Abstract
The long-term usefulness of Bacillus thuringiensis Cry toxins, either in sprays or in transgenic crops, may be compromised by the evolution of resistance in target insects. Managing the evolution of resistance to B. thuringiensis toxins requires extensive knowledge about the mechanisms, genetics, and ecology of resistance genes. To date, laboratory-selected populations have provided information on the diverse genetics and mechanisms of resistance to B. thuringiensis, highly resistant field populations being rare. However, the selection pressures on field and laboratory populations are very different and may produce resistance genes with distinct characteristics. In order to better understand the genetics, biochemical mechanisms, and ecology of field-evolved resistance, a diamondback moth (Plutella xylostella) field population (Karak) which had been exposed to intensive spraying with B. thuringiensis subsp. kurstaki was collected from Malaysia. We detected a very high level of resistance to Cry1Ac; high levels of resistance to B. thuringiensis subsp. kurstaki Cry1Aa, Cry1Ab, and Cry1Fa; and a moderate level of resistance to Cry1Ca. The toxicity of Cry1Ja to the Karak population was not significantly different from that to a standard laboratory population (LAB-UK). Notable features of the Karak population were that field-selected resistance to B. thuringiensis subsp. kurstaki did not decline at all in unselected populations over 11 generations in laboratory microcosm experiments and that resistance to Cry1Ac declined only threefold over the same period. This finding may be due to a lack of fitness costs expressed by resistance strains, since such costs can be environmentally dependent and may not occur under ordinary laboratory culture conditions. Alternatively, resistance in the Karak population may have been near fixation, leading to a very slow increase in heterozygosity. Reciprocal genetic crosses between Karak and LAB-UK populations indicated that resistance was autosomal and recessive. At the highest dose of Cry1Ac tested, resistance was completely recessive, while at the lowest dose, it was incompletely dominant. A direct test of monogenic inheritance based on a backcross of F1 progeny with the Karak population suggested that resistance to Cry1Ac was controlled by a single locus. Binding studies with 125I-labeled Cry1Ab and Cry1Ac revealed greatly reduced binding to brush border membrane vesicles prepared from this field population.
Transgenic Bacillus thuringiensis plants used commercially to date produce Cry toxins constitutively and have proved successful in terms of target control, reduction in the use of chemical insecticides, and environmental safety (4, 12, 14). One of the main issues related to use of this technology is the potential for development of resistance by target insect pests due to intense selection pressure (11, 41). Evolution of resistance to pesticides is often based on a substitution of alleles. This substitution can have a major effect at one or several loci (24, 40) and is favored under strong pesticide selection that acts on initial genotypic variation. It has been suggested that the extremely high selection pressure in the field favors resistance alleles with a major effect (34) but that the lower intensity of selection in laboratory selection experiments favors polygenic control of resistance. McKenzie and Batterham (35) proposed that an insecticide concentration that kills 90% of a susceptible population would be expected to produce polygenically based resistance.
The current “best practice” model for managing the evolution of resistance to transgenic B. thuringiensis crops is the high-dose-refuge strategy, which is to combine an expression of toxin sufficient to kill all heterozygous insects with the use of refuges (plots containing conventional crops not expressing B. thuringiensis toxins) (37, 54). This strategy has assumed that the inheritance of B. thuringiensis resistance in insects is recessive, so if resistance genes are predominantly rare and carried by heterozygotes, then these individuals will have no fitness advantage over susceptible homozygous insects (e.g., see the work of Gould [18]). However, a few studies have shown that resistance to Cry1A is incompletely dominant (17, 41, 44, 45). Genetic and biochemical studies with different insect species have shown that a recessive mode of inheritance of high levels of resistance and cross-resistance to Cry1A toxins is generally related to a lack of toxin binding to midgut receptors (14). In contrast, incompletely dominant alleles are also associated with a reduced activation of Cry toxin (43, 45). Models used to predict the evolution of resistance to Cry toxins often assume that resistance is due to one gene with two alleles (50), although studies of some insect populations have suggested more than one factor for resistance (19, 25, 38, 41, 44, 45, 50).
Knowledge of the mode of inheritance and ecology of resistance genes can help to devise and improve resistance management strategies. To examine these factors, a field population of the diamondback moth (Plutella xylostella) from Malaysia with a history of exposure to B. thuringiensis subsp. kurstaki (41) was collected and tested in the laboratory. The overall aim of the present work was to test whether the genetics, mechanisms, and stability of resistance to Cry1Ac differed between a field-selected strain and laboratory-selected or -reselected strains. In the first part of this study, field-evolved resistance to various B. thuringiensis toxins was evaluated and the mode of inheritance of resistance and number genes involved were determined. In the second part of the study, alteration of the binding of Cry1A toxins to larval midgut binding sites was tested as a possible mechanism of resistance. Finally, the stability of resistance was examined in large, replicated, outbred subpopulations of the field strain.
MATERIALS AND METHODS
Insects.
A field population (Karak) of P. xylostella moths was collected from the Karak area in Kuala Lumpur, Malaysia, in November 2001. An insecticide-susceptible population (LAB-UK) of P. xylostella was obtained from Rothamsted Research (Harpenden, Hertfordshire, United Kingdom), where it had been maintained in the laboratory for more than 150 generations. Insect larvae were reared and tested on 4- to 6-week-old pesticide-free, greenhouse-grown Chinese cabbage (Brassica pekinensis cv. Tip Top) at 20°C and ca. 65% relative humidity under a 16-h photophase.
B. thuringiensis products.
Pure activated toxins Cry1Aa, Cry1Ab, Cry1Ac, Cry1Ca, Cry1Fa, and Cry1Ja were obtained, purified, and prepared for bioassay and for binding experiments as described previously (16, 44). Two commercial products based on B. thuringiensis were used: Dipel and MVP. Dipel is based on B. thuringiensis subsp. kurstaki HD-1 (32,000 IU mg−1 [wettable powder]), which produces Cry1Aa, Cry1Ab, Cry1Ac, Cry2A, and vegetative insecticidal proteins (47), and was purchased from Biowise (Didcot, United Kingdom). MVP (10%; Mycogen, San Diego, Calif.) is a formulation of Cry1Ac protoxin expressed and encapsulated by transgenic Pseudomonas fluorescens. This product has been used as an alternative form of pure Cry1Ac protoxin in other published works (32, 49). Each test product was freshly prepared in distilled water with Triton X-100 (50 μg ml−1) added as a surfactant (45).
Toxicity bioassay.
All bioassays were conducted with third-instar larvae of P. xylostella on leaf disks as described by Sayyed et al. (44). Each leaf disk (4.8-cm diameter) was immersed in a test solution for 10 s and allowed to dry at ambient temperature for 1 to 1.5 h (45). Control leaf disks were immersed in distilled water with Triton X-100. The leaf disks were placed in individual petri dishes (5-cm diameter) containing moistened filter paper. Five larvae were placed in each dish, and each treatment was repeated eight times. Mortality was determined after 5 days. In order to characterize field resistance to Cry1Ac (MVP) and B. thuringiensis subsp. kurstaki, the Karak population was bulked up in the laboratory for a single generation (G1), and range-finding bioassays were conducted at G1 for Cry1Ac and Cry1Ca. Full bioassays were conducted at G2.
Stability of resistance.
A long-term study to examine the stability of resistance to Cry1Ac and to B. thuringiensis subsp. kurstaki in the Karak population was set up in January 2002. The experiment was started at G3 with eight replicated subpopulations reared in large culture cages (1 by 0.75 by 0.55 m). The insects were maintained continuously at 22°C on four to six Chinese cabbage plants per cage in the absence of any selection pressure. Each subpopulation was initiated with 320 pupae to avoid inbreeding. Plants were replaced at regular intervals to maintain a constant food supply. After 2 months, the size of the experiment was reduced to five subpopulations. The change in susceptibility to Cry1Ac and B. thuringiensis subsp. kurstaki was assessed with a leaf dip bioassay after G8 and G13. Bioassays used a minimum of four treatments, including one control per subpopulation and 25 to 30 insects per dose.
Evolution of maternal effects and sex linkage.
The responses of the F1 and F2 progenies to MVP were evaluated. Mass and single-pair reciprocal crosses between the Karak and LAB-UK populations produced the F1 progeny. The F2 progeny were produced by single-pair crosses with the Karak population. The larvae of both sexes were separated at the fourth instar based on the color of the fifth abdominal segment. F1 mass crosses used 50 adults of each sex and provided enough offspring for multiple-concentration testing and calculation of the 50% lethal concentration (LC50).
F1 progeny from the single-pair crosses between the LAB-UK and Karak populations were obtained. Single pairs consisted of a LAB-UK virgin male and a Karak virgin female or vice versa. F1 progeny from each family were reared on a separate Chinese cabbage plant. The F1 larvae were tested in a leaf dip bioassay with 0.1 and 0.5 μg of MVP ml−1. To obtain F2 progeny, single-pair crosses were made between F1 progeny (from mass crosses between the Karak and LAB-UK populations) and the Karak population. The F2 progeny from single-pair crosses were tested with 1 mg of MVP ml−1.
Tests of F1 and F2 progeny from single-pair crosses enabled the detection of genetic variation within parental strains, which was not possible with mass crosses (44). Detection of genetic variation within parental strains is important because standard methods for estimating dominance assume that the susceptible and resistant parental strains are homozygous (22). If the parental strains are genetically varied at the locus or loci controlling resistance, then estimates of dominance may be biased (44, 50). Calculation of minimum numbers of independently segregating loci was performed using Lande's method (28).
Estimation of degree of dominance.
The degree of dominance for the LC50 (DLC) was calculated as described by Bourguet et al. (5). Effective dominance (DML) was calculated from mortality values at a single concentration (5) as follows: DML = (MLRS − MLSS)/(MLRR − MLSS), where MLRR, MLRS, and MLSS are the mortality values at a particular toxin concentration for the field population, the F1 progeny, and the LAB-UK population, respectively. The DML values range from 0 (completely recessive resistance) to 1 (completely dominant resistance).
Binding experiments.
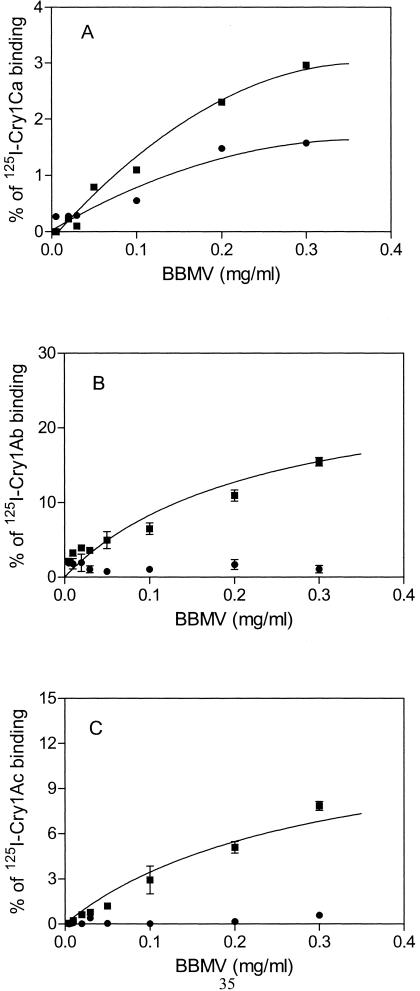
Brush border membrane vesicles (BBMV) from whole Karak and LAB-UK last-instar larvae were prepared by the differential magnesium precipitation method (58) as modified by Escriche et al. (13). BBMV were frozen in liquid nitrogen and kept at −80°C until used. The protein concentration in the BBMV was measured (6) using bovine serum albumin as a standard. Cry1Ab was 125I labeled by the chloramine-T method (57). Cry1Ac and Cry1Ca were 125I labeled by the Iodo-Bead (Pierce, Rockford, Ill.) method (30). A toxin can be inactivated depending on the labeling method used. For example, Cry1Ca is inactivated when it is labeled with the chloramine-T method (B. Escriche, unpublished). Specific activities of the labeled proteins were analyzed by an enzyme-linked immunosorbent sandwich assay (56). The specific activities for 125I-Cry1Ab, 125I-Cry1Ac, and 125I-Cry1Ca were 0.48, 0.08, and 0.02 mCi mg−1, respectively. Binding experiments were performed as described previously (44), except that the bound toxins were separated from unbound toxins by centrifugation at 11,000 × g for 10 min with two washes of 0.5 ml of cold binding buffer (1 mM KH2PO4, 10 mM Na2HPO4, 137 mM NaCl, 2.7 mM KCl [pH 7.4], 0.1% bovine serum albumin). The amounts of labeled toxin in the experiments were 7, 39, and 157 ng for 125I-Cry1Ab, 125I-Cry1Ac, and 125I-Cry1Ca, respectively. The radioactivity in the pellet was measured in a model 1282 Compugamma CS gamma counter (LKB Pharmacia). Two binding experiments were performed with Cry1Ab and Cry1Ac. A single Cry1Ca binding experiment was performed as a positive control for the activity of the BBMV (Fig. 1A). Nonspecific binding was determined by using 1,000 ng of unlabeled toxin, and the following values were obtained with 0.3 mg of BBMV/ml from the total binding: 10% for Cry1Ab, 1.2% for Cry1Ac, and 1.5% for Cry1Ca.
FIG. 1.
Specific binding of 125I-labeled pure activated toxins Cry1Ca (A), Cry1Ab (B), and Cry1Ac (C) as a function of P. xylostella BBMV concentration for the susceptible strain LAB-UK (▪) and resistant strain Karak (•). Nonspecific binding values were subtracted from each total binding point to obtain the specific binding. Each point represents the result from a single experiment for Cry1Ca and the mean of results of two experiments for Cry1Ab and Cry1Ac. The standard error of the mean is represented by bars.
Statistical analysis.
When necessary, bioassay data were corrected for control mortality (1). Estimates of LC50s and their 95% fiducial limits (FL) were obtained by maximum-likelihood logit regression analysis in a generalized linear model using the statistical package GLIM 3.77 (Numerical Algorithms Group), from which differences between sets were extracted by analysis of deviance (9). Differences between the LC50s of two sets were considered significant (P < 0.01) if their 95% FL did not overlap.
RESULTS
Toxicity to Cry toxins.
The LAB-UK and Karak populations were tested with six purified Cry toxins and two bioinsecticide formulations, B. thuringiensis subsp. kurstaki (containing Cry1Aa, Cry1Ab, Cry1Ac, Cry2A, and vegetative insecticidal proteins) and MVP (Cry1Ac) (Table 1). The LAB-UK population was significantly more susceptible to Dipel and purified Cry1Ac toxin than the other toxins tested. Cry1Ab, Cry1Aa, and Cry1Fa appeared to be the least toxic (Table 1).
TABLE 1.
Toxicity of B. thuringiensis subsp. kurstaki and Cry toxins to the susceptible laboratory (LAB-UK) and resistant field (Karak) populations of P. xylostella
| Population | Toxin used | LC50 (95% FL)a | Avg slope (±SE) | RRb | nc |
|---|---|---|---|---|---|
| LAB-UK | B. thuringiensis subsp. kurstaki | 0.0039 (0.0013-0.021) | 1.025 (0.23) | 180 | |
| MVP | 0.027 (0.012-0.060) | 1.72 (0.39) | 184 | ||
| Cry1Ac | 0.007 (0.002-0.016) | 1.36 (0.26) | 154 | ||
| Cry1Ab | 0.47 (0.20-1.77) | 1.02 (0.21) | 180 | ||
| Cry1Aa | 0.039 (0.0001-0.78) | 0.41 (0.18) | 180 | ||
| Cry1Fa | 0.20 (0.055-10.25) | 0.48 (0.37) | 181 | ||
| Cry1Ja | 0.031 (0.010-2.23) | 0.92 (0.35) | 178 | ||
| Cry1Ca | 0.07 (0.023-0.177) | 1.07 (0.16) | 185 | ||
| Karak | B. thuringiensis subsp. kurstaki | 2.97 (2.18-4.15) | 3.30 (0.60) | 770 | 185 |
| MVP | 9,800 (7,500-13,600) | 4.68 (1.49) | 363,000 | 180 | |
| Cry1Ac | >40d | >5,710 | 180 | ||
| Cry1Ab | 77.0 (57.9-110) | 3.75 (0.72) | 164 | 182 | |
| Cry1Aa | 32.9 (24.2-45.8) | 3.32 (0.54) | 845 | 180 | |
| Cry1Fa | 82.7 (67.2-103) | 5.29 (0.77) | 414 | 183 | |
| Cry1Ja | 0.84 (0.59-1.25) | 2.98 (0.44) | 27 | 180 | |
| Cry1Ca | 4.15 (2.87-5.70) | 3.46 (0.70) | 59 | 180 |
Cry toxin concentrations are in micrograms per milliliter. B. thuringiensis subsp. kurstaki data are in international units per milligram per milliliter.
RR, resistance ratio of the LC50 for the selected subpopulation to that for the LAB-UK population.
n, number of larvae used in the bioassay, including the controls.
Mortality at 40 μg ml−1 was 46% (logit analysis gave an LC50 of 75.8 with a 95% FL of 21.7 to 5,560).
When compared with LAB-UK, the Karak population showed significant levels of resistance to a number of pure Cry toxins, B. thuringiensis subsp. kurstaki (Dipel), and MVP (Table 1). Relative resistance to Cry1Ac (purified toxin or MVP) was much greater than to any of the other toxins tested. However, there was no significant (P > 0.01) difference among the LC50s of purified Cry1Ac, Cry1Ab, Cry1Aa, and Cry1Fa (Table 1). The LC50s for the Karak population at G2 were consistent with the results of preliminary assays of resistance from the previous generation (data not shown).
Stability of resistance.
After 11 generations without exposure to insecticides, the susceptibility of the Karak population to B. thuringiensis subsp. kurstaki had not changed significantly (Table 2). Measurements of the stability of resistance to Cry1Ac were more variable and depended on the generation assessed. After five generations without selection, the toxicity of Cry1Ac had increased 10-fold (P < 0.01), with a rate of decline in resistance of −0.17 (Table 2). At G13, the toxicity of Cry1Ac had decreased only threefold compared with that at G2, an overall rate of decline in resistance of −0.04 (Table 2).
TABLE 2.
Stability of resistance to B. thuringiensis subsp. kurstaki (Dipel) and Cry1Ac toxin (MVP) in the resistant field population Karak of P. xylostella
| Generation from field | Insecticide used | LC50 (95% FL)a | Avg slope (±SE) | RRb | Rc |
|---|---|---|---|---|---|
| G2 | B. thuringiensis subsp. kurstaki | 2.97 (2.18-4.15) | 3.30 (0.60) | 770 | |
| G2 | Cry1Ac | 9,800 (7,500-13,600) | 0.90 (0.28) | 363,000 | |
| G8 | B. thuringiensis subsp. kurstaki | 1.70 (1.03-2.75) | 0.96 (0.11) | 440 | −0.04 |
| G8 | Cry1Ac | 980 (420-2170) | 0.96 (0.11) | 36,900 | −0.17 |
| G13 | B. thuringiensis subsp. kurstaki | 2.98 (1.81-5.89) | 0.25 (0.08) | 770 | 0.00 |
| G13 | Cry1Ac | 3,220 (1,860-5,820) | 0.71 (0.08) | 119,000 | −0.04 |
Cry toxin concentrations are in micrograms per milliliter. B. thuringiensis subsp. kurstaki data are in international units per milligram per milliliter.
RR, resistance ratio of the LC50 for the selected subpopulation to those for the LAB-UK population.
R, log (final LC50 − initial LC50)n, where n is the number of generations in the population reared without insecticide exposure.
Maternal effects, sex linkage, and genetic variation in resistance to Cry1Ac.
Following reciprocal mass crosses, the LC50s and slopes obtained by exposure to Cry1Ac for F1 progeny of Karak females and LAB-UK males were not significantly (P > 0.01) different from those for F1 progeny of Karak males and LAB-UK females (Table 3); thus, inheritance of resistance was autosomal. Neither maternal effects nor sex linkage were evident. Similarly, single-pair crosses between Karak and LAB-UK populations indicated that there was no significant difference in mortality between families from both reciprocal crosses made between Karak and LAB-UK populations at the two doses tested (0.1 and 0.5 μg ml−1). Mean pool values of mortality proportions ± standard errors were 0.64 ± 0.07 for 0.1 μg ml−1 and 0.97 ± 0.06 for 0.5 μg ml−1 (Table 4).
TABLE 3.
Responses to MVP (mortality) of resistant (Karak) and susceptible (LAB-UK) P. xylostella larvae and their hybrid F1 progeny
| Strain | LC50 (μg ml−1) | 95% FL | Avg slope (±SE) | DLCa |
|---|---|---|---|---|
| Karak field population | 3,790 | 2,490-6350 | 2.29 (0.32) | |
| LAB-UK population | 0.027 | 0.012-0.060 | 1.72 (0.39) | |
| F1 (Karak female × LAB-UK male) | 0.028 | 0.010-0.06 | 1.71 (0.43) | 0.0039 |
| F1 (Karak male × LAB-UK female) | 0.057 | 0.017-0.15 | 1.28 (0.37) | 0.06 |
| F1 (pooled) | 0.039 | 0.019-0.07 | 1.45 (0.28) | |
| F1 × Karak population | 3.62 | 1.15-28.2 | 0.40 (0.20) |
DLC = (log LCRS − log LCss)/(log LCRR − log LCss) (DLC varies from 0 to 1, where 0 is completely recessive and 1 is completely dominant).
TABLE 4.
Dominance of resistance to MVP in the Karak population of P. xylostella as a function of the concentration of MVP for single-pair hybrid F1 families
| Single-pair F1 family(ies) | MVP concn
|
|||
|---|---|---|---|---|
| 0.1 μg ml−1
|
0.5 μg ml−1
|
|||
| Mortality (α) | DMLa | Mortality (∞) | DML | |
| Karak | 0.00 | 0.00 | ||
| LAB-UK | 0.80 | 1.00 | ||
| Karak female × LAB-UK male | 0.60 | 0.25 | 0.96 | 0.04 |
| Karak female × LAB-UK male | 0.60 | 0.25 | 1.00 | 0.00 |
| Karak female × LAB-UK male | 0.72 | 0.10 | 1.00 | 0.00 |
| Karak female × LAB-UK male | 0.72 | 0.10 | 1.00 | 0.00 |
| Karak male × LAB-UK female | 0.52 | 0.35 | 0.84 | 0.16 |
| Karak male × LAB-UK female | 0.68 | 0.15 | 0.96 | 0.04 |
| Karak male × LAB-UK female | 0.68 | 0.15 | 1.00 | 0.00 |
| Karak male × LAB-UK female | 0.60 | 0.25 | 1.00 | 0.00 |
Estimates of DML values range from 0 (completely recessive resistance) to 1 (completely dominant resistance).
Degree of dominance.
Bioassays of F1 progeny from mass and single-pair crosses between field and LAB-UK populations showed that dominance of resistance depended upon the concentration of Cry1Ac (Tables 3 and 5). It was incompletely dominant at the lowest concentration and completely recessive at the highest concentration tested. Although the resistance ratio was approximately 360,000-fold for the resistant Karak population, ratios were only 1- and 2-fold for F1 progenies. The LC50s for the Karak and LAB-UK populations and their F1 progenies from mass crosses yielded DLC values of 0.0039 and 0.006, respectively. This finding indicated that inheritance of resistance was completely recessive (Material and Methods; Table 3).
TABLE 5.
Dominance of resistance to MVP in the Karak population of P. xylostella as a function of the concentration of MVP
| Concn (μg ml−1) | Population | Mortalitya (%) | DML |
|---|---|---|---|
| 0.005 | Karak | 0 | |
| LAB-UK | 27 | ||
| F1 progeny | 10 | 0.63 | |
| 0.01 | Karak | 0 | |
| LAB-UK | 42 | ||
| F1 progeny | 33 | 0.21 | |
| 0.05 | Karak | 0 | |
| LAB-UK | 51 | ||
| F1 progeny | 42 | 0.18 | |
| 0.1 | Karak | 0 | |
| LAB-UK | 78 | ||
| F1 progeny | 55 | 0.29 | |
| 0.5 | Karak | 0 | |
| LAB-UK | 96 | ||
| F1 progeny | 89 | 0.07 |
Numbers of larvae tested were 25 for Karak and LAB-UK populations and 60 for F1 progeny.
The estimated DMLs from single-pair families ranged from 0 to 0.35 at 0.1 μg of Cry1Ac per ml and 0 to 0.16 at 0.5 μg/ml (Table 4).
Number of factors influencing resistance.
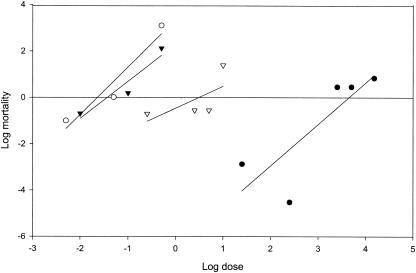
Analysis of backcross data (Tables 3 and 6 and Fig. 2) suggests that one locus conferred resistance to Cry1Ac in the field population. The direct test for a monogenic mode of inheritance of resistance showed no significant deviation (P > 0.05) between observed and expected rates of mortality at any concentration of Cry1Ac against backcross progeny (Karak moths × F1 progeny) (Table 6). This finding indicated that a single-gene model was an acceptable fit for the data at the concentrations tested.
TABLE 6.
Direct test of monogenic inheritance for resistance to Cry1Ac by comparing expected and observed mortalities of the backcross of F1 progeny and the Karak population of P. xylostella
| Concn (μg ml−1) | Observed mortality | Expected mortalitya | χ2 (df = 1)c | Pb |
|---|---|---|---|---|
| 0.025 | 10 | 7.00 | 1.28 | 0.26 |
| 0.25 | 10 | 9.00 | 0.11 | 0.74 |
| 2.5 | 11 | 15.00 | 1.07 | 0.30 |
| 5 | 11 | 19.00 | 3.37 | 0.07 |
| 15 | 24 | 21.75 | 0.23 | 0.63 |
The expected number of larvae dead at given dose is the starting number times 0.5 (the proportion of F1 larvae that die plus the proportion of field larvae that die).
Probability values were considered significantly different at a P of <0.05.
df, degree of freedom.
FIG. 2.
Response to MVP of P. xylostella larvae of the LAB-UK (○) and Karak (•) strains, F1 progeny (Karak × LAB-UK) (▾), and backcross progeny (Karak × F1) (▿).
Slope, variance, and minimum number of genes involved in resistance to Cry1Ac.
Estimates for the slope of logit mortality against toxin concentration were much lower for backcross progeny than for LAB-UK and Karak moths and their F1 hybrid progeny (Table 3). This pattern indicates increased genetic variance in the backcross progeny compared with that of parental populations and F1 progeny and also suggests that the number of loci with major effects on resistance to Cry1Ac was small (48). By using the method of Lande (28), the minimum number of independently segregating loci with equal and additive effects to resistance was estimated to be 0.60.
Binding assays.
Binding of pure, activated Cry1Ab, Cry1Ac, and Cry1Ca toxins to BBMV was evaluated in the resistant Karak and susceptible LAB-UK strains by incubation with increasing concentrations of BBMV with iodinated proteins (Fig. 1). The control toxin used (Cry1Ca) specifically bound to the BBMV of both strains (Fig. 1A). Experiments with labeled Cry1Ab and Cry1Ac showed a major difference in their levels of specific binding to vesicles from the two strains (Fig. 1B and C). At the highest concentration of BBMV used (0.3 mg ml−1), levels of specific binding of 15 and 8% were found for Cry1Ab and Cry1Ac, respectively, in the susceptible strain compared with 1 and 0.6%, respectively, in the Karak population.
DISCUSSION
High levels of resistance to B. thuringiensis toxins have evolved several times in field populations of P. xylostella moths (21, 26, 55). Until the present study, fully characterized resistance to B. thuringiensis or Bacillus sphaericus has been described only for populations that have undergone reselection in the laboratory (10, 14, 44, 45, 50). However, prolonged laboratory selection can produce insecticide resistance traits with different mechanisms and genetics from those of field-selected resistance (16, 34). Given the widespread use of laboratory-selected P. xylostella as a model system for testing theories of resistance management, especially in relation to B. thuringiensis transgenic crops, a comparison of the properties of laboratory- and field-selected populations would be instructive.
In the present study, lower levels of resistance to B. thuringiensis subsp. kurstaki than to purified Cry1Ac can be explained in part by the lower levels of resistance found to two other component toxins (Cry1Aa and Cry1Ab); the influence of other components of B. thuringiensis subsp. kurstaki (Cry2A and vegetative insecticidal proteins) was not investigated. B. thuringiensis subsp. kurstaki contains 32.2% Cry1Ac (33), and the proportion of Cry1Ac in 2.97 IU mg−1 ml−1 (the LC50 of B. thuringiensis subsp. kurstaki) is therefore 29 mg ml−1, whereas the LC50 of the purified toxin is greater than 40 mg ml−1.
Cross-resistance between toxins of the Cry1A family might have been expected, as these are known to bind to the same receptor site in the insect midgut epithelium (3) and share more than 80% homology (11). Loss of receptor binding is the most commonly reported cause of resistance to Cry1A toxins in P. xylostella (16, 17, 25, 44, 50, 59). However, further studies are required to determine the causes of resistance in the Karak population, since the possibility of other mechanisms cannot be excluded. The low level of resistance to Cry1Ca compared with that to Cry1A toxins and Cry1Fa was not due to loss of binding, since the binding assays showed that the Karak population still maintained a significant level of binding. However, it is not possible to exclude the possibility of reduced binding as the resistance mechanism for Cry1Ca. It has been shown that Cry1Ca recognizes a binding site different from those of Cry1A toxins in P. xylostella (3, 47). The low level of resistance to Cry1Ca may be explained by the more limited use of products based on B. thuringiensis subsp. aizawai, which contains Cry1Aa, Cry1Ab, Cry1Da, and Cry1Ca (47). These results are in agreement with those of other studies where it has been shown that the resistance to Cry1Ca is not due to loss of binding (31, 59). Lack of cross-resistance between Cry1Ca and Cry1A toxins has also been reported for three other P. xylostella populations collected from lowland Malaysia (44, 45, 59).
Resistance in the Karak population declined very slowly (Cry1Ac) or was stable (B. thuringiensis subsp. kurstaki) over 11 generations in the absence of selection. Typically, high levels of resistance to B. thuringiensis have been reported to decline very quickly (8, 21, 26, 45, 52, 55), irrespective of whether populations have been selected in the laboratory or the field. A slow loss of resistance (R < −0.05) is normally associated only with low-to-moderate B. thuringiensis resistance (<100-fold), as has been found for Plodia interpunctella (36). The stability of resistance in the Karak strain has at least two nonmutually exclusive explanations. First, fitness costs expressed by resistance strains can be environmentally dependent and may not occur under ordinary laboratory culture conditions (7). Alternatively, resistance in the Karak population may have been near fixation, leading to a very slow increase in heterozygosity. The variation in resistance between cages and sampling dates does, however, lend more support to the former explanation. In similar microcosm experiments over 12 generations with another Malaysian population, SERD4, that had been reselected in the laboratory, resistance declined rapidly at a rate of −0.40 (8). Previous stability studies have cultured B. thuringiensis-resistant lepidoptera on either Brassica napus (26, 55), Brassica oleracea (51), radish, Raphanus sativus (21), or an artificial diet (27), whereas all our experiments with P. xylostella populations from Malaysia have used the plant species on which they were collected, namely, B. pekinensis (8, 46). B. oleracea offers more natural resistance to attack by P. xylostella than most B. pekenensis varieties, and ongoing studies suggest that resistance genes in the Karak population impose increased fitness costs when larvae are cultured on B. oleracea (B. Raymond, A. H. Sayyed, and D. J. Wright, unpublished data).
The inheritance of resistance in the Karak population in this study was autosomal and monogenic. All other studies of B. thuringiensis resistance have also shown an autosomal mode of inheritance (25, 41, 44, 48), although a few have suggested paternal or maternal influences (31, 40, 44). Monogenic inheritance has been demonstrated for at least three populations, two laboratory-reselected (42, 53) and one field-selected (21) strain of P. xylostella. Polygenic inheritance has been found to be equally distributed in field (26)- and laboratory (16, 45)-reselected populations. Thus, both major and minor factors may contribute to field- and laboratory-selected B. thuringiensis resistance, as theoretical findings predict (20). Major genes, which by definition have a larger effect on fitness, tend to respond to any kind of selection more quickly than minor genes.
Resistance in the Karak population was found to be recessive, a mode of inheritance that is an important basis of the high-dose-refuge strategy for the management of resistance in transgenic crops (50). However, the dominance of resistance genes has been found to increase with the reduction in dose of B. thuringiensis toxin encountered in this, and other, populations from Malaysia (41, 44, 45). Inconsistent expression of toxins in transgenic plants, especially in earlier transgenic crop varieties, has been reported (2, 15, 18). These results highlight the importance of maintaining a high level of expression of toxin in B. thuringiensis plants throughout the season, since a late season reduction in expression could allow sizeable increases in fitness for resistant heterozygotes with this type of inheritance.
Despite some evidence to the contrary (29, 45, 59), the most likely cause of resistance to Cry1A toxins in P. xylostella is reduced binding of toxins to midgut membranes (25, 44, 50). Results obtained from the binding assays imply that a major mechanism of resistance to Cry1Ab and Cry1Ac in the Karak strain is a reduction of the binding of these toxins to midgut membrane binding sites. Toxin binding is necessary, although not sufficient, for the toxicity of a toxin (39). Loss of toxin binding has been established as a major resistance mechanism to Cry1A toxins (14). Tabashnik et al. (51) classified such a resistance mechanism as “mode 1,” which is characterized by a high level of resistance to at least one Cry1A toxin, recessive inheritance of resistance, little or no cross-resistance to Cry1C, and reduced binding to at least one Cry1A toxin. Mode 1 resistance has been reported for laboratory-selected NO-QA, PEN, and Loxa-A strains of P. xylostella (50, 55), the PHI strain of P. xylostella against Cry1Ab (50), the YHD2 strain of Heliothis virescens (23), and the 343R strain of Plodia interpunctella (56).
Overall, there appear to be no empirical grounds to support the view that the genetics and/or nature of resistance to B. thuringiensis differs between laboratory- and field-selected populations. What is remarkable about the Karak population is the very high, and apparently stable, level of resistance observed after field collection, although whether this stability is maintained under field conditions remains to be investigated. Low and cryptic fitness costs associated with B. thuringiensis resistance have already been described for another Malaysian population, SERD4 (46). The environmental and agricultural conditions associated with Brassica cultivation in Malaysia may be particularly prone to selecting for resistance to B. thuringiensis. High humidity and high mean temperatures allow P. xylostella to complete its life cycle in less than 18 days. Moreover, continuous cropping throughout the year may also be a factor. Other authors have already noted how continuous cropping (21) or the particularly conducive conditions in greenhouses (27) may be associated with the development of B. thuringiensis resistance.
Acknowledgments
We thank Neil Crickmore (University of Sussex) for supplying Cry1Ac toxin and Dzolkhifli Omar (Universiti Putra Malaysia) for helping to collect the Karak field population.
This work was conducted under plant health license PHL 189/3973 (October 2001, amended February 2002).
Work in the United Kingdom was supported by the Biotechnology and Biological Sciences Research Council (grant D15960). Work in Spain was supported by the Spanish Ministerio de Ciencia y Tecnología with FEDER resources (grant AGL2003-09282-C03-01) and with a research contract for B.E. from the Ramón y Cajal Program. M.S.I.-P. was supported by the Ministerio de Educación, Cultura y Deportes with a fellowship (AP 2001-0972).
REFERENCES
- 1.Abbott, W. 1925. A method of computing the effectiveness of insecticide. J. Econ. Entomol. 18:265-267. [Google Scholar]
- 2.Akhurst, R. J., W. James, L. Bird, and C. Beard. 2003. Resistance to the Cry1Ac δ-endotoxin of Bacillus thuringiensis in the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Econ. Entomol. 96:1290-1299. [DOI] [PubMed] [Google Scholar]
- 3.Ballester, V., F. Granero, B. E. Tabashnik, T. Malvar, and J. Ferré. 1999. Integrative model for binding of Bacillus thuringiensis toxins in susceptible and resistant larvae of the diamondback moth (Plutella xylostella). Appl. Environ. Microbiol. 65:1413-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betz, F. S., B. G. Hammond, and R. L. Fuchs. 2000. Safety and advantages of Bacillus thuringiensis-protected plants to control insect pests. Regul. Toxicol. Pharmacol. 32:156-173. [DOI] [PubMed] [Google Scholar]
- 5.Bourguet, D., A. Genissel, and M. Raymond. 2000. Insecticide resistance and dominance levels. J. Econ. Entomol. 93:1588-1595. [DOI] [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Carriere, Y., C. Ellers-Kirk, A. L. Patin, M. A. Sims, S. Meyer, Y. B. Liu, T. J. Dennehy, and B. E. Tabashnik. 2001. Overwintering cost associated with resistance to transgenic cotton in the pink bollworm (Lepidoptera: Gelechiidae). J. Econ. Entomol. 94:935-941. [DOI] [PubMed] [Google Scholar]
- 8.Cerda, H., A. H. Sayyed, and D. J. Wright. 2003. Laboratory culture conditions affect stability of resistance to Bacillus thuringiensis Cry1Ac in Plutella xylostella (Lepidoptera: Plutellidae). J. Appl. Entomol. 127:142-145. [Google Scholar]
- 9.Crawley, M. J. 1993. GLIM for ecologists. Blackwell, London, United Kingdom.
- 10.Darboux, I., Y. Pauchet, C. Castella, M. H. Silva-Filha, C. Nielsen-LeRoux, J. F. Charles, and D. Pauron. 2002. Loss of the membrane anchor of the target receptor is a mechanism of bioinsecticide resistance. Proc. Natl. Acad. Sci. USA 99:5830-5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Maagd, R. A., A. Bravo, and N. Crickmore. 2001. How Bacillus thuringiensis has evolved specific toxins to colonize the insect world. Trends Genet. 17:193-199. [DOI] [PubMed] [Google Scholar]
- 12.De Maagd, R. A., D. Bosch, and W. Stiekema. 1999. Bacillus thuringiensis toxin-mediated insect resistance in plants. Trends Plant Sci. 4:9-13. [DOI] [PubMed] [Google Scholar]
- 13.Escriche, B., F. J. Silva, and J. Ferré. 1995. Testing suitability of brush border membrane vesicle prepared from whole larvae from small insects for binding studies with Bacillus thuringiensis Cry1Ab crystal protein. J. Invertebr. Pathol. 65:318-320. [Google Scholar]
- 14.Ferré, J., and J. Van Rie. 2002. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 47:501-533. [DOI] [PubMed] [Google Scholar]
- 15.Fitt, G. P. 2000. An Australian approach to IPM in cotton: integrating new technologies to minimise insecticide dependence. Crop Prot. 19:793-800. [Google Scholar]
- 16.González-Cabrera, J., S. Herrero, and J. Ferré. 2001. High genetic variability for resistance to Bacillus thuringiensis toxins in a single population of diamondback moth. Appl. Environ. Microbiol. 67:5043-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Cabrera, J., B. Escriche, B. E. Tabashnik, and J. Ferré. 2003. Binding of Bacillus thuringiensis toxins in resistant and susceptible strains of pink bollworm (Pectinophora gossypiella). Insect Biochem. Mol. Biol. 33:929-935. [DOI] [PubMed] [Google Scholar]
- 18.Gould, F. 1998. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annu. Rev. Entomol. 43:701-726. [DOI] [PubMed] [Google Scholar]
- 19.Gould, F., A. Anderson, A. Reynolds, L. Bumgarner, and W. Moar. 1995. Selection and genetic analysis of a Heliothis virescens (Lepidoptera: Noctuidae) strain with high levels of resistance to Bacillus thuringiensis toxins. J. Econ. Entomol. 88:1545-1559. [Google Scholar]
- 20.Groeters, F. R., and B. E. Tabashnik. 2000. Roles of selection intensity, major genes, and minor genes in evolution of insecticide resistance. J. Econ. Entomol. 93:1580-1587. [DOI] [PubMed] [Google Scholar]
- 21.Hama, H., K. Suzuki, and H. Tanaka. 1992. Inheritance and stability of resistance to Bacillus thuringiensis formulations of the diamondback moth, Plutella xylostella (Linnaeus) (Lepidoptera: Yponomeutidae). Appl. Entomol. Zool. 27:355-362. [Google Scholar]
- 22.Hartl, D. L. 1988. A primer of population genetics, 2nd ed. Sinauer, Sunderland, Mass.
- 23.Heckel, D. G., L. J. Gahan, F. Gould, and A. Anderson. 1997. Identification of a linkage group with a major effect on resistance to Bacillus thuringiensis Cry1Ac endotoxin in the tobacco budworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 90:75-86. [Google Scholar]
- 24.Heckel, D. G., L. J. Gahan, Y. B. Liu, and B. E. Tabashnik. 1999. Genetic mapping of resistance to Bacillus thuringiensis toxins in diamondback moth using biphasic linkage analysis. Proc. Natl. Acad. Sci. USA 96:8373-8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrero, S., B. Oppert, and J. Ferré. 2001. Different mechanisms of resistance to Bacillus thuringiensis toxins in the Indianmeal moth. Appl. Environ. Microbiol. 67:1085-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imai, K., and Y. Mori. 1999. Levels, inheritance and stability of resistance to Bacillus thuringiensis formulation in a field population of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) from Thailand. Appl. Entomol. Zool. 34:23-29. [Google Scholar]
- 27.Janmaat, A. F., and J. Myers. 2003. Rapid evolution and the cost of resistance to Bacillus thuringiensis in greenhouse populations of cabbage loopers, Trichoplusia ni. Proc. R. Soc. Lond. B 270:2263-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lande, R. 1981. The minimum number of genes contributing to quantitative variation between and within populations. Genetics 99:541-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, Y. B., B. E. Tabashnik, L. Masson, B. Escriche, and J. Ferré. 2000. Binding and toxicity of Bacillus thuringiensis protein Cry1C to susceptible and resistant diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 93:1-6. [DOI] [PubMed] [Google Scholar]
- 30.MacIntosh, S. C., T. B. Stone, R. T. Jokerst, and R. L. Fuchs. 1991. Binding of Bacillus thuringiensis proteins to a laboratory-selected line of Heliothis virescens. Proc. Natl. Acad. Sci. USA 88:8930-8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez-Ramírez, A. C., B. Escriche, M. D. Real, F. J. Silva, and J. Ferré. 1995. Inheritance of resistance to a Bacillus thuringiensis toxin in a field population of diamondback moth (Plutella xylostella). Pestic. Sci. 43:115-120. [Google Scholar]
- 32.Morin, S., R. W. Biggs, M. S. Sisterson, L. Shriver, C. Ellers-Kirk, D. Higginson, D. Holley, L. J. Gahan, D. G. Heckel, Y. Carriére, T. J. Dennehy, J. K. Brown, and B. E. Tabashnik. 2003. Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. Proc. Natl. Acad. Sci. USA 100:5004-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masson, L., G. Prefontaine, L. Peloquin, P. C. Lau, and R. Brousseau. 1990. Comparative analysis of the individual protoxin components in P1 crystals of Bacillus thuringiensis subsp. kurstaki isolates NRD-12 and HD-1. Biochem. J. 269:507-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKenzie, J. A. 2000. The character or the variation: the genetic analysis of the insecticide-resistance phenotype. Bull. Entomol. Res. 90:3-7. [DOI] [PubMed] [Google Scholar]
- 35.McKenzie, J. A., and P. Batterham. 1994. The genetic, molecular and phenotypic consequences of selection for insecticide resistance. Trends Ecol. Evol. 9:166-169. [DOI] [PubMed] [Google Scholar]
- 36.McGaughey, W. H., and R. W. Beeman. 1988. Resistance to Bacillus thuringiensis in colonies of Indianmeal moth and almond moth (Lepidoptera: Pyralidae). J. Econ. Entomol. 81:28-33. [Google Scholar]
- 37.Onstad, D. W., C. A. Guse, P. Porter, L. L. Buschman, R. A. Higgins, P. E. Sloderbeck, F. B. Peairs, and G. B. Cronholm. 2002. Modeling the development of resistance by stalk-boring lepidopteran insects (Crambidae) in areas with transgenic corn and frequent insecticide use. J. Econ. Entomol. 95:1033-1043. [DOI] [PubMed] [Google Scholar]
- 38.Rahardja, U., and M. E. Whalon. 1995. Inheritance of resistance to Bacillus thuringiensis subsp. tenebrionis CryIIIA δ-endotoxin in Colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 88:21-26. [DOI] [PubMed] [Google Scholar]
- 39.Rajamohan, F., O. Alzate, J. A. Cotrill, A. Curtiss, and D. H. Dean. 1996. Protein engineering of Bacillus thuringiensis δ-endotoxin: mutations at domain II of Cry1Ab enhance receptor affinity and toxicity toward gypsy moth larvae. Proc. Natl. Acad. Sci. USA 93:14338-14343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roush, R. T., and J. A. McKenzie. 1987. Ecological genetics of insecticide and acaricide resistance. Annu. Rev. Entomol. 32:361-380. [DOI] [PubMed] [Google Scholar]
- 41.Sayyed, A. H., T. H. Schuler, and D. J. Wright. 2003. Inheritance of resistance to Bt canola in a field-derived population of Plutella xylostella. Pest Manag. Sci. 59:1197-1202. [DOI] [PubMed] [Google Scholar]
- 42.Sayyed, A. H., J. Ferré, and D. J. Wright. 2000. Mode of inheritance and stability of resistance to Bacillus thuringiensis var. kurstaki in a diamondback moth (Plutella xylostella) population from Malaysia. Pest Manag. Sci. 56:743-748. [Google Scholar]
- 43.Sayyed, A. H., R. Gatsi, T. Kouskoura, D. J. Wright, and N. Crickmore. 2001. Susceptibility of a field-derived, Bacillus thuringiensis-resistant strain of diamondback moth to in vitro-activated Cry1Ac toxin. Appl. Environ. Microbiol. 67:4372-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sayyed, A. H., R. Haward, S. Herrero, J. Ferré, and D. J. Wright. 2000. Genetic and biochemical approach for characterization of resistance to Bacillus thuringiensis toxin Cry1Ac in a field population of the diamondback moth, Plutella xylostella. Appl. Environ. Microbiol. 66:1509-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sayyed, A. H., and D. J. Wright. 2001. Cross-resistance and inheritance of resistance to Bacillus thuringiensis toxin Cry1Ac in diamondback moth (Plutella xylostella L) from lowland Malaysia. Pest Manag. Sci. 57:413-421. [DOI] [PubMed] [Google Scholar]
- 46.Sayyed, A. H., and D. J. Wright. 2001. Fitness costs and stability of resistance to Bacillus thuringiensis in a field population of the diamondback moth Plutella xylostella L. Ecol. Entomol. 26:502-508. [Google Scholar]
- 47.Schnepf, E., N. Crickmore, J. Van Rie, D. Lereclus, J. Baum, J. Feitelson, D. R. Zeigler, and D. H. Dean. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:775-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabashnik, B. E., Y. B. Liu, T. J. Dennehy, M. A. Sims, M. S. Sisterson, R. W. Biggs, and Y. Carrière. 2002. Inheritance of resistance to Bt toxin Cry1Ac in a field-derived strain of pink bollworm (Lepidoptera: Gelechiidae). J. Econ. Entomol. 95:1018-1026. [DOI] [PubMed] [Google Scholar]
- 49.Tabashnik, B. E., Y.-B. Liu, R. A. de Maagd, and T. J. Dennehy. 2000. Cross-resistance of pink bollworm (Pectinophora gossypiella) to Bacillus thuringiensis toxins. Appl. Environ. Microbiol. 66:4582-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tabashnik, B. E., Y. B. Liu, T. Malvar, D. G. Heckel, L. Masson, V. Ballester, F. Granero, J. L. Ménsua, and J. Ferré. 1997. Global variation in the genetic and biochemical basis of diamondback moth resistance to Bacillus thuringiensis. Proc. Natl. Acad. Sci. USA 94:12780-12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tabashnik, B. E., Y. B. Liu, T. Malvar, D. G. Heckel, L. Masson, and J. Ferré. 1998. Insect resistance to Bacillus thuringiensis: uniform or diverse? Philos. Trans. R. Soc. Lond. Ser. B 353:1751-1756. [Google Scholar]
- 52.Tabashnik, B. E., N. Finson, F. R. Groeters, W. J. Moar, M. W. Johnson, K. Luo, and M. J. Adang. 1994. Reversal of resistance to Bacillus thuringiensis in Plutella xylostella. Proc. Natl. Acad. Sci. USA 91:4120-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tabashnik, B. E., J. M. Schwartz, N. Finson, and M. W. Johnson. 1992. Inheritance of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera, Plutellidae). J. Econ. Entomol. 85:1046-1055. [Google Scholar]
- 54.Tang, J. D., H. L. Collins, T. D. Metz, E. D. Earle, J. Z. Zhao, R. T. Roush, and A. M. Shelton. 2001. Greenhouse tests on resistance management of Bt transgenic plants using refuge strategies. J. Econ. Entomol. 94:240-247. [DOI] [PubMed] [Google Scholar]
- 55.Tang, J. D., S. Gilboa, R. T. Roush, and A. M. Shelton. 1997. Inheritance, stability, and lack of fitness costs of field-selected resistance to Bacillus thuringiensis in diamondback moth (Lepdoptera: Plutellidae) from Florida. J. Econ. Entomol. 90:732-741. [Google Scholar]
- 56.Van Rie, J., W. H. McGaughey, D. E. Johnson, B. D. Barnett, and H. Van Mellaert. 1990. Mechanism of insect resistance to the microbial insecticide Bacillus thuringiensis. Science 247:72-74. [DOI] [PubMed] [Google Scholar]
- 57.Van Rie, J., S. Jansens, H. Höfte, D. Degheele, and H. Van Mellaert. 1989. Specificity of Bacillus thuringiensis delta-endotoxins. Importance of specific receptors on the brush border membrane of the midgut of target insects. Eur. J. Biochem. 186:239-247. [DOI] [PubMed] [Google Scholar]
- 58.Wolfersberger, M., P. Luthy, A. Maurer, P. Parenti, F. V. Sacchi, B. Giordana, and G. M. Hanozet. 1987. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp. Biochem. Physiol. 86A:301-308. [Google Scholar]
- 59.Wright, D. J., M. Iqbal, F. Granero, and J. Ferré. 1997. A change in a single midgut receptor in the diamondback moth (Plutella xylostella) is only in part responsible for field resistance to Bacillus thuringiensis subsp. kurstaki and B. thuringiensis subsp. aizawai. Appl. Environ. Microbiol. 63:1814-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]