β-lactamase gene and a class 1 integron. Antimicrob. Agents Chemother. 44:2911-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]