Abstract
Background
In glaucoma, non-intraocular pressure (IOP)-related risk factors can result in increased levels of extracellular glutamate, which triggers a cascade of neurodegeneration characterized by the excessive activation of N-methyl-D-aspartate (NMDA). The purpose of our study was to evaluate the glioprotective effects of memantine as a prototypic uncompetitive NMDA blocker on retinal astrocytes in the optic nerve crush (ONC) mouse model for glaucoma.
Material/Methods
Optic nerve crush was performed on all of the right eyes (n=8), whereas left eyes served as contralateral healthy controls (n=8) in Balb/c/Sca mice. Four randomly assigned mice received 2-μl intravitreal injections of memantine (1 mg/ml) after ONC in the experimental eye. One week after the experiment, optic nerves were dissected and stained with methylene blue. Retinae were detached from the sclera. The tissue was immunostained. Whole-mount retinae were investigated by fluorescent microscopy. Astrocyte counts for each image were performed manually.
Results
Histological sections of crushed optic nerves showed consistently moderate tissue damage in experimental groups. The mean number of astrocytes per image in the ONC group was significantly lower than in the healthy control group (7.13±1.5 and 10.47±1.9, respectively). Loss of astrocytes in the memantine-treated group was significantly lower (8.83±2.2) than in the ONC group. Assessment of inter-observer reliability showed excellent agreement among observations in control, ONC, and memantine groups.
Conclusions
The ONC is an effective method for investigation of astrocytic changes in mouse retina. Intravitreally administered memantine shows a promising glioprotective effect on mouse retinal astrocytes by preserving astrocyte count after ONC.
MeSH Keywords: Astrocytes, Glaucoma, Memantine, Neuroprotective Agents
Background
Glaucoma is a multifactorial optic neuropathy characterized by progressive loss of retinal ganglion cells (RGC), leading to gradual deterioration of visual function [1,2]. The exact etiological mechanism of glaucoma is still unknown, despite numerous scientific advances in recent decades. The main goal of currently available glaucoma treatment is to lower intraocular pressure (IOP). However, reducing increased IOP alone cannot entirely prevent the progression of neurodegeneration and ensuing vision loss [3]. Furthermore, a significant number of glaucoma patients have normal IOP, suggesting non-IOP-mediated mechanisms. Therefore, new treatment strategies targeting neuroprotection pathways are urgently needed [3].
Various animal models exist that mimic different pathophysiological aspects of glaucoma: optic nerve transection, optic nerve crush (ONC), injection of polystyrene beads into the anterior chamber, laser trabecular photocoagulation, and genetic models [4].
ONC triggers a series of events in the optic nerve and retina, characterized by primary acute axonal damage in some of the optic nerve axons and secondary retinal neurodegeneration [4]. Therefore, the ONC model is particularly well-suited for investigation of neuroprotective approaches to glaucomatous optic neuropathy.
Retinal astrocytes are located in the nerve fiber layer below the inner limiting membrane, covering the lamina cribrosa and expanding throughout the optic tract [5].
Astrocytes are capable of transmitting electric signals and maintaining highly specialized connections to RGCs that control synaptic strength and provide a source of energy [6]. Astrocytes have processes reaching RGC synapses and interact with RGCs through G protein-coupled receptors. In glaucoma, RGC damage is accompanied by astrocyte activation and hypertrophy [7], resulting in changes in gene expression [8]. Reactive astrocytes release neurotrophic factors and serve as a mechanical scafold for the remaining neurons. Astrocytes are also capable of keeping the blood-brain barrier intact by sealing damaged areas [9]. Elucidation of astrocytic changes in glaucoma is thus critical for devising novel anti-glaucoma therapies.
Glutamate excitotoxicity is the primary factor responsible for neuronal cell death in neurodegenerative diseases [1,2,10], and glutamate is known to exert toxic effects on the retina, mainly on RGCs [14–17]. In glaucoma, non-IOP-related risk factors can result in increased levels of extracellular glutamate [18–20], which triggers a cascade of neurodegeneration characterized by excessive activation of N-methyl-D-aspartate (NMDA) receptors [2,21], glutamate excitotoxicity [22–25], and retinal ischemia. The associated increase in the intracellular calcium concentration ultimately induces neuronal death by either apoptotic or necrotic mechanisms [13,26].
Memantine (1-amino-3,5-dimethyladamantane) is a potent neuroprotective agent that is approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for moderate-to-severe Alzheimer disease [27,28]. As an uncompetitive “open channel blocker” of NMDA receptors, memantine does not compete with glutamate for binding sites, and possesses a relatively low affinity and fast kinetics [29]. Thus, memantine has almost no effect when extracellular glutamate levels are normal and only becomes effective in the presence of excess glutamate. While memantine showed great promise in preclinical glaucoma research, it failed to meet expectations in phase III clinical trials for open-angle glaucoma (NCT00141882; NCT00168350).
While most research groups have studied the effects of memantine on neurons, only a few studies have analyzed its effect on astroglia. Wu et al. performed a study showing there are two main neuroprotective effects of memantine [30]. They proved that memantine increased the release of neurotrophic factors, such as glial cell line-derived neurotrophic factor [30], which has an important role in neuronal survival [31]. Also, memantine anti-inflammatory properties are mediated through the reduction of pro-inflammatory factors such as superoxide, ROS, TNF-α, NO, and PGE2, release, thus resulting in inhibition of microglial over-activation [30]. Therefore, astroglia is a crucial component in neuronal protection.
The purpose of our study was to evaluate the glioprotective effects of memantine as a prototypic uncompetitive NMDA blocker on retinal astrocytes in the ONC mouse model for glaucoma.
Material and Methods
Animals
All procedures and animal care were carried out according to the European Convention of Animal Care and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All experiments were approved by the Lithuanian State Food and Veterinary Service (No. G2-23). Eight healthy 6-month old male Balb/c/Sca mice were used for the study. Mice were housed in a vivarium (Veterinary Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania) and maintained on a 12-h light-dark cycle with food and water ad libitum. All procedures were performed under deep intraperitoneal anesthesia using 1 mg/kg medetomidine hydrochloride (Domitor 1mg/ml, Orion Corporation Orion Pharma, Finland) and 75 mg/kg ketamine (Ketamidor 10%, Richter Pharma AG, Austria). We used artificial tears (Systane Ultra UD, Alcon Inc., USA) to avoid corneal dryness. The animals were sacrificed under deep anesthesia by cervical dislocation 7 days after the ONC.
Optic nerve crush
We performed ONC on all of the right eyes (n=8), whereas left eyes served as contralateral healthy controls (n=8). An incision in the conjunctivae was made in the superolateral part, bluntly dissected posteriorly, the muscle cone was entered, and the optic nerve was clearly exposed. The optic nerve was crushed with cross-action Dumont tweezers [27] for 3 seconds, approximately 2 mm posterior to the globe.
Four randomly assigned mice received 2-μl intravitreal injections of memantine (1 mg/ml) immediately after ONC in the experimental eye. Special care was taken to protect surrounding blood vessels. After the procedure, the mouse was placed into the cage for full recovery after anesthesia.
Tissue preparation
One week after the initial experiment, mice were euthanized by cervical dislocation under general intraperitoneal anesthesia; both eyes were enucleated and post-fixed in 4% paraformaldehyde (PFA) solution for 3 h. Optic nerves were dissected from the eyeball, fixated in glutaraldehyde overnight (O/N), and stained with methylene blue. Optic nerves were cut in semi-thin sections and cover-slipped.
Retinae were detached from the sclera and were post-fixed in the 4% PFA solution O/N. Subsequently, the retinas were washed in 0.1 M phosphate buffer solution (PBS), pH 7.4, 4 times for 5 min. Then the tissue was incubated in 10% normal goat serum (NGS; Colorado Serum Company, CO) for 30 min, after which the retinas were washed in 0.1 M PBS, pH 7.4 4 times for 5 min, and incubated in rabbit anti-glial fibrillary acidic protein (GFAP; 1:10 000) overnight at 4°C. Retinae were subsequently washed in 0.1 M PBS, pH 7.4, 4 times for 5 min and incubated in anti-rabbit IgG Alexa Fluor 488 (1:500) overnight at room temperature. Following the incubation, retinae were washed in 0.1 M PBS, pH 7.4 4 times for 5 min and flat-mounted on a glass slide facing astrocytes layering up, topping it with glycerol and cover-slipped.
Imaging
Immunostained whole-mount retinae were investigated by fluorescent microscopy using a Zeiss Axio Imager M2 (Carl Zeiss AG, Jena, Germany) (Figure 1). Astrocyte counts for each image were performed manually using ImageJ 1.49m software (Wayne Rasband, National Institutes of Health, USA). Twelve randomly chosen images of 222×170 μm in size were taken from each retina for further evaluation (Figure 1). Astrocytic somata were manually counted from each image. To achieve credible results, astrocyte somas were counted in the whole image, including left and lower borders. Astrocytic somata that crossed right and upper borders were excluded. Counts were taken manually and in a blinded manner by 2 examiners.
Figure 1.

Retinal whole-mount. Black squares indicate randomly chosen parts of the retina: periphery, mid-periphery, and central. Scale bar – 1 mm.
Semi-thin sections of the optic nerves were analyzed by light microscopy (Zeiss Axio Imager M1, Carl Zeiss AG, Jena, Germany), under 100x magnification. We randomly acquired 10 images of each optic nerve and evaluated ONC damage by grading it as control, mild, moderate, or severe [32].
Inter-observer variation
To assess inter-observer variation, manual astrocyte counts were performed by 2 independent observers blinded to the treatment condition. All observations were performed using the same microscope.
Statistical analysis
IBM SPSS Statistics 22.0 Program Package (IBM Corporation, USA) was used for statistical analysis. The Kruskal-Wallis test was used to compare the 3 groups. A P value of p<0.05 was considered statistically significant. Inter-observer variability was examined using intra-class correlation coefficient (ICC) statistics. ICC cut-off values were defined as follows: <0.40 – poor agreement; 0.41–0.60 – moderate agreement; 0.61–0.79 – good agreement; and ≥0.80 – excellent agreement [33,34].
Results
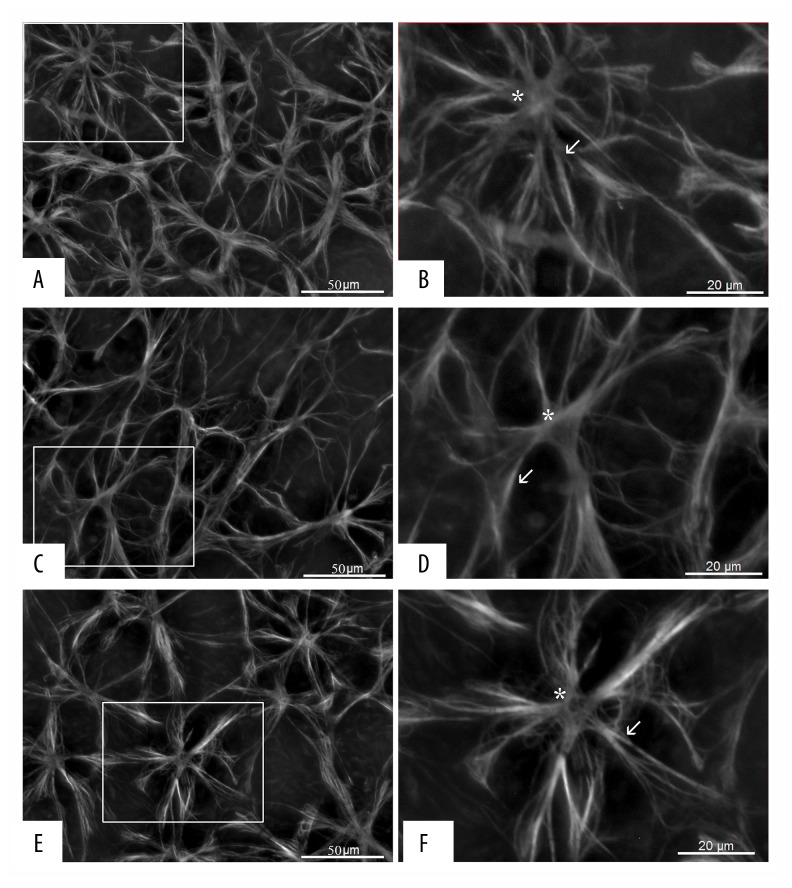
Memantine significantly reduced the loss of retinal astrocytes after ONC. The mean number of astrocytes per image in the control group was 10.47±1.9 (mean ±SD). ONC reduced the number of astrocytes to 7.13±1.5. Loss of astrocytes in the memantine-treated group was significantly attenuated (8.83±2.2; n=4, ANOVA, p<0.001) (Figure 2). Post hoc analysis using the Kruskal-Wallis test revealed a statistically significant difference between the untreated (p<0.001) and memantine-treated ONC condition (p=0.048) compared with the control condition (Figure 3). Furthermore, the loss of astrocytes in the memantine condition was significantly attenuated compared to the untreated condition (p=0.047).
Figure 2.
Representative images of GFAP immunoreactivity in whole-mount retinae showing astrocyte loss after ONC (C) compared with the control condition (A) and glioprotection by memantine (E). Scale bar 50 μm. Boxed areas are enlarged (B, D, F). White arrows point to astrocyte processes, and asterisks indicate astrocyte soma (B, D, F). GFAP – glial fibrillary acidic protein; ONC – optic nerve crush.
Figure 3.

Memantine significantly reduced the loss of retinal astrocytes after ONC. ONC significantly reduced the mean number of astrocytes compared to the control condition (p<0.001). Memantine treatment resulted in a statistically significant smaller loss of the number of astrocytes compared to the untreated ONC condition (p=0.047). Data are shown as means with the 95% CI. F=16.041, df=2, p<0.001; χ2=26.143, df=2, p<0.001; *,**,*** p<0.05. ONC – optic nerve crush.
Assessment of inter-observer reliability showed excellent agreement among observations in control, ONC, and memantine groups (Table 1).
Table 1.
Inter-observer reliability for manual retinal astrocyte count between 2 observers.
| Group | Intra-class correlation | 95% confidence interval |
|---|---|---|
| Control | 0.942 | 0.908–0.964 |
| ONC | 0.892 | 0.427–0.979 |
| Memantine | 0.956 | 0.918–0.977 |
ONC – optic nerve crush.
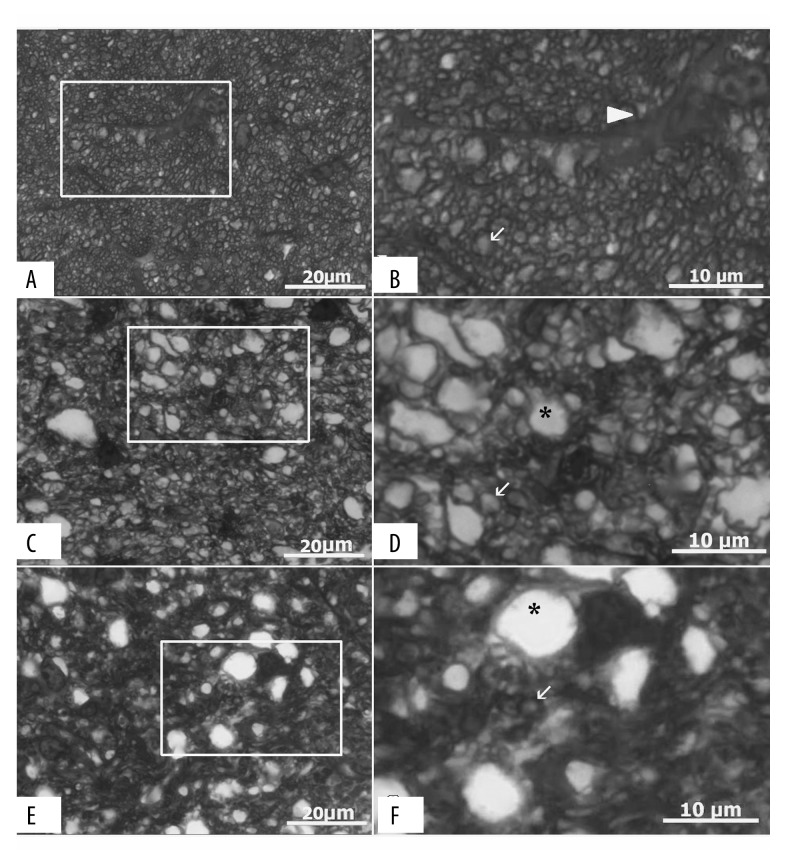
To exclude effects resulting from variability in execution of the ONC model, we graded optic nerve damage severity. We found a moderate severity optic nerve crush in all right eyes (ONC and memantine groups) on which ONC was performed. Representative images of semi-thin sections are shown in Figure 4.
Figure 4.
Representative photomicrographs (100× magnification) showing optic nerve sections from control (A), ONC (C), and memantine (E) groups. Scale bar 50 μm. Boxed areas are enlarged (B, D, F). Images A and B show a healthy optic nerve, while C–F were graded as moderate optic nerve damage. Arrow head indicates astrocyte (B), arrows – axons (B, D, F), and asterisks point to swollen axons (D, F). ONC – optic nerve crush.
Discussion
Our study investigated the effect of intravitreal memantine injection on the number of astrocytes in the mouse retina after ONC. Our study revealed that intravitreally administered memantine preserves retinal astrocytes after ONC in Balb/c mice. To the best of our knowledge, this is the first study to show evidence of glioprotective effects by memantine in an experimental glaucoma model.
To ascertain the successful and consistent execution of ONC, we evaluated and graded all optic nerve histological sections by severity of tissue damage [32]. Crushed optic nerves were graded as moderate severity, thus indicating that the ONC was performed uniformly, and confounding effects of the experimental model were excluded. The moderate ONC severity better mimics glaucomatous changes in comparison with optic nerve transection [35].
Memantine is an uncompetitive NMDA receptor blocker that prevents deleterious glutamate excitotoxicity on the target damage site and in surrounding cells [36]. ONC induces ionic concentration changes in RGCs, which begin with sodium influx and intracellular calcium elevation [37]. This leads to the sequence of events resulting in the further release of glutamate, elevation of the intracellular calcium concentration, and, eventually, death of RGCs. These processes cause primary neurodegeneration of RGCs, which induces astrocyte activation through junctions with RGCs axons [38]. Zhang et al. reported that astrocyte activation leads to both primary and secondary RGC death [39], while Ridet et al. suggested that activa ted astrocytes create conditions for axonal regeneration [40]. However, the exact role of astrocytes and the mechanisms of astrocyte activation in glaucoma are not yet fully understood.
Our study shows a greater reduction in astrocyte number following ONC in the untreated vs. the memantine group. It is likely that this is due to greater RGC death [41]; however, further studies analyzing both astrocytes and RGCs counts at the same time are needed.
It is well established that astrocytes release signals that are needed for neuronal survival, provide energy supply, and play an active role in synapse formation [42]. Furthermore, the elevated intracellular calcium concentration of activated astrocytes can result in potent vasoactive effects that help maintain the blood-retinal barrier after pathologic stress and/or injury. Depending on the trigger, different mechanisms are activated which result in either vascular dilatation or constriction [38–42]. Thus, it is crucial to understand these mechanisms and how memantine affects them, for the development of novel anti-glaucoma therapies.
To fully describe the effect of memantine on astrocytes and RGCs after ONC, larger-scale studies are needed to overcome some limitations of the present study: it was a short-term study, with a small sample size, and used a single memantine injection.
Conclusions
Herein, we present the first report of the glioprotective effects of intravitreally administered memantine on retinal astrocytes in the mouse ONC model. Despite its limitations, our study provides an important foundation for future studies that should include the systematic morphological, biochemical, immunological, and electrophysiological characterization of the signaling between retinal astrocytes and RGCs.
Footnotes
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Source of support: This research was supported in part by a grant from the Research Council of Lithuania, for a project entitled “The competitive funding of short-term researcher visits”, Nr. VP1-3.1-ŠMM-01-V-02-001
References
- 1.Gupta N, Ang LC, Noel de Tilly L, et al. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol. 2006;90:674–78. doi: 10.1136/bjo.2005.086769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA, Nickells RW, Kerrigan LA, et al. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–86. [PubMed] [Google Scholar]
- 3.Chang EE, Goldberg JL. Glaucoma 2.0. Neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology. 2012;119(5):979–86. doi: 10.1016/j.ophtha.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishikawa M, Yoshitomi T, Zorumski CF, Izumi Y. Experimentally induced mammalian models of glaucoma. BioMed Res Intl. 2015;2015:281214. doi: 10.1155/2015/281214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling TL, Stone J. The development of astrocytes in the cat retina: Evidence of migration from the optic nerve. Brain Res Dev Brain Res. 1988;44(1):73–85. doi: 10.1016/0165-3806(88)90119-8. [DOI] [PubMed] [Google Scholar]
- 6.Yoles E, Schwartz M. Degeneration of spared axons following partial white matter lesion: Implications for optic nerve neuropathies. Exp Neurol. 1988;153(1):1–7. doi: 10.1006/exnr.1998.6811. [DOI] [PubMed] [Google Scholar]
- 7.Jourdain P, Bergersen LH, Bhaukaurally K, et al. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10(3):331–39. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- 8.Prasanna G, Krishnamoorthy R, Yorio T. Endothelin, astrocytes and glaucoma. Exp Eye Res. 2011;93(2):170–77. doi: 10.1016/j.exer.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu J, Jakobs TC. The time course of gene expression during reactive gliosis in the optic nerve. PLoS One. 2013;8(6):e67094. doi: 10.1371/journal.pone.0067094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voskuhl RR, Peterson RS, Song B, et al. Reactive astrocytes form scar-like perivascular barriers to leucocytes during adaptive immune inflammation of the CNS. J Neurosci. 2009;29:11511–22. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tezel G. The immune response in glaucoma: a perspective on the roles of oxidative stress. Exp Eye Res. 2011;93:178–86. doi: 10.1016/j.exer.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haberg A, Qu H, Saether O, et al. Differences in neurotransmitter synthesis and intermediary metabolism between glutamatergic and GABAergic neurons during 4 hours of middle cerebral artery occlusion in the rat: The role of astrocytes in neuronal survival. J Cereb Blood Flow Metab. 2001;21(12):1451–63. doi: 10.1097/00004647-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1(8):623–34. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 14.Meldrum B, Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990;11(9):379–87. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- 15.Lipton SA, Rosenberg RA. Mechanisms of disease: Excitatory amino acids as a final common pathway in neurologic disorders. N Engl J Med. 1994;330(9):613–22. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 16.Lucas DR, Newhouse JP. The toxic effect of sodium L-glutamate on the inner layers of the retina. AMA Arch Ophthalmol. 1957;58(2):193–201. doi: 10.1001/archopht.1957.00940010205006. [DOI] [PubMed] [Google Scholar]
- 17.Olney JW. Glutamate-induced retinal degeneration in neonatal mice. Electron microscopy of the acutely evolving lesion. J Neuropathol Exp Neurol. 1969;28(3):455–74. doi: 10.1097/00005072-196907000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Vorwerk CK, Lipton SA, Zurakowski D, et al. Chronic low-dose glutamate is toxic to retinal ganglion cells. Toxicity blocked by memantine. Invest Ophthalmol Vis Sci. 1996;37(8):1618–24. [PubMed] [Google Scholar]
- 19.Hare WA, Wheeler L. Experimental glutamatergic excitotoxicity in rabbit retinal ganglion cells: block by memantine. Invest Ophthalmol Vis Sci. 2009;50(6):2940–48. doi: 10.1167/iovs.08-2103. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto T, Kitazawa Y. Vascular pathogenesis of normal-tension glaucoma: A possible pathogenic factor, other than intraocular pressure, of glaucomatous optic neuropathy. Prog Retinal Eye Res. 1998;17:127–43. doi: 10.1016/s1350-9462(97)00009-8. [DOI] [PubMed] [Google Scholar]
- 21.Massey SC, Miller RF. Glutamate receptors of ganglion cells in the rabbit retina: Evidence for glutamate as a bipolar cell transmitter. J Physiol. 1988;405:635–55. doi: 10.1113/jphysiol.1988.sp017353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung HS, Harris A, Evans DW, et al. Vascular aspects in the pathophysiology of glaucomatous optic neuropathy. Surv Ophthalmol. 1999;43:43–50. doi: 10.1016/s0039-6257(99)00050-8. [DOI] [PubMed] [Google Scholar]
- 23.Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retinal Eye Res. 2002;21:359–93. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 24.Otori Y, Wei JY, Barnstable CJ. Neurotoxic effects of low doses of glutamate on purified rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 1998;39:972–81. [PubMed] [Google Scholar]
- 25.Luo X, Heidinger V, Picaud S, et al. Selective excitotoxic degeneration of adult pig retinal ganglion cells in vitro. Invest Ophthalmol Vis Sci. 2001;42:1096–106. [PubMed] [Google Scholar]
- 26.Aizenman E, Frosch MP, Lipton SA. Responses mediated by excitatory amino acid receptors in solitary retinal ganglion cells from rat. J Physiol. 1988;396:75–91. doi: 10.1113/jphysiol.1988.sp016951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trump BF, Berezesky IK. Calcium-mediated cell injury and cell death. FASEB J. 1995;9(2):219–28. doi: 10.1096/fasebj.9.2.7781924. [DOI] [PubMed] [Google Scholar]
- 28.Parsons CG, Gruner R, Rozental J, et al. Patch clamp studies on the kinetics and selectivity of N-methyl-D-aspartate receptor antagonism by memantine (l-amino-3,5-dimethyladamantane) Neuropharmacology. 1993;32:1337–50. doi: 10.1016/0028-3908(93)90029-3. [DOI] [PubMed] [Google Scholar]
- 29.Schanne FA, Kane AB, Young EE, Farber JL. Calcium dependence of toxic cell death: a final common pathway. Science. 1979;206(4419):700–2. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- 30.Wu HM, Tzeng NS, Qian L, et al. Novel neuroprotective mechanisms of memantine: increase in neurotrophic factor release from astroglia and anti-inflammation by preventing microglial activation. Neuropsychopharmacology. 2009;34(10):2344–57. doi: 10.1038/npp.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen PS, Peng GS, Yang S, et al. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol Psychiatry. 2006;11(12):1116–25. doi: 10.1038/sj.mp.4001893. [DOI] [PubMed] [Google Scholar]
- 32.Kalesnykas G, Oglesby EN, Zack DJ, et al. Retinal ganglion cell morphology after optic nerve crush and experimental glaucoma. Invest Ophthalmol Vis Sci. 2012;53(7):3847–57. doi: 10.1167/iovs.12-9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haber M, Barnhart HX, Song J, Gruden J. Observer variability: A new approach in evaluating interobserver agreement. Journal of Data Science. 2005;3:69–83. [Google Scholar]
- 34.Landis JR, Koch GG. The measurement of observer agreement foe categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 35.McKinnon SJ, Schlamp CL, Nickells RW. Mouse models of retinal ganglion cell death and glaucoma. Exp Eye Res. 2009;88:816–24. doi: 10.1016/j.exer.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao L, Chen X, Tang Y, et al. Neuroprotective effect of memantine on the retinal ganglion cells of APPswe/PS1ΔE9 mice and its immunomodulatory mechanisms. Exp Eye Res. 2015;135:47–58. doi: 10.1016/j.exer.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Wells J, Kilburn MR, Shaw JA, et al. Early in vivo changes in calcium ions oxidative stress markers, and ion channel immunoreactivity following partial injury to the optic nerve. J Neurosci Res. 2012;90:606–18. doi: 10.1002/jnr.22784. [DOI] [PubMed] [Google Scholar]
- 38.Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–26. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Cheng M, Chintala SK. Optic nerve ligation leads to astrocyte-associated matrix metalloproteinase-9 induction in the mouse retina. Neurosci Lett. 2004;356:140–44. doi: 10.1016/j.neulet.2003.10.084. [DOI] [PubMed] [Google Scholar]
- 40.Ridet JL, Malhotra SK, Privat A, Gage FG. Reactive astrocytes: Cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–77. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 41.Naskar R, Quinto K, Romann I, et al. Phenytoin blocks retinal ganglion cell death after partial optic nerve crush. Exp Eye Res. 2002;74(6):747–52. doi: 10.1006/exer.2002.1173. [DOI] [PubMed] [Google Scholar]
- 42.Blondel O, Collin C, Mccarran WJ, et al. A glia-derived signal regulating neuronal differentiation. J Neurosci. 2000;20:8012–20. doi: 10.1523/JNEUROSCI.20-21-08012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]