Abstract
2,4,6-Trichlorophenol (2,4,6-TCP) is a hazardous pollutant that is efficiently degraded by some aerobic soil bacterial isolates under laboratory conditions. The degradation of this pollutant in soils and its effect on the soil microbial community are poorly understood. We report here the ability of a previously unexposed forest soil microbiota to degrade high levels of 2,4,6-TCP and describe the changes in the soil microbial community found by terminal restriction fragment length polymorphism (T-RFLP) analysis. After 30 days of incubation, about 50% degradation of this pollutant was observed in soils amended with 50 to 5,000 ppm of 2,4,6-TCP. The T-RFLP analysis showed that the soil bacterial community was essentially unchanged after exposure to up to 500 ppm of 2,4,6-TCP. However, a significant decrease in richness was found with 2,000 and 5,000 ppm of 2,4,6-TCP, even though the removal of this pollutant remained high. The introduction of Ralstonia eutropha JMP134 or R. eutropha MS1, two efficient 2,4,6-TCP degraders, to this soil did not improve degradation of this pollutant, supporting the significant bioremediation potential of this previously unexposed, endogenous forest soil microbial community.
2,4,6-Trichlorophenol (2,4,6-TCP) is a toxic, persistent, and bioaccumulable pollutant. Large amounts are released into the environment because it is widely used as a preservative for leather and textile goods. 2,4,6-TCP is also a precursor for the synthesis of the fungicides pentachlorophenol and prochloraz (28). In addition, this chlorophenol is one of the main components in chlorine bleaching Kraft pulp mill effluents (15). Therefore, 2,4,6-TCP has been considered a priority environmental pollutant (11).
Aerobic bacteria in pure cultures and microbial consortia have been reported to degrade 2,4,6-TCP and in several cases to use 2,4,6-TCP as a sole carbon and energy source (4, 5, 18, 20, 21). In theory, these microorganisms can be useful in eliminating this trichlorophenol and thus detoxifying polluted environments. Although significant levels of 2,4,6-TCP degradation have been reported in aqueous environments (10) and in bleached Kraft mill effluents (29), there are very few studies of the natural degradation of 2,4,6-TCP in soils. It has been shown previously that 5 ppm of 2,4,6-TCP is completely removed in pristine soils (16), and up to 250 ppm of this trichlorophenol is removed in soils amended with a bacterial strain able to degrade this pollutant (2). Here we report the intrinsic capability of a forest soil to degrade this chlorophenol and assess the effect of the presence of up to 5,000 ppm of this pollutant in the forest soil microbial community, using a culture-independent approach. The effect of bioaugmentation in this forest soil with two 2,4,6-TCP-degrading strains was also evaluated.
One forest soil located in a coast-to-inland transect from central Chile (sampling site located at 37°S, 73°W), which has not been exposed to anthropogenic chloroorganic compounds, was selected for this study. The chemical analysis results, pH, and water content of this soil have been previously reported (16). Three grams of soil was mixed with 1 ml of an aqueous solution containing different amounts of 2,4,6-TCP, thus making up soil microcosms containing 0, 50, 500, 2,000, or 5,000 ppm of this pollutant. Before incubation, soils were air dried and sieved (1-mm internal pore diameter). Soil incubations were performed in open beakers (25 ml), and soils were kept at 20°C and 65% relative humidity for 7, 15, 30, and 60 days. Every 2 to 3 days, water was added to field capacity. Soil incubations were performed in four replicates, two for high-pressure liquid chromatography (HPLC) analysis and the other two for terminal restriction fragment length polymorphism (T-RFLP) analysis.
The removal of 2,4,6-TCP and accumulation of its potential derivatives were determined by HPLC analysis of soil extracts. These extracts were prepared according to the Environmental Protection Agency ultrasonic extraction protocol (12), with some modifications. In detail, 1 g of soil and 1 g of anhydrous sodium sulfate were mixed and 2 ml of an acetone-hexane (1:1, vol/vol) solution was added. This mixture was subjected to sonication (two times) with a Vibracell apparatus (Sonics & Materials, Inc.) and then centrifuged at 6,000 × g for 5 min. Samples for HPLC (20 μl) were injected into a 126/166 System Gold Beckman liquid chromatograph equipped with a Waters Symmetry C18 4.6-μm column (Beckman Instruments, Fullerton, Calif.). A methanol-water (80:20) mixture containing phosphoric acid (0.3%, vol/vol) was used as the solvent, at a flow rate of 1 ml min−1. The column effluent was monitored by absorbance at 210 nm. The retention volume for 2,4,6-TCP was 8.2 ml. 2,4,6-Tribromophenol was the internal standard. The recovery was around 95%.
It has been previously shown that 5 ppm of 2,4,6-TCP, as part of a chloroaromatic mixture, is significantly (>80%) degraded in this forest soil, after 30 days of incubation (16). Therefore, it was interesting to evaluate if the microorganisms from this soil were also able to degrade higher levels of 2,4,6-TCP. After 30 days of incubation, about 50% of pollutant was degraded in soils exposed to 500, 2,000, or 5,000 ppm of the trichlorophenol, and more than 90% of the trichlorophenol was degraded in the soil spiked with 50 ppm (Table 1). The microbial nature of this phenomenon is indicated by the characteristic time course of the degradation and also by the lack of pollutant removal in incubations carried out with sterile soils (data not shown). The HPLC profile did not show 2,4,6-TCP-derived material, indicating that accumulation of metabolic intermediates did not take place. It should be noted, however, that some intermediates from aerobic trichlorophenol catabolism (such as chlorohydroxyquinols or chlorohydroquinones) would be not stable enough for detection in soils by the use of standard analytical tools (26, 31).
TABLE 1.
Removal of 2,4,6-TCP in a forest soil inoculated or not with 2,4,6-TCP-degrading Ralstonia strainsa
| 2,4,6-TCP concn (ppm) | % in soil:
|
||
|---|---|---|---|
| Noninoculated | Inoculated with strain JMP134 | Inoculated with strain MS1 | |
| 50 | 6.6 | 5.9 | 16.3 |
| 500 | 55.8 | 54.5 | 48.2 |
| 2,000 | 49.1 | ND | ND |
| 5,000 | 49.1 | ND | ND |
Values are the percentages (averages of two replicates) of residual 2,4,6-TCP in soils, after 30 days of incubation with respect to the amount initially spiked. Standard deviations were lower than 5%. ND, not determined.
The results reported here indicate the existence of a significant endogenous 2,4,6-TCP-degradative potential in the soil microbial community. This ability can be explained by the presence of bacteria that use this pollutant as a sole carbon and energy source (4, 5, 18, 20, 21) and also by less efficient bacterial strains that degrade 2,4,6-TCP (13, 23). The presence of such bacterial strains in this soil is supported because 102 CFU g−1 was observed in agar plates containing 2,4,6-TCP as a sole carbon source. In addition, several strains have been isolated from this forest soil, by the use of a 2,4,6-TCP enrichment (23). In this context, it is interesting to speculate that bacteria can be adapted to degrade 2,4,6-TCP because chlorophenols are naturally occurring in forest soils. This idea is supported by the increasing evidence that insects and fungi produce chlorophenols (14).
It should be noted that microorganisms of this forest soil had never been exposed before to human-generated chloroorganic compounds (16) and that their considerable catabolic potential enables them to efficiently degrade up to 5,000 ppm of 2,4,6-TCP. However, the presence of this pollutant may affect the forest soil microbial community. Total cultivable bacterial counts in these soil incubations, typically 109 CFU g of soil−1 as determined in R2A agar plates, were not significantly affected by any of the concentrations of 2,4,6-TCP. However, as the determination of cultivable counts allows detection of only 1 to 5% of soil microorganisms (1), it is possible that the presence of this chlorophenol does affect the microbial community and that such changes remain undetected.
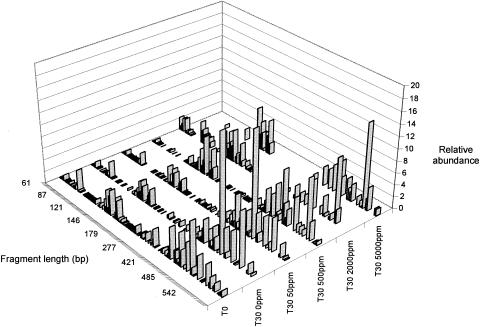
To assess the effect of 2,4,6-TCP in the soil bacterial community, a culture-independent technique, detecting T-RFLPs in the 16S ribosomal DNA, was carried out as described elsewhere (16). T-RFLP profiles were plotted as peak area (relative abundance) against fragment size. The fragment sizes were estimated using as reference the internal standard ROX 1000. As proposed previously, terminal restriction fragment sizes with a peak height lower than 80 fluorescence units were not considered in the analysis (3). By using the statistical tool of the Ribosomal Database Project web page (6), the T-RFLP profiles obtained from replicates were found significantly similar, and therefore, the relative areas of same-sized fragments from replicate profiles were averaged. The T-RFLP profiles from soil incubated for 30 days (Fig. 1) were compared using the Friedman nonparametric analysis of variance (7). The 30-day T-RFLP profiles of soils spiked with 0, 50, or 500 ppm of 2,4,6-TCP were essentially similar (P > 0.05), although there were statistically significant differences (P < 0.05) between the T-RFLP profiles from the nonincubated soil (T0) and those from the incubated soils.
FIG. 1.
T-RFLP profiles in bacterial community DNA obtained from soil incubated in the absence or the presence of 50, 500, 2,000, or 5,000 ppm of 2,4,6-TCP. T0, nonincubated soil; T30, soil incubated for 30 days. The soil microbial community DNA was amplified using primer pairs 8F and 1392R and digested with the enzyme MspI. Fragment sizes (base pairs) that can be putatively assigned to bacterial genera with reported catabolic ability towards 2,4,6-TCP were the following: 141 to 143 (Burkholderia), 150 (Sphingomonas), 152 (Rhodococcus), 159 to 161 (Streptomyces), 159 (Nocardia), 163 (Arthrobacter), and 490 to 494 (Pseudomonas). The fragment size of 430 bp corresponding to Ralstonia was not found.
We determined richness and diversity to detect more subtle effects of the presence of 2,4,6-TCP on this forest soil microbial community. Although it is known that each terminal restriction fragment may represent more than one phylotype and that the relative peak area does not necessarily correspond to the abundance of each phylotype, because different phylotypes may have different 16S ribosomal DNA dosages, richness and diversity were determined by the total number of different fragment sizes (between 60 and 600) and the relative areas, respectively. The Shannon-Weiner diversity (H) index was calculated as follows: H = Σ(pi)(log2pi), where p is the proportion of an individual peak area relative to the sum of all peak areas (22).
The H index decreased from 5.51 in the nonincubated soil to 4.92, 5.12, and 5.02 in the soils incubated for 30 days with 0, 50, and 500 ppm, respectively. After 30 days, the changes in the H index were mainly due to decreases in richness: 102 signals in T0, compared with 79, 93, and 81 signals found in soils spiked with 0, 50, and 500 ppm of 2,4,6-TCP, respectively. These results show that this forest soil microbial community is essentially undisturbed by 2,4,6-TCP levels that are even higher than those reported in the literature for polluted soils (19, 30). However, when this forest soil was exposed to 2,000 or 5,000 ppm of 2,4,6-TCP, the richness after 30 days of incubation decreased abruptly, 52 and 33 signals, respectively (Fig. 1). The H index for the soil spiked with 5,000 ppm of 2,4,6-TCP was 4.65, the lowest found for all soil incubation conditions. Notably, the H index for the soil exposed to 2,000 ppm (5.21) was unexpectedly high. The latter, mainly due to the presence of new signals corresponding to terminal fragment sizes of 90 to 130 bp (Fig. 1), may correspond to an increase in the abundance of microbial populations that would be favored either by the presence of this concentration of pollutant or by the inhibition of antagonist, competitive microorganisms. A similar observation on population dynamics has been recently reported for soil microbial communities exposed to drying-rewetting stress and crop protection products (27).
The T-RFLP profiles of this soil showed signals that can be putatively assigned by Ribosomal Database Project analysis (6) to bacterial genera with reported abilities to degrade 2,4,6-TCP (Fig. 1, legend). For example, the main signals correspond to fragment sizes (490 to 494 bp) that can be assigned to Pseudomonas species. Furthermore, signals that are relatively more abundant in soils incubated with high levels of 2,4,6-TCP also correspond to fragment sizes (141 to 163 bp) that can be assigned to bacterial genera that reportedly degrade this pollutant. In addition, Sphingomonas and Pseudomonas strains able to degrade 2,4,6-TCP have been isolated from this forest soil (23). These observations further support the idea that this forest soil has a significant potential to degrade this pollutant.
Some soil incubations were inoculated (108 CFU g of soil−1) with Ralstonia eutropha JMP134(pJP4) or R. eutropha MS1, two bacterial strains able to efficiently degrade 2,4,6-TCP (23). Both strains possess the same 2,4,6-TCP catabolic pathway, encoded by tcp genes, but strain MS1 can grow in the presence of higher 2,4,6-TCP concentrations than strain JMP134 can (23). Despite the large number of viable cells inoculated into the soil, no significant differences in pollutant removal with respect to the noninoculated soil were detected (Table 1). Andreoni et al. (2) have reported that addition of the Alcaligenes eutrophus TCP strain improved the rate of 2,4,6-TCP degradation in soil microcosms. However, complete removal of 2,4,6-TCP was observed in both inoculated and noninoculated soil, suggesting that strain TCP may not play a significant role in 2,4,6-TCP degradation, compared with the indigenous microbiota.
It is possible that strains JMP134 and MS1 did not improve the removal of 2,4,6-TCP from this soil because they do not survive well in the soil incubation. Viable counts for strains JMP134 and MS1 were determined using agar plates containing 2,4,6-TCP-tellurite. The tellurite marker, introduced in strains JMP134 and MS1 by using a mini-Tn5 delivery system, was selected because noninoculated soils gave no cultivable counts with these selection media. After 30 days of incubation, both strains reached levels of 103 CFU g of soil−1 that continued stable until day 60. Such a 5-order-of-magnitude decrease in cultivable counts indicated poor survival of both Ralstonia strains in this forest soil. It has been reported that 400 ppm, but not 100 or 40 ppm, of 2,4,6-TCP decreased the survival of R. eutropha JMP134 in bleached Kraft mill effluent microcosms (29). In lake water (17) and in seawater (25), this strain has also a limited survival capacity. In soil incubations amended with 100 ppm of 2,4-dichlorophenoxyacetic acid (2,4-D), R. eutropha JMP134 reached levels of 104 CFU g of soil−1 (8). However, no decrease in the levels of R. eutropha JMP134 was found in a different soil amended with 100 ppm of 2,4-D (24), although these determinations were carried out in short incubations (3 to 8 days). In contrast, DiGiovanni et al. (9) did not detect cells of R. eutropha JMP134 after 1 week of incubation in microcosm soils exposed to 1,000 ppm of 2,4-D. The poor survival of these Ralstonia strains in soils, reported in this work and in the literature, indicates that use of well-studied catabolic strains is not always an appropriate bioremediation strategy to clean up soils exposed to high 2,4,6-TCP levels.
In summary, this is the first report that shows the ability of a forest soil microbiota to degrade very high levels of 2,4,6-TCP. The significant endogenous degradative activity towards 2,4,6-TCP helps to explain the fact that no significant changes in the diversity of the soil microbial community were observed with levels of this pollutant that are higher than those found in polluted sites. The results reported here also indicate that addition of specialized, catabolic bacteria is not always required for bioremediation of soils exposed to pollutants that have natural counterparts or can be naturally produced.
Acknowledgments
This work was supported by FONDAP-FONDECYT (grant 1501-0001, program 7) and by contract ICA4-CT-2002-10011 (ACCESS) of the European Union. M.A.S. is a CONICYT Ph.D. fellow.
REFERENCES
- 1.Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreoni, V., G. Baggi, M. Colombo, L. Cavalca, M. Zangrossi, and S. Bernasconi. 1998. Degradation of 2,4,6-trichlorophenol by a specialized organism and by indigenous soil microflora: bioaugmentation and self-remediability for soil restoration. Lett. Appl. Microbiol. 27:86-92. [DOI] [PubMed] [Google Scholar]
- 3.Blackwood, C. B., T. Marsh, S.-H. Kim, and E. A. Paul. 2003. Terminal restriction fragment length polymorphism data analysis for quantitative comparison of microbial communities. Appl. Environ. Microbiol. 69:926-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bock, C., R. M. Kroppennstedt, U. Schmidt, and H. Diekmann. 1996. Degradation of prochloraz and 2,4,6-trichlorophenol by environmental bacterial strains. Appl. Microbiol. Biotechnol. 45:257-262. [DOI] [PubMed] [Google Scholar]
- 5.Clément, P., V. Matus, L. Cárdenas, and B. González. 1995. Degradation of trichlorophenols by Alcaligenes eutrophus JMP134. FEMS Microbiol. Lett. 127:51-55. [DOI] [PubMed] [Google Scholar]
- 6.Cole, J. R., B. Chai, T. L. Marsh, R. J. Farris, Q. Wang, S. A. Kulam, S. Chandra, D. M. McGarrell, T. M. Schmidt, G. M. Garrity, and J. M. Tiedje. 2003. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 31:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conover, W. J. 1980. The one sample or matched pairs case: the Friedman test, p. 299-309. In W. J. Conover (ed.), Practical nonparametric statistics. John Wiley & Sons, New York, N.Y.
- 8.Daane, L. L., and M. M. Haggblom. 1999. Earthworm egg capsules as vectors for the environmental introduction of biodegradative bacteria. Appl. Environ. Microbiol. 65:2376-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiGiovanni, G. D., J. W. Neilson, I. L. Pepper, and N. A. Sinclair. 1996. Gene transfer of Alcaligenes eutrophus JMP134 plasmid pJP4 to indigenous soil recipients. Appl. Environ. Microbiol. 62:2521-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domínguez, V. M., J. Correa, G. Vidal, A. López, and M. Martínez. 2002. 2,4,6-Trichlorophenol degradation by river sediment exposed to bleached kraft mill discharge. Bull. Environ. Contam. Toxicol. 69:463-470. [DOI] [PubMed] [Google Scholar]
- 11.Environmental Protection Agency. 1984. Method 604. Phenols in Federal Register. Part VIII, 40 CFR Part 136, p. 58-66. Environmental Protection Agency, Washington, D.C.
- 12.Environmental Protection Agency. 2000. Method 3550C. First revision. Ultrasonic extraction. Environmental Protection Agency, Washington, D.C.
- 13.Godoy, F. A., M. Bunster, V. Matus, C. Aranda, B. González, and M. Martínez. 2003. Poly-β-hydroxyalkanoates consumption during degradation of 2,4,6-trichlorophenol by Sphingopixis chilensis S37. Lett. Appl. Microbiol. 36:315-320. [DOI] [PubMed] [Google Scholar]
- 14.Gribble, G. W. 1994. The natural production of chlorinated compounds. Environ. Sci. Technol. 28:310A-319A. [DOI] [PubMed] [Google Scholar]
- 15.Huynh, V. B., H. M. Chang, T. W. Joyce, and T. K. Kirk. 1985. Dechlorination of chloro-organics by a white-rot fungus. TAPPI J. 68:98-102. [Google Scholar]
- 16.Jordan, M., M. A. Sánchez, L. Padilla, R. Céspedes, M. Osses, and B. González. 2002. Kraft mill residues effects on Monterey pine growth and soil microbial activity. J. Environ. Qual. 31:1004-1009. [DOI] [PubMed] [Google Scholar]
- 17.Kandel, A., O. Nybroe, and O. F. Rasmussen. 1992. Survival of 2,4-dichlorophenoxyacetic acid degrading Alcaligenes eutrophus AEO106 (pR0101) in lake water microcosms. Microb. Ecol. 24:291-303. [DOI] [PubMed] [Google Scholar]
- 18.Kharoune, L., K. Kharoune, and J. M. Lebeault. 2002. Aerobic degradation of 2,4,6-trichlorophenol by a microbial consortium—selection and characterization of microbial consortium. Appl. Microbiol. Biotechnol. 59:112-117. [DOI] [PubMed] [Google Scholar]
- 19.Kitunen, V. H., R. J. Valo, and M. S. Salkinoja-Salonen. 1987. Contamination of soil around wood-preserving facilities by polychlorinated aromatic compounds. Environ. Sci. Technol. 21:96-101. [Google Scholar]
- 20.Kiyohara, H., T. Hatta, Y. Ogawa, T. Kakuda, H. Yokohama, and N. Takizawa. 1992. Isolation of Pseudomonas pickettii strains that degrade 2,4,6-trichlorophenol and their dechlorination of chlorophenols. Appl. Environ. Microbiol. 58:1276-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, D.-Y., J. Eberspächer, B. Wagner, J. Kuntzer, and F. Lingens. 1991. Degradation of 2,4,6-trichlorophenol by Azotobacter sp. strain GP1. Appl. Environ. Microbiol. 57:1920-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margalef, R. 1958. Information theory in ecology. Gen. Syst. 3:36-71. [Google Scholar]
- 23.Matus, V., M. A. Sánchez, M. Martínez, and B. González. 2003. Efficient degradation of 2,4,6-trichlorophenol requires a set of catabolic genes related to tcp genes from Ralstonia eutropha JMP134(pJP4). Appl. Environ. Microbiol. 69:7108-7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neilson, J. W., K. L. Josephson, I. L. Pepper, R. B. Arnold, G. D. Di Giovanni, and N. A. Sinclair. 1994. Frequency of horizontal gene transfer of a large catabolic plasmid (pJP4) in soil. Appl. Environ. Microbiol. 60:4053-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nybroe, O., K. Einarson, and T. Ahl. 1996. Growth and viability of Alcaligenes eutrophus JMP134 in seawater is affected by substrate and nutrient amendment. Lett. Appl. Microbiol. 22:366-370. [DOI] [PubMed] [Google Scholar]
- 26.Padilla, L., V. Matus, P. Zenteno, and B. González. 2000. Degradation of 2,4,6-trichlorophenol via chlorohydroxyquinol in Ralstonia eutropha JMP134 and JMP222. J. Basic Microbiol. 40:243-249. [DOI] [PubMed] [Google Scholar]
- 27.Pesaro, M., G. Nicollier, J. Zeyer, and F. Widmer. 2004. Impact of soil drying-rewetting stress on microbial communities and activities and on degradation of two crop protection products. Appl. Environ. Microbiol. 70:2577-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sittig, M. 1981. Handbook of toxic and hazardous chemicals. Noyes Publications, Park Ridge, N.J.
- 29.Valenzuela, J., U. Bumann, R. Céspedes, L. Padilla, and B. González. 1997. Degradation of chlorophenols by Alcaligenes eutrophus JMP134(pJP4) in bleached Kraft mill effluent. Appl. Environ. Microbiol. 63:227-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valo, R. J., V. H. Kitunen, M. S. Salkinoja-Salonen, and S. Räisänen. 1984. Chlorinated phenols as contaminants of soil and water in the vicinity of two Finnish sawmills. Chemosphere 13:835-844. [Google Scholar]
- 31.Xun, L., and C. M. Webster. 2003. A monooxygenase catalyzes sequential dechlorinations of 2,4,6-trichlorophenol by oxidative and hydrolytic reactions. J. Biol. Chem. 279:6696-6700. [DOI] [PubMed] [Google Scholar]