Abstract
Although arsenite [As(III)] is non-essential and toxic for plants, it is effectively absorbed through various transporters into the roots. Here we identified a calcium-dependent protein kinase (CPK31) response for As(III) tolerance in Arabidopsis. We identified CPK31 as an interacting protein of a nodulin 26-like intrinsic protein (NIP1;1), an aquaporin involved in As(III) uptake. Similarly to the nip1;1 mutants, the loss-of-function mutants of CPK31 improved the tolerance against As(III) but not As(V), and accumulated less As(III) in roots than that of the wild-type plants. The promoter-β-glucuronidase and quantitative Real-Time PCR analysis revealed that CPK31 displayed overlapping expression profiles with NIP1;1 in the roots, suggesting that they might function together in roots. Indeed, the cpk31 nip1;1 double mutants exhibited stronger As(III) tolerance than cpk31 mutants, but similar to nip1;1 mutants, supporting the idea that CPK31 might serve as an upstream regulator of NIP1;1. Furthermore, transient CPK31 overexpression induced by dexamethasone caused the decrease in As(III) tolerance of transgenic Arabidopsis lines. These findings reveal that CPK31 is a key factor in As(III) response in plants.
Introduction
Arsenic (As) is a metalloid and present as pentavalent arsenate [As(V)] and trivalent arsenite [As(III)] that reversibly convert depending on the redox status of the environment. Typically, As(V) and As(III) are the prevalent form in aerobic or anaerobic soils, respectively[1]. Although As is a non-essential element in plants, it can accumulate in crop plants, specially rice, and deposit into grains when the soil and water are contaminated [2,3]. Crops exhibit a range of As toxicity symptoms that are detrimental to growth and yield once As accumulation reaches beyond an optimal level, and may eventually pose a potential health risk to their consumers including animals and humans [3]. Due to its toxicity, universal distribution, and tendency to accumulate in animals and humans, As is ranked as one of the most toxic elements and defined as a group I carcinogen [4,5].
As(V), as a phosphate analogue, has a strong affinity with iron oxides or hydroxides in the soil, and its concentration is normally under 2.3 μM even in a highly As-contaminated soil [6,7]. As(V) thus enters plant roots mainly through the high-affinity phosphate transporters, such as PHT1;1 in Arabidopsis and OsPT8 in rice [8,9]. After acquisition, As(V) is rapidly reduced to As(III) by the enzyme arsenate reductase, and As(III) consequently acts as the predominant form of As for As extrusion, long-distance transport and sequestration in plants [4,10]. As(III) is uncharged at neutral pH in flooded paddy soils, and is more soluble and mobile compared with As(V). As(III) enters the cells mainly through nodulin 26-like intrinsic proteins (NIPs) subfamily of aquaporin proteins that belong to the major intrinsic proteins (MIPs), a family essentially facilitates the diffusion of water and small uncharged solutes in all domains of life [4,10–11]. Plant NIPs are remarkably well conserved across species, and can be divided into five well-defined subgroups (specified as NIP1-NIP5) according to phylogenetic analysis [11]. In addition to As(III), NIPs are also involved in the uptake and extrusion of other metalloids, including antimonite, boron, silicon and selenium, into or out of the plants and distribution within the plant body [11,12].
Several NIPs are permeable for As(III). Exogenous expression of AtNIP1;1 enhanced the As(III) uptake into the oocytes, as did its close homologue AtNIP1;2, and OsNIP1;1 and OsNIP3;1 from rice [2, 13]. In addition, NIP1;2, NIP3;1, NIP5;1, NIP6;1, and NIP7;1 of Arabidopsis, OsNIP2;1 and OsNIP3;2 from Oryza sativa, HvNIP1;2 from barley, and LjNIP5;1 and LjNIP6;1 from Lotus japonicas are all permeable to As(III) in the yeast expression system [14–16]. Among nine members of the NIP subfamily in Arabidopsis, NIP1;1 was firstly identified as a determinant of As(III) tolerance using a forward genetics screen [13]. NIP1;1 is highly expressed in roots and localized at the plasma membrane, and its loss-of-function mutants displayed an As(III)-tolerant phenotype with strong growth of roots [13], indicating that NIP1;1 is a critical As(III) transporter that mediates toxic As(III) uptake into roots. However, little is known about potential signaling mechanisms that allow roots to regulate NIP1;1 activity in response to As(III) toxicity.
GmNod26, a NIP family member abundantly targeted to the symbiosome membrane of nitrogen-fixing nodules of soybean roots, has been shown to be phosphorylated by a symbiosome membrane-associated calcium-dependent protein kinase (CPK) on Ser-262 of C-terminal [17, 18]. CPKs contain calmodulin-like Ca2+-binding domains and a kinase domain in a single protein, and have therefore been recognized as the major transducers of calcium signals and catalyze the phosphorylation of multiple proteins, including membrane channels, pumps, and a number of metabolic enzymes [19]. More studies have shown that several members of CPKs physically associate with ion channels and transporters to effectively regulate their activities [20–24]. Therefore, it is conceivable that CPKs fulfills critical functions in regulating the activity of NIP1;1 involved in As(III) uptake in the roots.
In the present study, we report the identification of CPK31 as a major component controlling As(III) tolerance in Arabidopsis. Our genetic and biochemical studies indicate that CPK31 fulfils this function by interaction with NIP1;1, providing a novel role of CPK31 in controlling As(III) toxicity in plants.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana wild-type and the T-DNA insertion mutants nip1;1–1 (SALK_016617), nip1;1–2 (SALK_017916), cpk31-1 (SALK_049228), cpk31-2 (SALK_007777) and cpk31-3(SALK_049236) were obtained from the Arabidopsis Biological Resource Center. The ecotype of Arabidopsis thaliana lines is Columbia-0 (Col-0). Homozygous individuals were identified by RT-PCR using the primers listed in S1 Table. To obtain the cpk31 nip1;1 double mutants, cpk31-1 and nip1;1–1 mutants were crossed. We identified cpk31 nip1;1 double mutants by antibiotic selection and RT-PCR analysis using the primers listed in S1 Table.
Surface-sterilized seeds were plated on half-strength MS medium containing various sodium arsenite concentrations with 1% (w/v) sucrose and pH was adjusted to 5.7, and solidified using 0.8% (w/v) agar. Seedlings were grown on vertically placed plates in the chambers at 23–24°C with light intensity of 150 μmol·m−2·s−1 and 16:8 h light/dark photoperiods. The seedlings grown for 14 days after germination were imaged for measuring primary root lengths of plants using the ImageJ software (http://rsb.info.nih.gov/ij/), and then were collected for weighing the whole-plant biomass. For soil culture, 3-day-old seedlings germinated on half-strength MS medium were carefully removed to the nutrient-rich soil. For hydroponic culture, 7-day-old seedlings grown on half-strength MS medium were transferred into liquid 1/6 MS medium for further growth. The soil and hydroponic cultured plants were grown in the greenhouse under 150 μmol·m−2·s−1 light intensity with a 16-h light/8-h dark photoperiod at 22°C.
Yeast-two-hybrid assay
Cytoplasmic C-terminal region of NIP1;1 and the CPK31 full-length cDNA were cloned into the activation domain vector pGADT7 and the DNA-binding domain vector pGBKT7. The recombinant vectors were transformed into yeast strain AH109 by the lithium acetate method reported as previous [24–27]. Transformants were selected the synthetic complete agar medium (SC) minus leucine and tryptophan and grown at 30°C for 4 d, and then transferred on SC minus histidine, leucine and tryptophan, or SC minus adenine, histidine, leucine and tryptophan supplemented with 10 mM 3-amino-1,2,4,-triazole and 40 μM X-α-gal. For serial dilution assay, exponentially grown yeast cells were harvested and adjusted to OD600 = 0.5 with sterilized double-distilled water and diluted to 1/10, 1/100, and 1/1000. Each yeast two-hybrid assay has been independently repeated at least three times. All primers used in this study are summarized in S1 Table.
Subcellular location and bimolecular fluorescence complementation (BiFC) Assay
For subcellular location of CPK31 and NIP1;1, the coding sequence of CPK31 and NIP1;1 was respectively amplified without the stop codon from wild-type Arabidopsis cDNA and subcloned into pEZS-NL vector. To generate the BiFC constructs, NIP1;1 and CPK31 full length cDNA with no stop codon were subcloned into 35S-SPYNE(R)173 and 35S-SPYCE(M) vectors, respectively [28]. For transient expression, constructs were transformed into protoplasts of Arabidopsis suspension cultured cells according to the described methods [29]. For microscopic analyses, the transfected protoplasts incubated after 16 h at 22°C were imaged by confocal microscopy (LSM 710, Zeiss) equipped with an argon/krypton laser. The excitation wavelengths for GFP and YFP signals were 488 and 514 nm, respectively.
Measurements of As (III) content
Three-week-old seedlings grown under liquid 1/6 MS medium were transferred for the solutions containing 10 μM As(III). After 0 h, 12 h, 24 h, 48 h and 6 days of incubation, the plants were washed three times with distilled water. The shoots and roots were collected and dried for 72 h at 80°C. After weighed, the samples were digested in ultrapure HNO3 (Sigma-Aldrich). As(III) contents were determined using the inductively coupled plasma Atomic Fluorescence Spectrometry (PerkinElmer). As(III) contents in the samples were calculated as the ratio of values to dry weights.
Histochemical GUS analysis
For histochemical analysis of CPK31, a 2004-bp fragment upstream of starting codon was amplified with the primers listed in S1 Table, and subcloned into the pBI-101.1 binary vector. The Agrobacterium tumefaciens cells (GV3101) carrying the recombinant vector were used to transform Arabidopsis ecotype Col-0 plants with a floral dip method described previously [30]. Transformed Arabidopsis lines were selected based on kanamycin resistance. The GUS staining was done according to published protocols [31] with slight modification. In brief, T3 transgenic seedlings were incubated in GUS staining solution (2 mM 5-bromo-4-chloro-3-indolyl-β-D-glucuronide, 1 mM K3(Fe(CN)6), 1 mM K4Fe(CN)6·3H2O, 10 mM Na2EDTA, 0.1% Triton X-100, and 50 mM Na3PO4, pH 7.00) at 37°C for 12 h. After sufficiently decolorized with 75% (vol/vol) ethanol, individual representative plant tissues were photographed with an Olympus SZX12 microscope equipped with a camera.
Quantitative real-time PCR analysis
Total RNA was extracted from the plantlets grown in the hydroponic system using the TRIzol reagent (Invitrogen) following the manufacturer’s instructions. The first-strand cDNA was synthesized by M-MLV Reverse Transcriptase (Promega) with anchored oligo(dT18). Quantitative real-time PCR was performed on a CFX Connect Real-ime System (Bio-Rad) using the QuantiFast SYBR Green PCR Kit (Qiagen). All primers for expression assays were listed in S1 Table. The expression value of every sample was quantified with the 2−ΔΔCT method [32] using ACTIN2 as an internal reference.
Construction of dexamethasone-inducible transgenic Arabidopsis lines
For the inducible expression of CPK31, the full-length CPK31 CDS was amplified by PCR from a wild-type cDNA pool using specific primers and then was cloned into the binary vector pTA7002. The generated construct was further sequenced for confirmation and introduced into Agrobacterium tumefaciens (strain GV3101) cells for transformation into the cpk31-1 mutant plants. More than 50 independent lines were selected for hygromycin resistance. Homozygous transformants were confirmed by both segregation and PCR analysis. Three representative lines were used for phenotypic analysis and molecular characterization. The seeds were planted at half-strength MS medium containing 10 μM dexamethasone (DEX). 7-day-old seedlings were gathered for photograph and analysis of CPK31 expression and root length.
Results
CPK31 physically interacts with NIP1;1
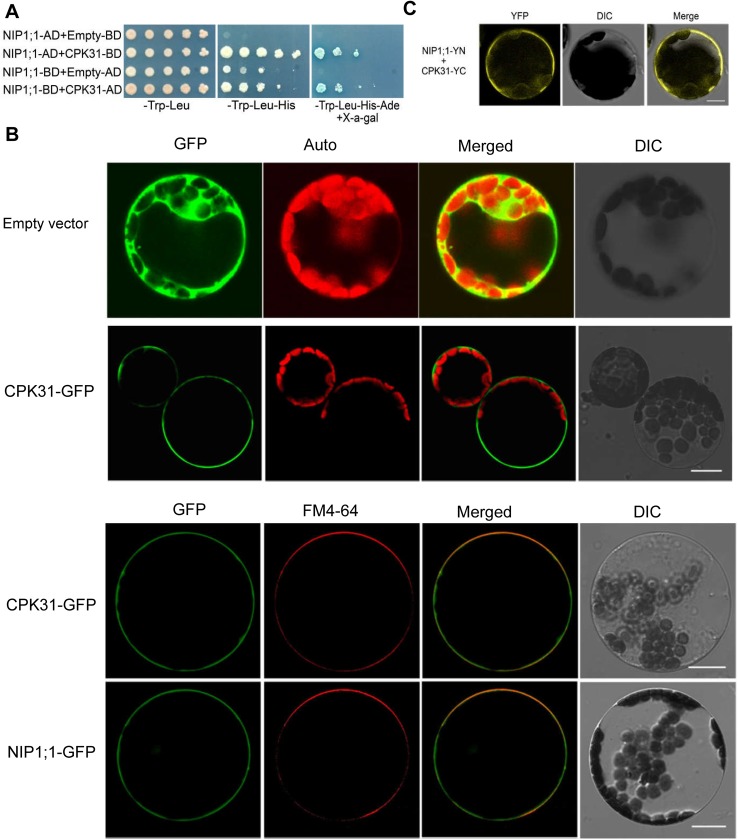
CPKs act as major calcium sensors and transducer, and play a fundamental role in growth, development and stress responses in plants. We have established a yeast two-hybrid system and successfully identified a number of interaction partners between the kinases and ion channels [24–27]. We hypothesized that some of CPKs may interact with and regulate the activity of AtNIP1;1 involving in As(III) uptake in the roots. To test this possibility, we thus screened all CPK members in Arabidopsis to find potential candidates physically interacting with AtNIP1;1 by using the yeast two-hybrid assay. Among the 34 members of CPKs tested, we found that only CPK31 (AT4G04695) interacted physically with the putative cytoplasmic hydrophilic domain at N-terminal of AtNIP1;1 (Fig 1A).
Fig 1. Interactions between CPK31 and NIP1;1 at the plasma membrane.
(A) Yeast two-hybrid assay of the interactions between CPK31 and NIP1;1. Yeast cells were cotransformed with various combinations of pGBT9- and pGADGH-fusion constructs as indicated on the left of each row. Serial decimal dilutions of corresponding yeast cells were spotted on the synthetic complete agar medium (SC) minus leucine and tryptophan (-Trp-Leu; left lane), SC minus leucine, tryptophan and histidine (-Trp-Leu-His; middle lane), or SC minus leucine, tryptophan, histidine, adenine and contained 40 μM X-α-gal (-Trp-Leu-His-Ade; right lane). Photographs were taken after cultivation at 30°C for 4 days. (B) Subcellular location of CPK31-GFP and NIP1;1-GFP in Arabidopsis cells. The GFP coding sequence was fused to the C-terminus of CPK31 or NIP1;1 coding sequence without stop codon in the pEZS-NL vector, and then transformed into protoplasts of Arabidopsis suspension cultured cells. Empty vector was transformed as a control. To further conform potential plasma membrane location, the protoplasts were stained with the plasma membrane-specific dye FM4-64 (red). (C) CPK31::35S-SPYCE co-expressed with NIP1;1::35S-SPYNE in plasma membrane of Arabidopsis protoplasts observed by bimolecular fluorescence complementation (BiFC). CPK31::35S-SPYCE (CPK31-YC) and NIP1;1::35S-SPYNE (NIP1;1-YN) were transformed into the protoplasts isolated from Arabidopsis suspension cultured mesophylls. The fluorescence signals shown at (B) and (C) were imaged under a Zeiss confocal microscope as green and yellow, respectively (Scale bar: 10 μm).
CPK family members were detected at multiple subcellular locations, including cytosol and plasma membrane [19]. To determine the subcellular localization of CPK31 in the Arabidopsis cells, we transiently expressed a CPK31-GFP fusion protein in Arabidopsis protoplasts. The CPK31-GFP signal appeared at the plasma membrane, while the control GFP was localized to the cytoplasm under confocal laser scanning microscopic analysis (Fig 1B). Moreover, CPK31-GFP green signal was colocalized with the red signal of FM4-64, a plasma membrane-specific dye (Fig 1B), confirming that CPK31 appears in the plasma membrane. This result is consistent with the prediction of membrane subcellular location of CPK31 with a myristoylation motif at N-terminal [19]. Similarly, GFP-tagged AtNIP1;1 signal appeared at the plasma membrane and overlapped with the FM4-64 signal (Fig 1B), exhibiting plasma membrane localization at the mesophyll protoplast, consistent with the observation in the root epidermal cells of the transgenic Arabidopsis lines [13].
To further confirm that the interaction between CPK31 and AtNIP1;1 could occur in plant cells, bimolecular fluorescence complementation (BiFC) assay was performed in protoplasts of suspension cultured Arabidopsis mesophyll. The fluorescence signals were primarily detected at plasma membrane of the cells co-expressing AtNIP1;1 and CPK31, while no fluorescence signals were detected in the cells expressing the fusion of AtNIP1;1-SPYNE or CPK31-SPYCE (Fig 1C). Therefore, these results suggest that the interaction between of CPK31 and AtNIP1;1 primarily occurs at the plasma membrane of plant cells.
The cpk31 mutants are more tolerant to As (III) than the wild-type plants
Work by Kamiya et al (2009) demonstrated that NIP1;1 plays a negative role in plant survival under high-As(III) conditions, because the nip1;1 loss-of-function mutants are more As-resistant than the wild-type [13]. It is believed that NIP1;1 mediates As(III) uptake into the roots thus contributes to the toxicity of external As(III). However, the mechanisms for activating NIP1;1 activity are still unknown. Some CPKs have been shown to activate channels by interacting with the channel proteins [20,21,23,24], and some inhibit the activity of channels [22]. Based on our findings of interaction of CPK31 and AtNIP1;1, we tested the possible regulatory mechanism of CPK31 on AtNIP1;1 by using transgenic Arabidopsis lines.
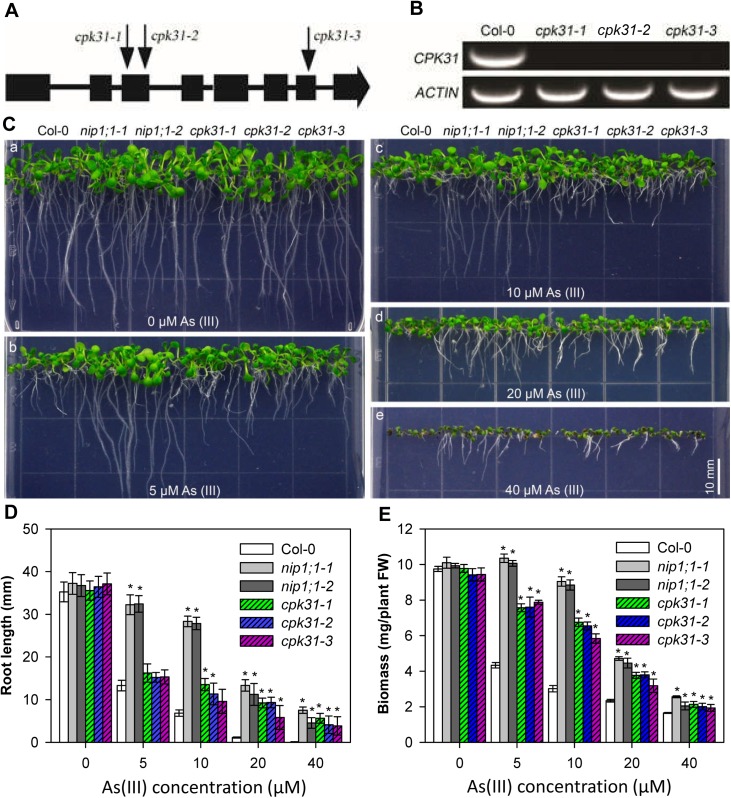
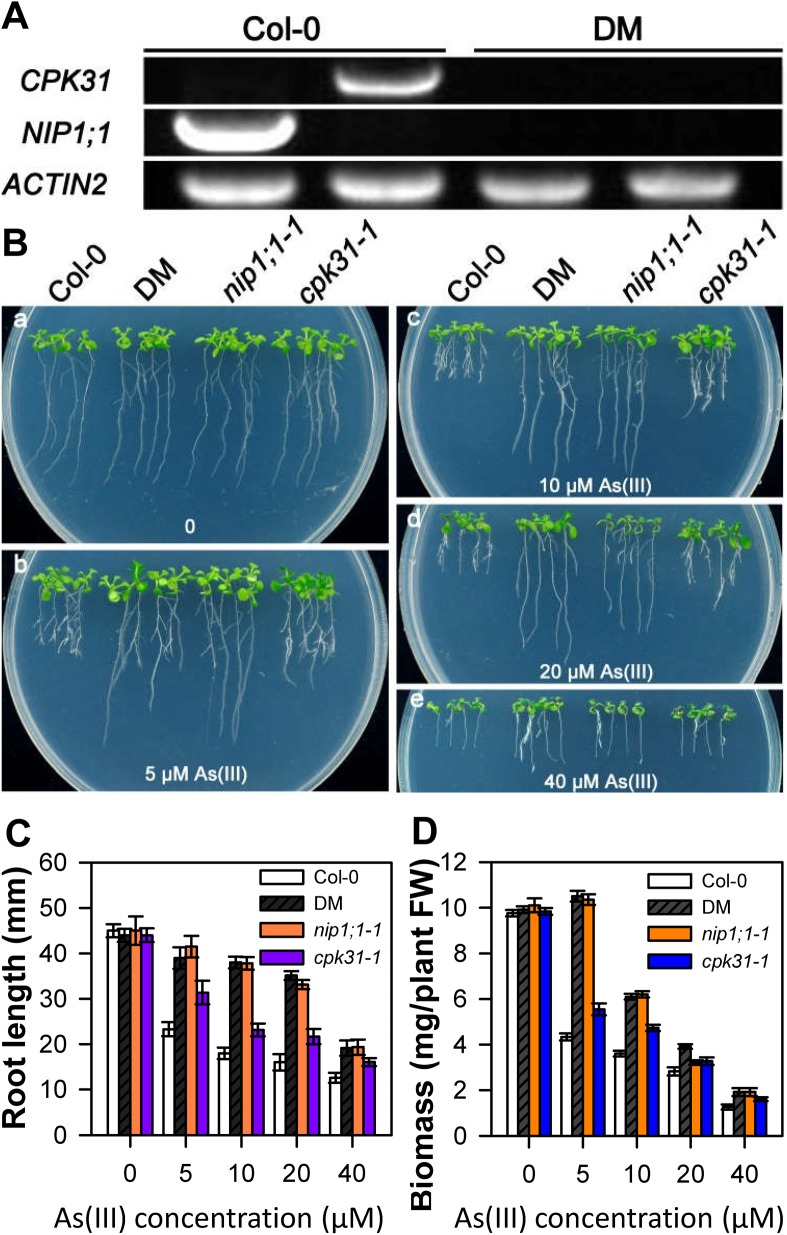
We isolated and characterized T-DNA insertional alleles in the CPK31 gene, including cpk31-1 (SALK_049228), cpk31-2 (SALK_007777) and cpk31-3(SALK_049236) (Fig 2A). CPK31 mRNA was not detectable by RT-PCR in homozygous plants of either T-DNA allele (Fig 2B), indicating that T-DNA insertions inside the gene disrupted the expression of CPK31 transcripts. To investigate the role of the CPK31 gene in the regulation of As(III) tolerance, we used two As(III) toxicity resistant lines, nip1;1–1 and nip1;1–2 T-DNA inserted mutants used in the previous study [13], as positive controls. When grown on the normal medium without As(III), all cpk31 and nip1;1 mutant seedlings showed similar phenotype to the wild-type (Fig 2C). However, in the presence of 5 and 10 μM As(III), the growth of cpk31-1, cpk31-2 and cpk31-3 seedlings, although not as good as nip1;1–1 and nip1;1–2 mutant seedlings, was better than the wild-type, indicating CPK31 may regulate As(III) uptake under these conditions. With the increase in As(III) concentrations, the cpk31 mutant seedlings displayed more similar phenotype to nip1;1–1 and nip1;1–2 mutant plants (Fig 2C–2E). Fig 2D and 2E respectively showed that root growth and biomass in the cpk31-1, cpk31-2 and cpk31-3 seedlings were significantly more tolerant to 20 and 40 μM As(III) treatment compared to the wild-type, and are similar to the nip1;1 mutant plants. These findings support a model that CPK31 might interact with and regulate AtNIP1;1 activity in As(III) transport.
Fig 2. Isolation of cpk31 T-DNA insertional mutants and As (III) resistance assays.
(A) Scheme of the Arabidopsis CPK31 gene structure and schematic T-DNA insertion sites of Arabidopsis lines SALK_049228, SALK_007777 and SALK_049236. Solid boxes and lines indicate exons and introns, respectively. The positions of T-DNA insertion in SALK_049228(cpk31-1), SALK_007777(cpk31-2) and SALK_049236 (cpk31-3) are indicated by arrows. (B) RT-PCR analysis of CPK31 and ACTIN2 mRNA levels in wild type (Col-0) and the three mutant lines (cpk31-1, -2, and -3). Three-week-old seedlings grown at half-strength MS were gathered for mRNA concentration analysis. (C) Comparative As(III) sensitivity analysis of wild-type, nip1;1 and cpk31 mutants. The seeds of wild-type (Col-0), nip1;1–1, nip1;1–2, and three cpk31 mutant lines were plated at half-strength MS agar plates containing 0 (a), 5 (b), 10 (c), 20 (d), or 40 μM (e) As(III), and 8-day-old seedlings after germination were used for the photographs. Seedlings of wild-type (Col-0), nip1;1–1, nip1;1–2, and three cpk31 mutant lines were plated on vertical half-strength strength MS agar plates containing 0 (a), 5(b), 10 (c), 20 (d), or 40 μM (e) As(III) for 8 days before taking the photographs. Scale bars = 10 mm. Length of primary roots (D) and whole-plant biomass (E) of wild-type (Col-0), two nip1;1 and three cpk31 mutant lines under various As(III) concentrations. The root length and biomass of 8-day-old seedlings cultured at the conditions described as (C) were measured (n >5 for each condition). Data are mean ± SD of four replicate experiments. Data are mean ± SD of four replicate experiments. Asterisks (*P< 0.05, Student’s t test,) represent statistically significant differences compared with Col-0 as the control.
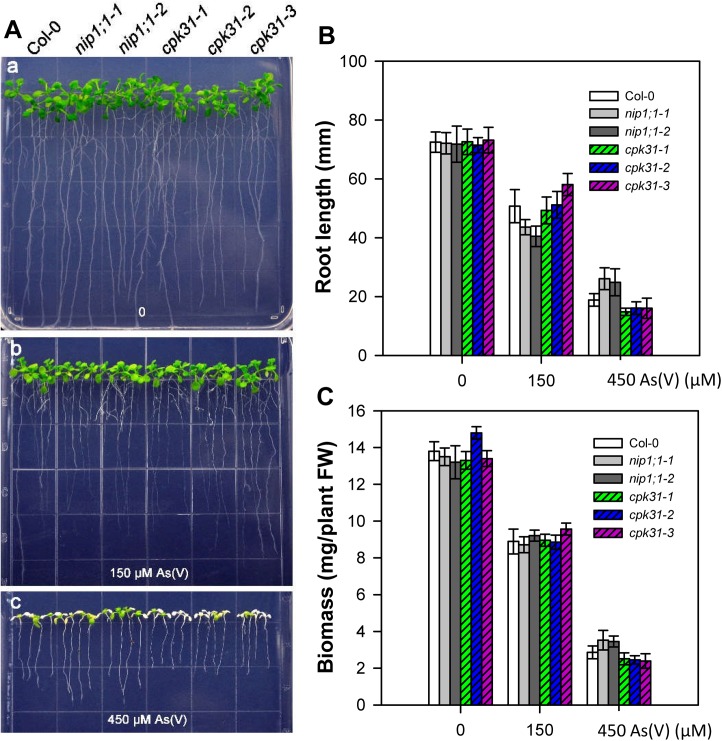
Earlier studies have shown that As(V) and As(III) enter plant roots via two different systems: As(V) is taken up via phosphate transport family (PHT), while As(III) is transported by NIPs [6–8]. Furthermore, after absorbed into roots, As(V) was widely accumulated in the endodermis, the apex (meristem) and cap of roots, whereas As(III) was mostly accumulated in the endodermis as detected by X-ray fluorescence microscopy [33]. Considering that As(V) is one of the most present forms of As absorbed by roots from soils [7,8], we also tested whether CPK31 also participates in As(V) response in Arabidopsis. We analyzed the growth of cpk31-3 mutants grown on half-strength MS medium containing various As(V) concentrations. As shown in Fig 3, cpk31-1, cpk31-2 and cpk31-3 seedlings showed similar growth to the wild-type under 0, 150, or 450 μM As(V), indicating that CPK31 may not regulate As(V) tolerance. Similarly, disruption of NIP1;1 did not enhance survival against As(V) stress (Fig 3), supporting further the notion that CPK31 may function with NIP1;1 specifically in As(III) response.
Fig 3. Growth phenotype of cpk31 and nip1;1 mutants under variable As(V) conditions.
(A) Growth phenotype of 7-day-old wild-type (Col-0), nip1;1–1, nip1;1–2, cpk31-1, cpk31-2, and cpk31-3 mutants in half-strength MS agar medium containing 0 (a), 150 (b), or 450 μM As (V) (c). Length of primary roots (B) and whole-plant biomass (C) of wild-type (Col-0), two nip1;1 and three cpk31 mutant lines grown for 10 days under 0, 150, or 450 μM As (V). Plants were grown at the normal half-strength MS for 3 days before transferred to the medium containing As(V). Data are mean ± SD of four replicate experiments. It was not statistically significant difference compared with Col-0 as the control (P< 0.05, Student’s t test).
The cpk31 mutants contain less As(III)
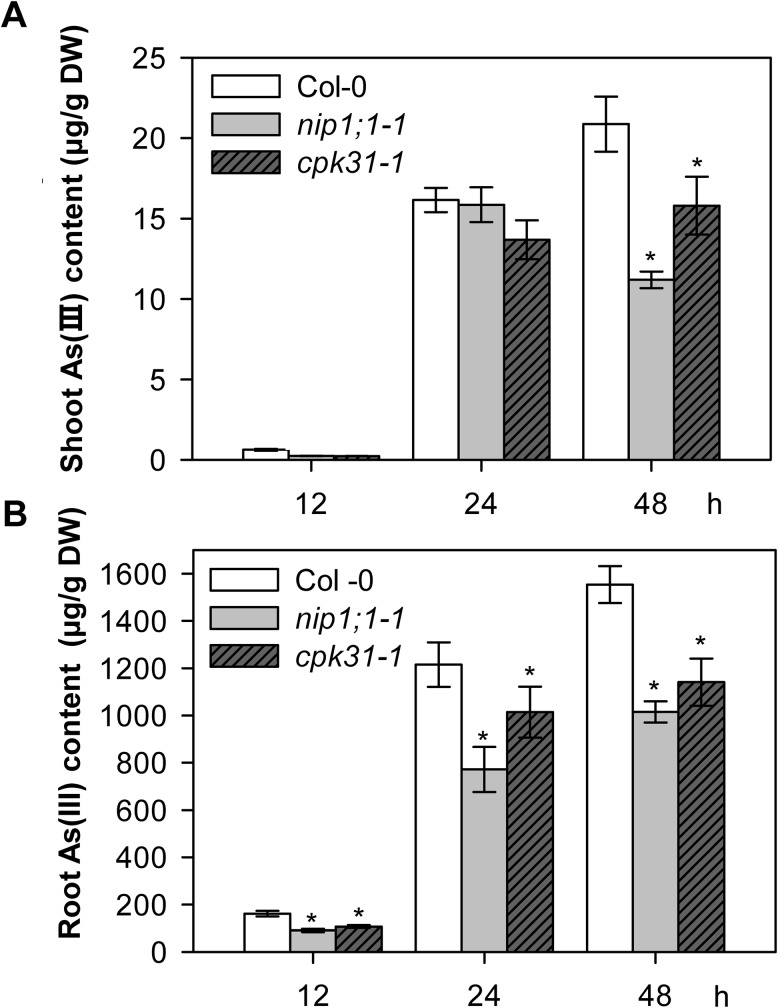
As(III) tolerance is determined by multiple processes, including acquisition and distribution. It was reported that As(III) tolerance in the nip1;1 mutant lines resulted from the decrease in As(III) uptake from the environments [13,16]. If As(III) acquisition is altered in cpk31 mutant plants through the AtNIP1;1 pathway, As(III) content in these plants may be reduced as well. Indeed, lower As(III) contents were observed in cpk31-1 mutant roots as compared with the wild-type after exposure to As(III), although As(III) content in the cpk31 mutants was still higher than the nip1;1–1 mutants at 12, 24 and 48 h after As(III) exposure (Fig 4B). This result suggests that As(III) uptake and accumulation from the medium are less efficient in cpk31 mutant plants.
Fig 4. cpk31-1 and nip1;1–1 mutants contain less As (III) compared with the wild-type.
As (III) contents in shoots (A) and roots (B) of seedlings under As(III) conditions. Three-week-old seedlings of wild-type (Col-0), nip1;1–1 and cpk31-1 mutant grown under the hydroponic medium were transferred to the medium containing 10 μM As (III) for 12, 24, or 48 h. Samples were collected at indicated time points after treatment and As(III) contents were determined. Summarized As(III) content in (A) and in (B) was respectively deduced from results of 5 seedlings/condition of two or four replicate experiments among three independent experiments. Data are mean ± SD, and asterisks indicate statistically significant difference between Col-0 and nip1;1 or cpk31 mutant plants (*P< 0.05, Student’s t test).
We further determined whether CPK31 is involved in As(III) translocation between roots and shoots after As(III) exposure. Three-week-old Arabidopsis seedlings grown in the hydroponic medium were transferred to the medium containing 10 μM As(III). After 12 h and 24 h As(III) exposure, As(III) contents in shoots were not significantly different among wild-type, nip1;1, and cpk31 mutants (Fig 4A), while those in the roots of nip1;1, and cpk31 mutants were less than wild-type (Fig 4B). However, shoots of nip1;1 and cpk31 mutants accumulated much less As(III) than the wild-type when these seedlings were exposed to As(III) for 48h (Fig 4B). These results suggest that CPK31 distributes As(III) within Arabidopsis body, probably companies with NIP1;1, to decrease As(III) toxicity.
CPK31 expression pattern overlapped with that of NIP1;1 in the roots
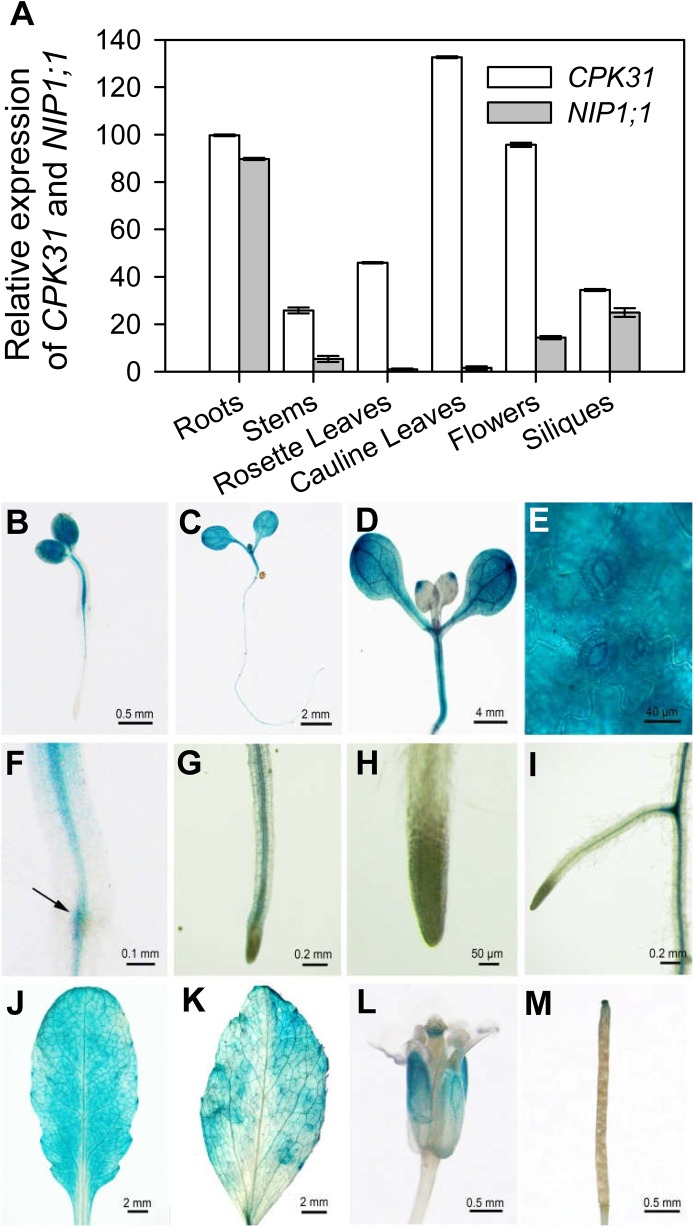
The results on protein-protein interaction and As(III) sensitivity suggest that CPK31 and NIP1;1 may function together in the same pathways. To gain further insights into the function of these proteins, we examined their expression pattern in plant tissues using qRT-PCR analysis. As shown in Fig 5A, the qRT-PCR analysis of gene transcripts indicated that CPK31 was ubiquitously expressed in roots, stems, rosette leaves, cauline leaves, flowers and siliques of 4-week-old Arabidopsis plants. On the other hand, NIP1;1 was expressed mostly in roots with low levels in other tissues, consistent with the earlier result that NIP1;1 is preferably expressed in roots [13]. To further analyze the expression pattern of CPK31, we analyzed transgenic Arabidopsis plants expressing CPK31 promoter-driving β-glucuronidase (GUS) reporter, CPK31 promoter was active in cotyledons, rosette leaves, the vascular bundle of primary roots, and the junction of lateral roots and primary roots of 2-, 3-, and 7-day-old seedlings (Fig 5B–5I). In addition to expressed in primary roots, CPK31 promoter the junction of primary root and lateral roots showed strong GUS activity (Fig 5I), It was noted that the activity of the CPK31 promoter was very high in guard cells (Fig 5E), overlapping with the expression pattern of the NIP1;1 genes [13]. We subsequently observed CPK31 gene promoter in leaves of the four-week-old seedlings, and found that the activity of promoter was highly expressed in the vascular tissues of mature leaves with higher levels at cauline leaves (Fig 5J) than rosette leaves (Fig 5K), consistent with the qRT-PCR analysis (Fig 5A). Furthermore, CPK31 was primarily expressed at petals and stigma of flower (Fig 5L), and the tip of siliques (Fig 5M).
Fig 5. Analysis on tissue expression pattern of CPK31.
(A) Quantification analysis of CPK31 and NIP1;1 transcripts in various organs of the wild-type plants. Total RNA was isolated from various tissues (root, leaf, stem, flower and silique) of 4-week-old wild-type plants (Col-0) grown in soil under long-day conditions. Values were normalized to ACTIN2, and the relative mRNA expression levels were calculated as the ratio of NIP1;1 or CPK31 mRNA level to the NIP1;1 level in rosette leaves of plants (as 1.0). Data are mean ± SD of four replicate experiments. (B-M) Histochemical analysis of CPK31 promoter–GUS expression in transgenic plants. GUS staining in 2-day-old (B), 3-day-old (C), and 7-day-old (D) transgenic seedlings grown on half-strength MS agar plates. The enlarged part of the cotyledon (E), root–hypocotyl junction (F), primary root (G), the tip of primary root (H), and lateral-primary root junction (I) of 7-day-old seedlings. GUS staining in cauline leaf (J), rosette leaf (K), flower (L), and silique (M) of mature CPK31::GUS transgenic plants. To obtain adult plants for staining, 7-day-old seedlings grown on 1/2 MS agar plates were transferred into soil, and samples used for GUS analysis were collected for (J) to (M) on the 21th day after the transfer.
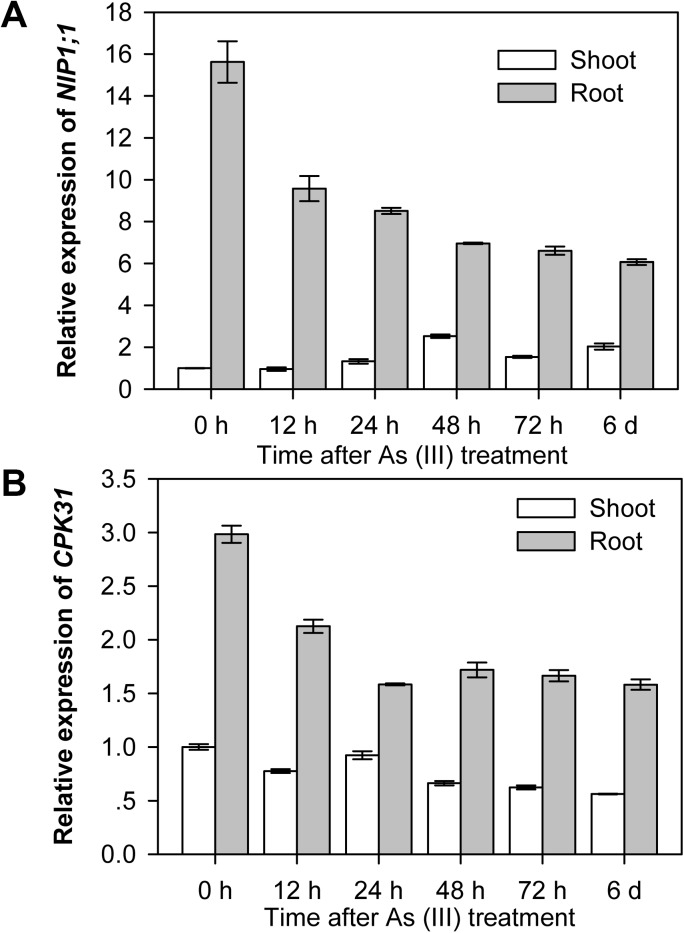
The assays described above indicated overlapping expression pattern of CPK31 and NIP1;1 in roots. We subsequently investigated whether mRNA levels of those two genes are regulated by As(III) treatment. After grown in normal hydroponic medium for 3 weeks, plants were transferred to the medium with 10 μM As(III). The roots and shoots of plants were collected at different time points and their mRNA levels were examined by qRT-PCR analysis. When exposed to As (III), both CPK31 and NIP1;1 mRNA levels in roots decreased within 12 hours (Fig 6). Interestingly, CPK31 and NIP1;1 in shoots responded to As(III) differently: CPK31 mRNA levels stayed relatively stable, but the mRNA of NIP1;1 increased. However, the mRNA levels of CPK31 and NIP1;1 altered more in roots than in shoots in response to As(III) (Fig 6). These results suggest that CPK31 and NIP1;1 might be functionally relevant, at least in roots, to decrease As(III) uptake under conditions of As(III) toxicity.
Fig 6. Expression of CPK31 and NIP1;1 is sensitive to As(III).
The levels of expression of NIP1;1(A) and CPK31(B) in leaves and roots of the seedlings. 3-week-old plants grown at normal hydroponic medium were transferred to the medium with 10 μM As (III), and the roots and shoots of plants incubated for 0, 12, 24, 48, 72, or 144 h were gathered separately for mRNA measurement. After normalized to ACTIN2, the relative expression of NIP1;1 or CPK31 was calculated as the ratio of the normalized expression of NIP1;1 or CPK31 in shoot or root of seedlings treated with As(III) to that in shoot before treated with As(III). Data are mean ± SD of four replicate experiments with n = 3 for each experiment.
As(III) sensitivity of cpk31 nip1;1 double mutants and transient CPK31 overexpression lines
CPK31 interacts physically with NIP1;1 and their genes are both expressed in roots. Together with the finding that both nip1;1 and cpk31 mutants showed similar phenotype in As(III) tolerance, we speculated that CPK31 and NIP1;1 might function in the same pathway. To address this hypothesis, we generated a cpk31 nip1;1 double mutant from cpk31-1 and nip1;1–1 single mutants (Fig 7A). The homozygous plants of the cpk31 nip1;1 double mutant did not produce detectable levels of transcripts of CPK31 and NIP1;1 as analyzed by RT-PCR, indicating disruption of both CPK31 and NIP1;1 expression.
Fig 7. Growth phenotype of cpk31 nip1;1 double mutants under variable As(III) conditions.
(A) qRT-PCR analysis of CPK31, NIP1;1 and ACTIN2 gene expression from the wild-type (Col-0) and cpk31 nip1;1 double mutants (DM). Expression of ACTIN2 was analyzed as a quantitative control. (B) Growth phenotype of 10-day-old wild-type (Col-0), cpk31 nip1;1 double mutants (DM), nip1;1–1, and cpk31-1 mutants grown in half-strength MS agar medium containing 0 (a), 5 (b), 10 (c), 20 (d), or 40μM As(III) (e). The seeds of wild-type (Col-0), cpk31 nip1;1 double mutants (DM), nip1;1–1, and cpk31-1 mutants were firstly planted at the normal half-strength MS agar medium without As(III) for 4 days, and were then transferred for the medium containing various As(III) contents. Length of primary roots (C) and whole-plant biomass (D) of wild-type (Col-0), cpk31 nip1;1 double mutants (DM), nip1;1–1, and cpk31-1 mutants seedlings grown for 10 days under 0, 5, 10, 20, or 40 μM As(III). Data are mean ± SD of four replicate experiments. Asterisks indicate statistically significant difference between Col-0 and mutant plants exposed various contents of As (III) (*P< 0.05, Student’s t test).
To analyze whether CPK31 is one of crucial regulators for NIP1;1 activity, we examined the As(III)-tolerant phenotype of wild-type, nip1;1, cpk31 nip1;1 double mutant seedlings grown on 1/2 strength MS agar plates under As(III) conditions. As shown in Fig 7, cpk31 nip1;1 double mutants significantly enhanced root growth as compared with cpk31 mutants, with longer primary root length and more biomass. Additionally, the growth phenotype of cpk31 nip1;1 double mutants is similar to that of nip1;1 mutants treated with As(III). Together, these results suggest that CPK31 might be one of factors for mediating NIP1;1 activity response for As(III) stress in vivo.
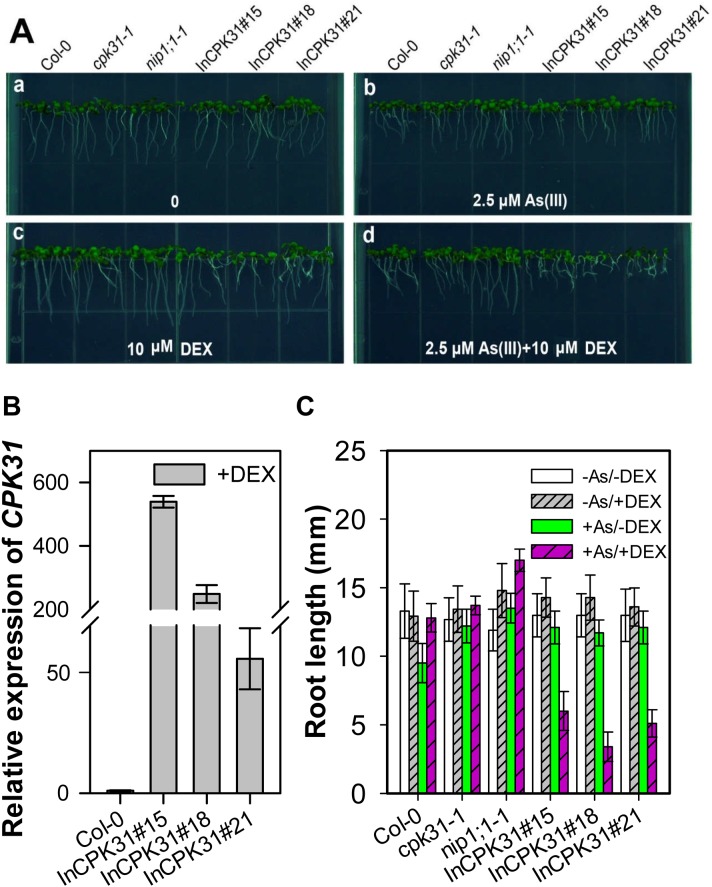
Because the knockout of CPK31 caused an increase in As(III) tolerance, we tried to find whether overexpression of CPK31 had a contrary response to As(III) in Arabidopsis. Thus, we constructed a dexamethasone (DEX)-inducible CPK31 expression system (InCPK31) in the cpk31-1 mutant background (Fig 8). We selected three lines of plants transformed with InCPK31, InCPK31#15, InCPK31#18, and InCPK31#21, as their CPK31 expression was significantly induced by DEX (Fig 8B). The inducible expression system did not cause the changes in growth at the absence of DEX (Fig 8A and 8C). However, the root length of plants transformed with CPK31 expression system (InCPK31), InCPK31#15, InCPK31#18, and InCPK31#21 was much shorter when these seedlings were grown at the medium containing DEX (Fig 8A and 8C), at which CPK31 expression was significantly induced (Fig 8B). These results again supports the hypothesis that the negative roles of CPK31 in As(III) tolerance in Arabidopsis.
Fig 8. Dexamethasone (DEX)-induced transient CPK31 expression caused a decrease in As(III) tolerance.
(A) Growth phenotype of 7-day-old wild-type (Col-0), cpk31-1, nip1;1–1, cpk31-1 mutant plants transformed with CPK31 expression system (InCPK31) lines 15# (InCPK31#15), 18# (InCPK31#18), and 21# (InCPK31#21) in half-strength MS agar medium containing 0 (a), 2.5 μM As (III) (b), 10 μM DEX (c), or 2.5 μM As (III) and 10 μM DEX (d). (B) Relative expression level of CPK31 in wild-type (Col-0), InCPK31#15, InCPK31#18, and InCPK31#21 lines treated with 10 μM DEX. The seedlings were obtained after 7 day DEX treatment for mRNA measurement. The relative expression of CPK31 was calculated as the ratio of CPK31 in InCPK31 lines to that in the wild type. The data are mean ± SD of three biological replicates. (C) The root length of the wild-type (Col-0), cpk31-1, nip1;1–1, InCPK31#15, InCPK31#18, and InCPK31#21 after 7 days of 10 μM DEX treatment. The data are mean ± SD of three biological replicates.
Discussion
Calcium-dependent protein kinases (CPKs) are crucial signaling molecules that mediate responses to diverse endogenous and environmental cues. In the present study, we provide evidence that CPK31 appears to regulate As (III) uptake in roots through interaction with As (III)-permeable aquaporin NIP1;1. This finding provides evidences of key roles of CPK31 in the regulation of As(III) toxicity in plants.
CPKs comprise a large family of serine/threonine kinases in plants (34 genes in Arabidopsis) with the conserved Ca2+-binding EF hands in the calmodulin-like domain. This unique molecular structure allows that CPKs could be directly activated by Ca2+, and function as a Ca2+ sensor [19]. Some studies proposed that CPKs could be involved in As(V) toxicity to some extent [34]. However, whether CPKs are involved in As(III)-mediated signaling pathway and their potential functions in defending against As(III) toxicity remain to be discovered. We found that CPK31 was down-regulated by As(III) treatment (Fig 6), whereas several CPKs were up-regulated by As(V) stress in the rice roots by the transcriptomic analysis [34], suggesting that CPKs may have contrary roles in As(III) and As(V) tolerance. Three homozygous T-DNA-insert knockout lines of CPK31 (CPK31-1, -2 and -3) all had the enhanced As(III)-tolerant phenotype (Fig 2), and cpk31-1 mutants accumulated As at a significantly lower rate in both roots and shoots than the wild-type plants (Fig 4). Furthermore, transient CPK31 overexpression induced by DEX caused the decrease in As(III) tolerance of transgenic Arabidopsis lines (Fig 8). These results indicate that CPK31 involving in As(III) uptake in roots and distribution in shoots.
As distinct Ca2+ sensor in plants, several CPKs, including CPK2, CPK13, CPK20, CPK21, CPK23, and CPK32, physically interacted with various channels [20–24]. Our results in this paper showed that CPK31 specifically interacts with NIP1;1 using yeast two hybrid screening, and used BiFC-based assays to confirm that the this interaction exists in Arabidopsis cells (Fig 1). CPK31 contains a myristoylation site at its N-terminus [19], known for targeting proteins to cellular membranes. The site could recruit CPK31 to the plasma membrane, where its anchoring target NIP1;1 lies (Fig 1)[13]. Therefore, it will be interesting to further disclose whether the existence of CPK31-involving Ca2+ signaling pathway for NIP1;1 functions.
CPK31 is mainly expressed at the vascular bundle but not root tips of the primary roots detected by histochemical staining of promoter-CPK31:GUS (Fig 5), with the similar patterns to NIP1;1 [13]. Moreover, RT-PCR analysis of CPK31 and NIP1;1 transcripts in root tissues indicated both expression of CPK31 and NIP1;1 was down-regulated once the roots were exposed to 10 μM As(III) (Fig 6). The decrease of NIP1;1 and CPK31 expression in presence of As(III) might result from the idea that NIP1;1 may not be "originally" designed to be As(III) carrier. For example, NIP1;1 also transports Sb(III) and determines the Sb(III) sensitivity of A. thaliana [12]. Thus, plants adapt to As(III) toxicity by developing this "avoidance" mechanism.
The overlapping expression profiles of CPK31 and NIP1;1 in the roots are consistent with the interaction with each other. Interestingly, most As(III) was accumulated in these sites of roots exposed to As(III) [33]. The loss-of-function mutants of CPK31 improved the specific tolerance of roots against As(III) but not As(V) (Figs 2 and 3), and accumulated less As(III) in roots than those of the wild-type plants (Fig 4). It is noteworthy that cpk31 mutants contained more As(III) with lower As(III) tolerance in the roots compared with nip1;1 mutants, indicating that CPK31 might be one of the kinases involving in As(III) uptake by NIP1;1. This hypothesis is further supported by the observations that roots of cpk31 nip1;1 double mutants showed stronger As(III) tolerance than cpk31 mutants, whereas similar to nip1;1 mutants (Fig 7). Moreover, as CPK31 is also present in other tissues such as the vascular tissues of leaves, stems and flowers, we speculate that it may function together with other components in those tissues as well, for example in the long-distance transport and distribution of As(III) throughout the whole plant, the functions of NIP1;1 was not involved [16].
As (III) is high toxic to organisms, due to its high affinity to bind with sulfhydryl groups of many proteins and then disrupting many key metabolic processes in the cells [4]. Several members of NIP aquaporins have been shown to function in As(III) uptake, including AtNIP1;1, AtNIP3;1 and AtNIP7;1 in Arabidopsis, and OsNIP2;1 in rice [2, 15, 16]. It has been reported that GmNOD26 could be activated by the unknown CPKs in soybean [17], but very little is known about potential signaling mechanisms that regulate these NIP aquaporins. Our study here has demonstrated that CPK31 might target and regulate NIP1;1 for As(III) tolerance, providing the first case for this notion that CPKs regulate As(III) sensitivity
Supporting information
(DOC)
Acknowledgments
We thank Jiangsu Collaborative Innovation Center for Modern Crop Production for technical support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China Grant, 31271626 and by the Ministry of Education of the People's Republic of China, Doctoral Fund of Ministry of Education of China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cullen WR, Reimer KJ. Arsenic speciation in the environment. Chem Rev. 1989; 89:713–764. [Google Scholar]
- 2.Ma JF, Yamaji N, Mitani N, Xu X-Y, Su Y-H, McGrath SP, et al. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci U S A. 2008; 105: 9931–9935. 10.1073/pnas.0802361105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao FJ, McGrath SP, Meharg AA. Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol. 2010;61:535–59. 10.1146/annurev-arplant-042809-112152 [DOI] [PubMed] [Google Scholar]
- 4.Ali W, Isayenkov SV, Zhao FJ, Maathuis FJ. Arsenite transport in plants. Cell Mol Life Sci. 2009; 66: 2329–2339. 10.1007/s00018-009-0021-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh P, Banerjee M, Giri AK, Ray K. Toxicogenomics of arsenic: classical ideas and recent advances. Mutat Res. 2008;659:293–301. 10.1016/j.mrrev.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 6.Tripathi R, Srivastava S, Mishra S, Singh N, Tuli R, Gupta D, et al. Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotech. 2007; 25(4):158–165. [DOI] [PubMed] [Google Scholar]
- 7.Zhao F, Ma J, Meharg A, McGrath S. Arsenic uptake and metabolism in plants. New Phytol. 2009;181: 777–794. 10.1111/j.1469-8137.2008.02716.x [DOI] [PubMed] [Google Scholar]
- 8.Catarecha P, Segura MD, Franco-Zorrilla JM, García-Ponce B, Lanza M, Solano R, et al. A mutant of the Arabidopsis phosphate transporter PHT1;1 displays enhanced arsenic accumulation. Plant Cell. 2007; 19: 1123–1133. 10.1105/tpc.106.041871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z, Ren H, McGrath SP, Wu P, Zhao F-J. Investigating the contribution of the phosphate transport pathway to arsenic accumulation in rice. Plant Physiol. 2011;157: 498–508. 10.1104/pp.111.178921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li N, Wang J, Song W-Y. Arsenic uptake and translocation in plants. Plant Cell Physiol. 2016; 57:4–13. 10.1093/pcp/pcv143 [DOI] [PubMed] [Google Scholar]
- 11.Maurel C, Boursiac Y, Luu DT, Santoni V, Shahzad Z, Verdoucq L. Aquaporins in plants. Physiol Rev. 2015;95(4):1321–58. 10.1152/physrev.00008.2015 [DOI] [PubMed] [Google Scholar]
- 12.Kamiya T and Fujiwara T. Arabidopsis NIP1;1 transports antimonite and determines antimonite sensitivity. Plant Cell Physiol. 2009;50:1977–81. 10.1093/pcp/pcp130 [DOI] [PubMed] [Google Scholar]
- 13.Kamiya T, Tanaka M, Mitani N, Ma JF, Maeshima M, Fujiwara T. NIP1;1, an aquaporin homolog, determines the arsenite sensitivity of Arabidopsis thaliana. J Biol Chem. 2009; 284: 2114–2120. 10.1074/jbc.M806881200 [DOI] [PubMed] [Google Scholar]
- 14.Bienert GP, Thorsen M, Schüssler MD, Nilsson HR, Wagner A, Tamás MJ, et al. A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol. 2008; 6:26 10.1186/1741-7007-6-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isayenkov SV, Maathuis FJ. The Arabidopsis thaliana aquaglyceroporin AtNIP7; 1 is a pathway for arsenite uptake. FEBS Lett. 2008; 582:1625–1628. 10.1016/j.febslet.2008.04.022 [DOI] [PubMed] [Google Scholar]
- 16.Xu W, Dai W, Yan H, Li S, Shen H, Chen Y, et al. Arabidopsis NIP3; 1 plays an important role in arsenic uptake and root-to-shoot translocation under arsenite stress conditions. Mol Plant. 2015; 8:722–733. 10.1016/j.molp.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 17.Weaver CD, Roberts DM. Determination of the site of phosphorylation of nodulin 26 by the calcium-dependent protein kinase from soybean nodules. Biochemistry. 1992; 31: 8954–8959. [DOI] [PubMed] [Google Scholar]
- 18.Guenther JF, Chanmanivone N, Galetovic MP, Wallace IS, Cobb JA, Roberts DM. Phosphorylation of soybean nodulin 26 on serine 262 enhances water permeability and is regulated developmentally and by osmotic signals. Plant Cell. 2003;15: 981–991. 10.1105/tpc.009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng SH, Willmann MR, Chen HC, Sheen J. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 2002; 129:469–85. 10.1104/pp.005645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geiger D, Scherzer S, Mumm P, Marten I, Ache P, Matschi S, et al. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc Natl Acad Sci U S A. 2010;107: 8023–8. 10.1073/pnas.0912030107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demir F, Horntrich C, Blachutzik JO, Scherzer S, Reinders Y, Kierszniowska S, et al. Arabidopsis nanodomain-delimited ABA signaling pathway regulates the anion channel SLAH3. Proc Natl Acad Sci U S A. 2013;110:8296–301. 10.1073/pnas.1211667110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ronzier E, Corratgé-Faillie C, Sanchez F, Prado K, Brière C, Leonhardt N, et al. CPK13, a noncanonical Ca2+-dependent protein kinase, specifically inhibits KAT2 and KAT1 shaker K+ channels and reduces stomatal opening. Plant Physiol.2014;166:314–26. 10.1104/pp.114.240226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutermuth T, Lassig R, Portes MT, Maierhofer T, Romeis T, Borst JW, et al. Pollen tube growth regulation by free anions depends on the interaction between the anion channel SLAH3 and calcium-dependent protein kinases CPK2 and CPK20. Plant Cell. 2013;25:4525–43. 10.1105/tpc.113.118463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou L, Lan W, Jiang Y, Fang W, Luan S. A calcium-dependent protein kinase interacts with and activates a calcium channel to regulate pollen tube growth. Mol Plant. 2014;7:369–76. 10.1093/mp/sst125 [DOI] [PubMed] [Google Scholar]
- 25.Lee SC, Lan W, Buchanan BB, Luan S. A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc Natl Acad Sci U S A. 2009; 106:21419–24. 10.1073/pnas.0910601106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lan WZ, Lee SC, Che YF, Jiang YQ, Luan S. Mechanistic analysis of AKT1 regulation by the CBL-CIPK-PP2CA interactions. Mol Plant. 2011;4:527–36. 10.1093/mp/ssr031 [DOI] [PubMed] [Google Scholar]
- 27.Li L, Kim BG, Cheong YH, Pandey GK, Luan S. A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. Proc Natl Acad Sci U S A. 2006;103:12625–30. 10.1073/pnas.0605129103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waadt R, Schmidt LK, Lohse M, Hashimoto K, Bock R, Kudla J. Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J. 2008; 56: 505–516. 10.1111/j.1365-313X.2008.03612.x [DOI] [PubMed] [Google Scholar]
- 29.Miao Y, Jiang L. Transient expression of fluorescent fusion proteins in protoplasts of suspension cultured cells. Nat Protoc. 2007; 2:2348–2353. 10.1038/nprot.2007.360 [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Henriques R, Lin SS, Niu QW, Chua NH. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc. 2006; 1:641–646. 10.1038/nprot.2006.97 [DOI] [PubMed] [Google Scholar]
- 31.Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987; 6:3901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods.2001;25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 33.Kopittke PM, de Jonge MD, Menzies NW, Wang P, Donner E, McKenna BA, et al. Examination of the distribution of arsenic in hydrated and fresh cowpea roots using two- and three-dimensional techniques. Plant Physiol. 2012;159:1149–58. 10.1104/pp.112.197277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang T-L, Nguyen QTT, Fu S-F, Lin C-Y, Chen Y-C, Huang H-J. Transcriptomic changes and signalling pathways induced by arsenic stress in rice roots. Plant Mol Biol. 2012;80: 587–608. 10.1007/s11103-012-9969-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.