Abstract
Samples from three submerged sites (MC, a core obtained in the methane seep area; MR, a reference core obtained at a distance from the methane seep; and HC, a gas-bubbling carbonate sample) at the Kuroshima Knoll in the southern Ryuku arc were analyzed to gain insight into the organisms present and the processes involved in this oxic-anoxic methane seep environment. 16S rRNA gene analyses by quantitative real-time PCR and clone library sequencing revealed that the MC core sediments contained abundant archaea (∼34% of the total prokaryotes), including both mesophilic methanogens related to the genus Methanolobus and ANME-2 members of the Methanosarcinales, as well as members of the δ-Proteobacteria, suggesting that both anaerobic methane oxidation and methanogenesis occurred at this site. In addition, several functional genes connected with methane metabolism were analyzed by quantitative competitive-PCR, including the genes encoding particulate methane monooxygenase (pmoA), soluble methane monooxygenase (mmoX), methanol dehydrogenese (mxaF), and methyl coenzyme M reductase (mcrA). In the MC core sediments, the most abundant gene was mcrA (2.5 × 106 copies/g [wet weight]), while the pmoA gene of the type I methanotrophs (5.9 × 106 copies/g [wet weight]) was most abundant at the surface of the MC core. These results indicate that there is a very complex environment in which methane production, anaerobic methane oxidation, and aerobic methane oxidation all occur in close proximity. The HC carbonate site was rich in γ-Proteobacteria and had a high copy number of mxaF (7.1 × 106 copies/g [wet weight]) and a much lower copy number of the pmoA gene (3.2 × 102 copies/g [wet weight]). The mmoX gene was never detected. In contrast, the reference core contained familiar sequences of marine sedimentary archaeal and bacterial groups but not groups specific to C1 metabolism. Geochemical characterization of the amounts and isotopic composition of pore water methane and sulfate strongly supported the notion that in this zone both aerobic methane oxidation and anaerobic methane oxidation, as well as methanogenesis, occur.
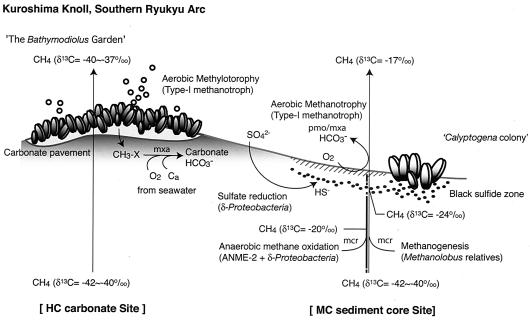
The Kuroshima Knoll is located in the southern Ryukyu arc near Ishigaki Island, Japan. One of the defining characteristics of this marine environment is the large amount of methane bubbling from numerous fissures in the large carbonate pavement; this methane is often associated with abundant clam (Calyptogena) and mussel (Bathymodiolus) colonies (20). In areas where methane bubbling has ceased, many fossilized mussels buried in the carbonate pavement can be found. The methane released from such subseafloor environments into the ocean and atmosphere is recognized as an important component of the global carbon cycle on Earth, so it is important to understand the dynamics of such systems and the factors that are involved in the production and consumption of methane.
To this end, we collected push cores from sediments adjacent to a Calyptogena colony (MC) and at a distance from any methane bubbling site (MR), as well as a massive piece of carbonate crust associated with a number of Bathymodiolus mussels (HC) (Fig. 1), with the goal of characterizing the pore water geochemistry, the populations present, and the presence of key indicator genes for prokaryotic methane metabolism.
FIG. 1.
Schematic diagram of the aerobic-anaerobic C1-metabolizing ecosystem in the Kuroshima Knoll in the southern Ryukyu arc.
The metabolic functions and activities of prokaryotic communities have a great impact on geochemical processes, including methane production and consumption, as well as sulfate reduction, in seafloor and subseafloor sediments (4). Molecular ecological analyses of other methane-rich environments have revealed that prokaryotic communities of archaea (ANME-1 group and ANME-2 lineages in the Methanosarcinales) and sulfate-reducing bacteria (δ-Proteobacteria) that naturally occur in shallow anoxic marine sediments are responsible for the anaerobic oxidation of methane according to the following equation: CH4 + SO42− → HCO3− + HS− + H2O (22). These archaeal communities are thought to oxidize methane by the reverse reaction of methanogenesis (1, 9, 29, 30, 31, 35). Both the ANME-1 and ANME-2 assemblages have the mcrA gene, which is a key functional gene for methanogenesis and encodes a methyl coenzyme M reductase (7). Recently, molecular analyses of DNA and proteins retrieved directly from natural methane seep environments in the Black Sea (25) revealed that the nickel-containing methyl coenzyme M reductase of ANME-1 is phylogenetically distinct from those of other methanogens, such as members of the orders Methanosarcinales and Methanococcales (18). Carbon isotopic characterization of archaeal lipids from ANME-1 and ANME-2 cells revealed the highly 13C-depleted lipids from the energy and carbon sources of ambient methane (9, 35). The strong disproportionation of the carbon of the ANME-1 and ANME-2 cells determined by fluorescence in situ hybridization with secondary ion mass spectrometry also demonstrated the presence of an anaerobic methane-consuming prokaryotic community (30, 31).
In addition to the ecological importance of the biological anaerobic methane oxidation (AMO) process, the C1-metabolizing microorganisms in oxic environments also play a significant role in the Earth's carbon cycling. Aerobic methanotrophic bacteria, including methylotrophs, can utilize methane and/or a variety of C1 compounds, such as methanol and methylated amines, as sole carbon and energy sources (8). These organisms are phylogenetically affiliated with two distinct lineages of the γ-Proteobacteria (type I methanotrophs) and the α-Proteobacteria (type II methanotrophs) and are widespread in terrestrial habitats (8), as well as in marine environments (13, 42). Almost all the known methanotrophs contain the pmoA and mxaF genes for aerobic methane oxidation. The pmoA gene encodes a particulate methane monooxygenase which is a key enzyme for the first step in aerobic methane oxidation (CH4 + 2H+ + O2 → CH3OH + H2O). The mmoX gene, which encodes a soluble methane monooxygenase, is also found in the type II methanotrophs and some type I members of the genus Methylococcus (8, 23, 26). Methanol is converted to formaldehyde in a second step by a methanol dehydrogenase encoded by mxaF. The mxaF gene has been found in all previously identified methanotrophs, as well as in methylotrophs (24), whereas the pmoA gene is not always involved in methylotrophic metabolism. Therefore, we used a combination of three functional genes (pmoA, mmoX, and mxaF) and the 16S rRNA gene to evaluate the aerobic methane-consuming bacterial communities.
Here we describe the distribution of C1-metabolizing organisms and genes in methane seep environments. We used 16S rRNA gene analyses to characterize the populations of archaea and bacteria and four structural genes to get a picture of the metabolic potential of the communities with regard to methane metabolism. The results suggest that the Kuroshima Knoll environment is complex, with methanogenesis, aerobic methane oxidation, and anaerobic methane oxidation all occurring in close proximity and with none of these processes abundant in reference sediment samples obtained at some distance from the active methane seeps.
MATERIALS AND METHODS
Sampling sites.
Two cores (MC and MR) and a surface sample (HC) were obtained in the Kuroshima Knoll area, which is located in the southern Ryukyu arc (24°07.5′N, 124°11.8′E) off Ishigaki Island, Japan (20), by using the manned submersible Shinkai 6500 (dives 6K761 and 6K764) during the YK03-05 scientific cruise of R/V Yokosuka in July 2003. The MC core (obtained 27.5 cm below the seafloor [bsf]) was collected from sediments beside a Calyptogena (clam) colony (diameter, <1 m) that was found on the western slope of the knoll at a depth of 686 m (24°07.809′N, 124°11.548′E). The core was mainly composed of black clay with fine sand, although the surface layer (0 to 3.0 cm bsf) was slightly reddish, indicating oxidized conditions. This layer was underlain by a black sulfide layer observed at a depth of 7.5 cm. In contrast, the reference (MR) core (length, 27.5 cm) was collected from the grayish sea bottom at a depth of 671 m (24°07.797′N, 124°12.039′E) and was uniformly composed of grayish clay. In addition, we collected a carbonate sample (HC) (approximately 2 kg) which was colonized by several mussels belonging to the Bathymodiolus sp. group from a very active methane bubbling site called The Bathymodiolus Garden at a depth of 636 m (24°07.777′N, 124°11.858′E). The HC sample was a porous columnar structure and relatively fragile. Methane gas diffusing from the HC carbonate was collected by using the WHATS system equipped with the submersible (37). The ambient seawater temperature was 6.1 to 6.8°C during the dives. The sediment cores were subsampled immediately by using sterilized syringes at 5-cm intervals and then prepared for microbiological and geochemical studies in the onboard laboratory as described below.
Characterization of pore water chemistry.
Pore water was extracted from the sediment core samples in an onboard laboratory within 3 h after sampling. At intervals of several centimeters, samples were extruded from each core, and the innermost zones were immediately transferred into airtight 50-ml plastic syringes to avoid degassing or air contamination. Pore water was then obtained by pressure filtration through a 0.45-μm-pore-size Millipore filter by using a stainless steel clamp (21) in a thermostatic refrigerator at 3 ± 1°C. Samples (∼2 ml) of the pore water were transferred into 3-ml glass vials, poisoned with HgCl2, and stored until the content and/or stable carbon isotopic composition of volatile components, such as methane, was measured. The residual pore fluids were kept in polypropylene bottles and used for analyses of dissolved components. All samples were refrigerated until analysis.
Cl− and SO42− were measured with an ion chromatograph (38) with an analytical precision of 0.3% and an accuracy of 3%, based on measurements of International Association for the Physical Sciences of the Oceans standard ocean water.
Compound-specific concentrations and stable carbon isotopic compositions of hydrocarbons were measured as previously described (40) by using an isotope-ratio-monitoring gas chromatograph-mass spectrometer. The analytical system consisted sequentially of an He-sparged bottle containing water (flow rate of helium carrier gas, 100 ml/min), a gas dryer (Nafion tubing), a CO2-trapping port with Ascarite II, a liquid N2 temperature trap (170-mm column packed with Porapak-Q), and a liquid O2 temperature mini gas chromatograph (10-mm column packed with Porapak-Q). With this system, methane was separated from the water, CO2, N2, and other noncondensable gases (such as O2 and CO) in the sample fluids. The hydrocarbons were then concentrated at the head of a PoraPLOT-Q analytical capillary column at the liquid O2 temperature and processed by separation by using an oven at 30°C. The eluted methane portion was quantitatively converted to CO2 by passing the gases through a 960°C combustion chamber (CuO/Pt catalyzer) that carried them directly into a Finnigan MAT 252 isotope-ratio-monitoring mass spectrometer.
The content of methane and ethane in a pore fluid sample was calculated by comparing the 44CO2 output of the isotope-ratio-monitoring mass spectrometer with the output measured during analyses of a working standard gas containing ca. 500 ppm of methane and ca. 27 ppm of ethane in nitrogen, which was made from a NIST RM 8560 (IAEA NGS2) standard. The precision of the concentration determination (1 sigma value for 10 determinations) was 5%. The analytical precision of the isotope analysis was 0.3‰. The systematic bias in the measured isotope ratio that depended on sample size was negligible. Analytical blanks of methane and ethane contained less than 3 pmol.
Nucleic acid extraction and PCR amplification of the mcrA, pmoA, mmoX, mxaF, and 16S rRNA genes.
Sediment samples for molecular analyses were stored at −80°C immediately onboard after subsampling. Bulk prokaryotic DNA was extracted from 10 g of thawed sediment by using a soil DNA Mega Prep kit (Mo Bio Laboratories, Inc., Solana Beach, Calif.) and then was purified and concentrated as previously described (12). DNA fragments encoding the mcrA, pmoA, mmoX, mxaF, and 16S rRNA genes of archaea and bacteria were amplified by using primer sets shown in Table 1. Amplification was performed with LA Taq polymerase with GC buffer I (TaKaRa, Tokyo, Japan) for each extracted DNA solution by using the GeneAmp 9600 PCR system (PE Applied Biosystems, Foster City, Calif.). The PCR conditions were as follows: denaturation at 96°C for 30 s, annealing at 47°C for 40 s, and extension at 72°C for 50 s for pmoA and mmoX amplification; denaturation at 96°C for 30 s, annealing at 50°C for 40 s, and extension at 72°C for 50 s for mxaF, mcrA, and archaeal 16S rRNA gene amplification; and denaturation at 96°C for 30 s, annealing at 52°C for 30 s, and extension at 72°C for 50 s for bacterial 16S rRNA gene amplification. Thirty-eight cycles were used for amplification of the pmoA, mmoX, mxaF, mcrA, and archaeal 16S rRNA genes, and 34 cycles were used for amplification of the bacterial 16S rRNA gene. PCR amplification without a DNA solution was used as a negative control to check for contamination.
TABLE 1.
Oligonucleotide sequences of PCR primers used in this study
| Target gene for PCR amplification | Primer | Oligonucleotide sequencea | Reference |
|---|---|---|---|
| 16S rRNA gene for archaea | Arc349f | 5′-GYGCASCAGKCGMGAAW-3′ | 33 |
| Arc806r | 5′-GGACTACVSGGGTATCTAAT-3′ | 33 | |
| 16S rRNA gene for bacteria | Bac349f | 5′-AGGCAGCAGTDRGGAAT-3′ | 33 |
| Uni806R | 5′-GGACTACYVGGGTATCTAAT-3′ | 33 | |
| Methyl coenzyme M reductase (mcrA) | ME1f | 5′-CGMATGCARATHGGWATGTC-3′ | 6 |
| ME2r | 5′-TCATKGCRTAGTTDGGRTAGT-3′ | 6 | |
| AOM39f | 5′-GCTGTGTAGCAGGAGAGTCA-3′ | 7 | |
| AOM40r | 5′-GATTATCAGGTCACGCTCAC-3′ | 7 | |
| Particulate methane monooxygenase (pmoA) | A189f | 5′-GGNGACTGGGACTTCTGG-3′ | 10 |
| A682r | 5′-GAASGCNGAGAAGAASGC-3′ | 10 | |
| Soluble methane monooxygenase (mmoX) | mmo882f | 5′-GGCTCCAAGTTCAAGGTCGAGC-3′ | 23 |
| mmo1403r | 5′-TGGCACTCGTAGCGCTCCGGCTCG-3′ | 23 | |
| Methanol dehydrogenase (mxaF) | mxa1003f | 5′-GCGGCACCAACTGGGGCTGGT-3′ | 24 |
| mxa1561r | 5′-GGGCAGCATGAAGGGCTCCC-3′ | 24 |
D = A, T, or G; H = A, T, or C; K = T or G; N = A, T, C, or G; M = C or A; R = A or G; S = G or C; V = A; C, or G; W = A or T; Y = C or T.
Quantification of mcrA, pmoA, mxaF, and 16S rRNA genes.
Copy numbers of functional genes were determined by quantitative competitive PCR (QC-PCR). A 400-bp competitor fragment was amplified by PCR by using a competitive DNA construction kit (TaKaRa), and then QC-PCRs were performed according to the manufacturer's instructions. The PCR conditions were the same as those described above. PCR products were separated by electrophoresis by using 2.0% (wt/vol) agarose with TBE buffer (90 mM Tris, 90 mM boric acid, 2 mM Na2-EDTA; pH 8.0) and were stained with SYBR Gold nucleic acid gel stain (Molecular Probes). The copy number of target genes was estimated by considering the band intensity, the length of the fragment, and the copy number of the competitor as described in the manufacturer's protocol.
Quantification of archaeal and bacterial 16S rRNA genes in bulk extracted DNA solutions was performed by quantitative real-time PCR by using universal and domain Archaea-specific TaqMan fluorogenic probes as described previously (33). PCR and monitoring of fluorescence signals were performed by using the GeneAmp 5700 sequence detection system (PE Applied Biosystems).
Cloning and sequencing.
Amplified products of the functional genes and the rRNA gene were subjected to agarose gel electrophoresis and then purified by using a Gel Spin DNA purification kit (Mo Bio Laboratories, Inc.) according to the manufacturer's protocol. Each gel-purified DNA fragment was then cloned in vector pCR2.1 by using an Original TA cloning kit (Invitrogen, Carlsbad, Calif.). The insert was amplified directly by PCR from a randomly selected colony by using M13 primers for the vector pCR2.1 (Invitrogen), treated with exonuclease I and shrimp alkaline phosphatase (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, United Kingdom), and then directly sequenced by using the forward primer and the dideoxynucleotide chain termination method with a dRhodamine sequencing kit (PE Applied Biosystems) as recommended by the manufacturer. The representative sequences for phylogenetic analysis were then determined by sequencing both strands.
Phylogenetic analysis.
The similarity among sequences was analyzed by using the FASTA program with the DNASIS software (Hitachi Software, Tokyo, Japan). Clone nucleotide sequences having ≥97% similarity were tentatively assigned to the same phylogenetic type (phylotype), and a representative clone sequence was selected for each phylotype. Phylogenetic analyses of the representative clone sequences were restricted to nucleotide positions that could be unambiguously aligned in all sequences. A least-squares distance matrix analysis, based on evolutionary distances, was carried out by using the correction of Kimura (16). A neighbor-joining analysis was performed by using the DDBJ CLUSTAL-X system (36). A bootstrap analysis was performed by using 100 trial replications to provide confidence estimates for phylogenetic tree topologies.
Nucleotide sequence accession numbers.
Nucleic acid sequences determined in this study have been deposited in the GenBank/EMBL/DDBJ databases. The accession numbers for representative sequences of the genes are as follows: mcrA, AB176925 to AB176932; pmoA, AB176933 to AB176940; mxaF, AB177996 to AB178005; archaeal 16S rRNA gene, AB178089 to AB178094; and bacterial 16S rRNA gene, AB178095 to AB178103.
RESULTS
Chemical characteristics.
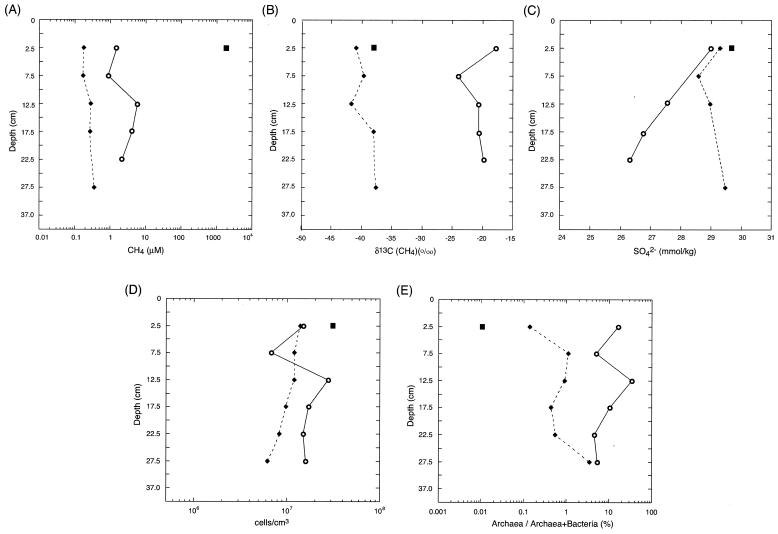
Pore water profiles show that there were low levels of methane in the MR core, 10-fold-higher levels in the MC core, and very high levels in the HC sample (Fig. 2A). The δ13C(CH4) values for the two cores were quite different; the values for the reference MR core (and HC sample) were close to the values for the methane venting at The Bathymodiolus Garden (around −40 ‰ Peedee belemnite [PDB]), and the values for the MC core were substantially higher and heterogeneous (−17.7 to −24.1 ‰ PDB) (Fig. 2B). These isotopic data suggest that the methane in the MC core was oxidized anaerobically in the sediments. The profile of methane concentrations in the MC core indicated an increasing tendency in deeper zones. The concentration of methane at 12.5 cm bsf (5.9 μM) was about three times higher than the concentration at 22.5 cm bsf (2.1 μM) (Fig. 2A), indicating that methanogenesis also occurred in the sediments. The chemical compositions of all gas samples indicated that the samples were composed of mostly methane. The levels of hydrogen and other hydrocarbon compounds were below the detection limit. Sulfate reduction was not apparent in the MR core or the HC sample, while the MC core was characterized by H2S production and slight SO42− depletion (Fig. 2C). Taken together, the chemical data suggest that both methanogenesis and anaerobic methane oxidation may occur in the sulfate reduction zone at the MC site (39).
FIG. 2.
Profiles of microbiological and geochemical parameters in the HC carbonate (▪), MC (○), and MR (♦) core sediments. (A) Concentrations of methane. (B) δ13C values of methane. (C) Concentrations of sulfate. (D) Total cell counts evaluated by direct counting of acridine orange-stained cells. (E) Percentages of archaeal 16S rRNA genes in the total prokaryote 16S rRNA genes estimated by quantitative real-time PCR analysis.
Microbial abundance.
The total cell counts (Fig. 2D) indicated that the sizes of the populations in the MC core samples were approximately two times greater than the sizes of the populations in the MR core samples (Fig. 2D). For the cell population in the MC core the minimum value (6.8 × 106 cells/cm3) was found in the black sulfide layer, and the concentration increased to 3.5 × 107 cells/cm3 below this layer. Thus, the total amounts of microbes, based on direct counts, were not dramatically different in any of the environments studied.
Quantitative and phylogenetic analyses of archaeal 16S rRNA genes.
Quantitative real-time PCR analysis demonstrated that the proportions of archaeal 16S rRNA genes in the universal prokaryotic rRNA gene communities in the MC core sediments were 4.6 to 34.0%, whereas those in the MR core sediments were 0.1 to 3.5% and the HC sample was nearly devoid of archaeal signals (Fig. 2E). Thus, while the total counts were similar, the compositions of the populations were very different. In the black sulfide layer, the proportion of the archaeal 16S rRNA genes in the prokaryotic DNA assemblages was 4.9%, and below this layer the proportion increased; however, overall, in the MC core the archaeal signals accounted for about 10% of the total signals. In contrast, in the MR core the proportion of archaeal rRNA increased with increasing depth of the sediment and was highest in the deepest sediments (Fig. 2E).
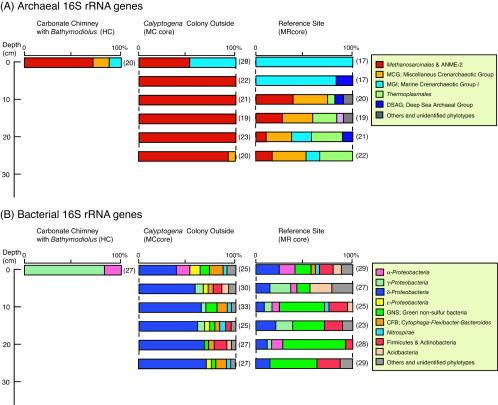
To study the nature of the archaeal rRNA community structures, a total of 269 clones of the archaeal 16S rRNA gene were sequenced. Phylogenetic analysis of the archaeal rRNA gene sequences revealed that the archaeal communities in both the HC and MC samples consisted of several predominant phylotypes belonging to the order Methanosarcinales, which were roughly classified into the family Methanosarcinaceae and the ANME-2 group (Fig. 3A). The most predominant archaeal phylotype was KAM1-5.10 (clone frequencies in the HC and MC libraries, 20 and 44%, respectively), which exhibited 91.1% similarity to Eel-36a2H11 in the Methanosarcinaceae (Table 2). KAHC-39 was the second most abundant phylotype (20% in the HC and MC clone libraries) and was closely related to the genus Methanolobus (97.9% similarity to Methanolobus oregonensis) (Table 2). In the MC core sediments, 16S rRNA gene sequences from members of the ANME-2a and ANME-2c subgroups were the dominant sequences detected (21.6 and 4.0% in the MC clone libraries, respectively), whereas the ANME-2 group was not detected in the HC carbonate sample. In addition, we did not detect ANME-2 sequences in the black sulfide layer at a depth of 7.5 cm, where the KAHC-39 phylotype was predominant. Sequences of crenarchaeota marine group I, which is the most abundant archaeal component in seawater (15, 34), were detected in the HC sample and the top layer of the MC sediment core (Fig. 3A).
FIG. 3.
Prokaryotic community structures based on the archaeal (A) and bacterial (B) 16S rRNA gene clone libraries. The number of clones examined in each library is indicated in parentheses.
TABLE 2.
Representative phylotypes of frequently retrieved 16S rRNA gene sequences in clone library analysis
| Phylogenetic group | Phylotype sequence | Related sequence in database | % Similarity | Habitata |
|---|---|---|---|---|
| Archaea | ||||
| Methanosarcinales | KAMC-5.10 | Eel-36a2H11 | 91 | HC (4/20), MC (59/133) |
| KAHC-39 | Methanolobus oregonensis | 98 | HC (9/20), MC (20/133) | |
| ANME-2a | KAMC-4.20 | BR34ARC_C05 | 98 | MC (28/133) |
| ANME-2c | KAMC-4.56 | Eel-36a2A5 | 99 | MC (6/133) |
| Thermoplasmales | KAMR-7.23 | AMOS1A_4113_D04 | 89 | MR (8/116) |
| Miscellaneous crenarchaeotic group | KAMR-4.07 | OHKA4.47 | 93 | MR (8/116) |
| Bacteria | ||||
| α-Proteobacteria | KBHC-26 | JTB131 | 88 | HC (4/27) |
| KBMR-2.29 | CS2.19 | 90 | MR (6/161) | |
| γ-Proteobacteria | KBHC-01 | Methylobacter luteus | 93 | HC (12/27) |
| KBHC-09 | Methylomonas methanica | 94 | HC (4/27) | |
| δ-Proteobacteria | KBMC-5.07 | Eel-36e1H1 | 97 | MC (35/167) |
| KBMC-1.18 | Eel-BE1B3 | 97 | MC (18/167) | |
| KBMC-3.16 | Hyd89-63 | 99 | MC (10/167) | |
| KBMC-4.05 | Desulfobulbus mediterraneus | 93 | MC (4/167), MR (4/161) | |
| Green nonsulfur bacteria | KBMC-6.16 | MB-C2-126 | 95 | MC (6/167), MR (27/161) |
The numbers in parentheses are the number of related clones having >97% similarity/number of clones analyzed. All samples were collected from the western slope of the Kuroshima Knoll in the southern Ryukyu arc.
In marked contrast, the archaeal 16S rRNA gene sequences obtained from the sediments in the MR core were mainly composed of members of marine group I (Fig. 3A). Clones belonging to the deep-sea archaeal group, previously determined to be the predominant archaeal components in the organic rich subseafloor clay sediments from the Sea of Okhotsk (12), were also detected at several depths (Fig. 3A). In the deeper zone of the MR sediment core, the archaeal rRNA community was composed of three dominant phylogenetic groups: Methanosarcinales, including Methanosarcinaceae and the ANME-2 group; Thermoplasmatales; and the miscellaneous crenarchaeotic group (Fig. 3A, Table 2).
Quantitative and phylogenetic analysis of the mcrA gene.
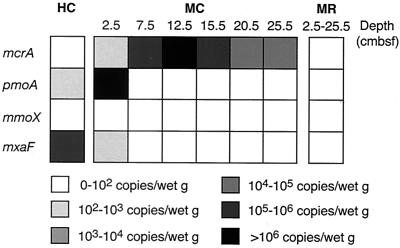
One archaeal gene (mcrA, which codes for methyl coenzyme M reductase) was detected by QC-PCR by using the ME1-ME2 primer set (Table 1) in all sections of the MC core sample, and this gene was not detected in the MR core or the HC sample. The maximum level of this gene was found at depths ranging from 7.5 to 17.5 cm (>106 copies/g [wet weight] of sediment) (Fig. 4), corresponding to the black sulfide layer and the layer just beneath it. The maximum value for the mcrA copy number at 12.5 cm was 2.5 × 106 copies per g (wet weight) of sediment. Not only were no PCR products obtained for the mcrA gene from the HC sample or the MR core sediments, but also no PCR amplicons were obtained when other primers were used (primers AOM39f and AOM40r designed for amplification of mcrA group b) (7) (Table 1).
FIG. 4.
Copy numbers of four functional genes (mcrA, pmoA, mmoX, and mxaF) for oxic-anoxic oxidation of methane estimated by quantitative competitive PCR analysis.
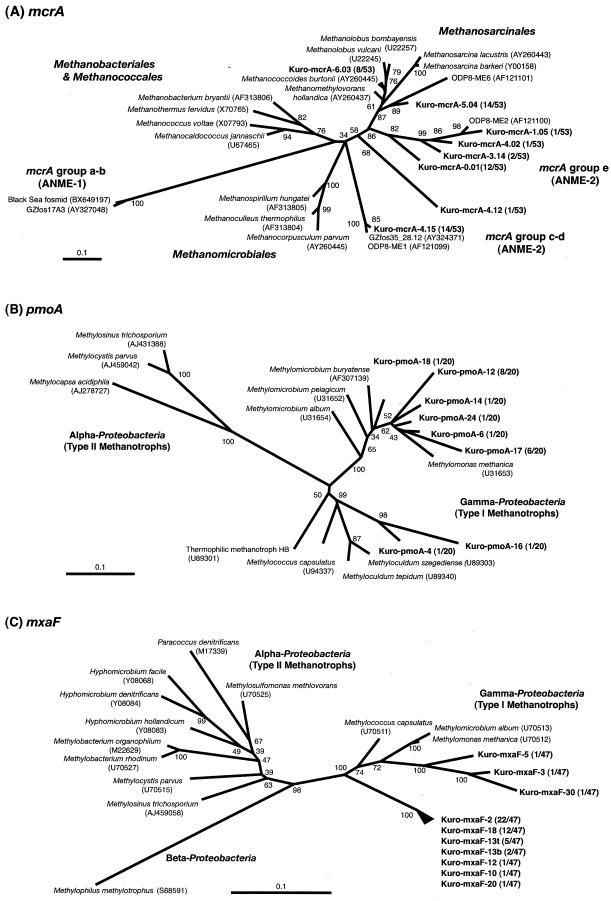
A total of 53 mcrA clones from the MC core sediments were sequenced, and eight representative phylotypes were determined. Phylogenetic analysis of the mcrA gene indicated that most of the sequences were affiliated with mcrA groups c-d and e, which were described previously by Hallam et al. (7); the only exception was a minor Kro-mcrA-4.12 phylotype (Fig. 5A). The mcrA sequence corresponding to the ANME-1 lineage was not detected in the MC core samples. Two representative phylotypes of the mcrA gene, Kuro-mcrA-6.03 and Kuro-mcrA-5.04, were related to the mcrA genes of the genera Methanococcoides and Methanolobus rather than to the mcrA genes of the ANME-2 group (Fig. 5A). The results for the mcrA and 16S rRNA genes consistently suggested the absence of ANME-1 lineages and the presence of both previously described mesophilic methanogens and ANME-2 in the order Methanosarcinales.
FIG.5.
Phylogenetic trees for McrA (A), PmoA (B), and MxaF (C) based on the partial amino acid sequences deduced from each functional gene. The numbers in parentheses are the accession numbers for nucleotide sequences in databases. The trees were constructed by using the neighbor-joining algorithm. The bootstrap values at the nodes are based on 100 trials. Scale bars = 0.1 amino acid substitution.
Bacterial 16S rRNA gene sequences.
Phylogenetic analysis of 355 bacterial 16S rRNA gene sequences revealed that the bacterial community structure in the HC sample was clearly different from that in the MC core sediments. The HC sample was dominated by the γ-Proteobacteria (85%), whereas the MC samples were dominated by δ-Proteobacteria, which comprised 40 to 70% of the community in the core sample (Fig. 3B).
The most common 16S rRNA gene sequences of γ-Proteobacteria in the HC sample were related to members of the type I methanotrophs, such as the genera Methylobacter, Methylomonas, and Methylophaga, and the similarity values were relatively low (90 to 94%) (Table 2). For example, the representative sequence of KBHC-01, which was the most abundant phylotype (12 of 27 clones in the HC clone library), exhibited 93.2% similarity with Methylobacter luteus (Table 2). Four clone sequences related to the α-Proteobacteria were also detected in the HC samples (15%); however, these sequences were not related to the type II methanotrophs. These clone sequences belonged to one phylotype (>97% similarity), KBHC-26, whose sequence exhibited low levels of similarity with all other sequences in the databases (88% similarity to JTB131) (Table 2).
The most common δ-Proteobacteria sequences in the MC sediment core were affiliated with the Desulfosarcina-Desulfococcus cluster in the family Desulfobacteriaceae, and they were closely related to sequences detected in other cold seep environments (e.g., Black Sea, Cascadia Margin, Eel River, Guaymas Basin, Gulf of Mexico, Japan Trench) (1, 11, 17, 19, 27, 29, 35). The predominant KBMC-5.07 and KBMC-1.18 phylotypes were most closely related to Eel-36e1H1 and Eel-BE1b3 (97% similarity), respectively (Table 2). These phylotypes were previously characterized as members of the putative AMO syntrophic sulfate-reducing bacterium group by well-defined phylogenetic and isotopic analyses (29). In the MC core clone libraries, besides the δ-Proteobacteria we detected a variety of minor sequences of α-, γ-, and ɛ-Proteobacteria, green nonsulfur bacteria, the Cytophaga-Flexibacter-Bacteroides group, Nitrospira, and gram-positive bacteria (Fig. 3B).
The percentage of clone sequences related to the δ-Proteobacteria was smaller in the MR reference core sediments (8 to 28%) than in the MC methane seep sediments (Fig. 2B), and the population was composed primarily of Desulfobulbus relatives and was essentially devoid of Desulfosarcina and Desulfococcus sequences. In contrast, the sequences affiliated with green nonsulfur bacteria were predominant bacterial components in the MR core clone libraries (7 to 68%) (Fig. 3B and Table 2). The representative phylotypes KBMC-6.16 and KBMR-3.18 were similar to MB-C2-126 (95%) and OHKB2.14 (96%), which were retrieved from hydrate-bearing deep sediments from the Nankai Trough (32) and pelagic clay subseafloor sediments from the Sea of Okhotsk (12), respectively (Table 2).
Quantitative and phylogenetic analyses of the pmoA, mmoX, and mxaF genes.
The distribution, phylogenetic diversity, and levels of three key functional genes for bacterial methanotrophic and/or methylotrophic metabolism were analyzed. The pmoA and mxaF genes were successfully amplified by PCR from the HC sample and the reddish surface layer of the MC sediment core (Fig. 4). The mmoX gene was not detected in any of the samples, which suggests that the abundance of the type II methanotroph and Methylococcus relatives might be quite low. QC-PCR analysis indicated that the pmoA gene was prominent in the MR core surface sediment (5.9 × 106 copies/g [wet weight]), while the amount of the mxaF gene was relatively small (102 to 103 copies/g [wet weight]) (Fig. 4). In contrast, for the HC carbonate sample, the QC-PCR analysis of the mxaF gene indicated that the copy number was high (7.1 × 106 copies/g [wet weight]) and that the copy number of the pmoA gene was about 1 order of magnitude lower (Fig. 4).
Phylogenetic analysis of the pmoA gene from the MR core sediment indicated that all pmoA sequences determined were closely related to the previously described pmoA sequences of the type I methanotrophs in the γ-Proteobacteria. As demonstrated by amplification of the mmoX gene, the sequence of the type II methanotroph was not detected in this study, which is consistent with the results of the bacterial 16S rRNA analysis. The results showed that the pmoA genes in the surface MR core sediment were phylogenetically diverse but consisted for the most part of two phylotypes, Kuro-pmoA-12 and Kuro-pmoA-17 (Fig. 5B).
A total of 47 mxaF clone sequences from the HC carbonate sample were analyzed. Phylogenetic analysis of the mxaF gene sequences revealed that most of the mxaF sequences in the HC sample were affiliated with the mxaF family of the γ-Proteobacteria; however, these sequences were clearly distinct from the previously described mxaF sequences (Fig. 5C). For example, phylogenetic analysis based on the amino acid sequences indicated that most mxaF lineages, which included Kuro-mxaF-18, were affiliated with a single deeply divergent branch with a 100% bootstrap value (Fig. 5C). None of the mxaF sequences related to the α- and β-Proteobacteria was detected in any sample in this study.
DISCUSSION
The Kuroshima Knoll methane seep system (20) is dominated by a δ13C value of approximately −40‰ PDB, which is almost certainly derived from the thermal degradation of buried organic matter. The sediment surfaces are characterized by communities of mussels (Bathymodiolus spp.), which use methanotrophic symbionts to grow on methane, and clams (Calyptogena spp.), which use sulfur-oxidizing lithotrophic symbionts to grow on sulfide (Fig. 1) (Y. Fujiwara, personal communication). Judging from the distribution of these shellfish, it appears that the mussels require a high concentration and flux of methane to support their methanotrophic symbionts. Similarly, the symbiotic clams need sulfide and carbon dioxide and probably require more efficiency in the lower flux of methane. Thus, it appears that the colonization of different shellfish with different trophic features may reflect the different methane fluxes in these habitats and serve as a guide to understanding the microbiology of this environment. Given that the energy flow in this system can support luxuriant colonies of shellfish, it was of interest to examine the nonsymbiotic populations in the underlying sediments whose activity should be directly linked to the geochemical processes of C1 compounds, which play a central role in the local carbon cycling in the Kuroshima Knoll.
Thus, the abundance of two archaeal genes (16S rRNA and mcrA) and four bacterial genes (16S rRNA, pmoA, mmoX, and mxaF) in combination with analyses of pore water chemistry was used to gain insight into the role(s) of microbes in the methane cycle. The results revealed that methane is oxidized by both aerobic and anaerobic communities and that methane production may also occur in some of the sediments. Three submerged samples were obtained, each of which seemed to tell a different story; these samples were a core obtained from the center of the methane seep zone (MC), a core obtained from a reference site at a distance from the active seep zone (MR), and a surface carbonate sample obtained from an area where there was active methane bubbling and emission (HC) (Fig. 1).
The simplest community was that of the HC carbonate sample, which was associated with Bathymodiolus mussels. In this environment, the methane gas bubbling from the fissures of the carbonate crust is thought to migrate directly from a deep source of methane, and the δ13C value of the methane (−37.8‰ PDP) likely represents the value for the deep source of methane in the absence of both aerobic and anaerobic microbial oxidation. Phylogenetic analyses of the bacterial 16S rRNA, pmoA, mmoX, and mxaF genes from the carbonate sample consistently indicated that the bacterial community was mainly composed of type I methylotrophs in the γ-Proteobacteria. Quantitative real-time PCR results suggested that the archaeal population was very small (0.01%) (Fig. 2E) and that bacterial community was composed mainly of γ-Proteobacteria, suggesting that there was no AMO community or the AMO community was very small. QC-PCR analyses indicated that the amount of mxaF (7.1 × 106 copies/g [wet weight]) was much greater than the amount of pmoA (3.2 × 102 copies/g [wet weight]) (Fig. 4). Both geochemical and molecular ecological analyses strongly suggested that the C1 metabolism in the carbonate structure is dominated by methylotrophy and that neither oxic nor anoxic methanotrophy is a major process.
In a previous study in which carbon and oxygen isotopic analysis was used, it was suggested that the carbonate in the Kuroshima Knoll was inorganically precipitated at the surface (28), almost all of which in this area (100 by 100m) is populated by mussels, leading to the name The Bathymodiolus Garden. Given the results presented here, it seems likely that the resident populations in the carbonate are fed by methylamine compounds, major tissue-dissolved components in marine invertebrates, secreted from the living individuals, buried dead mussels in the carbonate, and/or other one-carbon compounds in the upwelling water.
In marked contrast, the populations of bacteria found in the MC core indicate that there is a complex ecology that involves both aerobic and anaerobic oxidation of methane. This core was taken from the sediments just adjacent to a Calyptogena colony. Other than the reddish (oxidized) surface sediments, the prokaryotic community in the MC sediment core consistently contained the ANME-2 group and sulfate reducers in the δ-Proteobacteria (Fig. 3 and Table 2). No sequences indicating the presence of the ANME-1 group were found, and the high proportion of archaeal rRNA genes (Fig. 2B) and phylogenetic features of the mcrA and 16S rRNA genes of archaea and bacteria suggested the presence and function of an AMO community in the MC core sediments. Compared to the δ13C values of the methane gas in the bubbles and the pore water methane in the reference core sediments, the pore water in the MC core sediments contained highly 13C-enriched methane at all depths. This is a strong evidence that microbial methane oxidation occurred in the core and even in much deeper sulfate reduction zones of the sediments.
One potentially intriguing finding supported by the geochemical and phylogenetic results is that the AMO and methanogenesis may occur in very close proximity near the sulfate reduction interface. Phylogenetic analyses of the mcrA and archaeal 16S rRNA genes suggested the presence of Methanolobus relatives together with ANME-2 members (Table 2). As observed in the vertical comparison of the concentration and isotopic properties of the pore water methane in the MC core, the concentrations of methane at 12.5 and 15.5 cm bsf were about three times higher than the concentrations at 22.5 cm bsf (Fig. 2C), and the δ13C(CH4) value shifted 3.5‰ lower than the value beneath. These geochemical shifts of the pore water methane are consistent with the presence of a methane production zone and of Methanolobus relatives. If this is true, what is the source of energy for this community? In the order Methanosarcinales (3), there are isolates that are able to utilize methanol and methylamines. The genus Methanolobus is differentiated from the members of the family Methanosarcinaceae by producing methane from methanol and other methyl compounds in the absence of H2 (2). Considering the absence or extraordinarily low concentration of H2 in pore water and bubbling gases, the methanogenesis detected in the shallow sulfate interface of the core might have been due to Methanolobus relatives, whose energy sources might have depended on the invertebrate-derived methylamines provided by the Calyptogena communities colonizing the surface.
In the top layer of the MC sediment core, the heaviest methane (−17.67‰ PDB) was detected. On the basis of the 16S rRNA gene clone libraries, we detected ANME-2a and Desulfosarcina-Desulfococcus sequences, indicating the possible occurrence of an AMO community near the surface. In addition, we detected 16S rRNA gene sequences of γ-Proteobacteria by PCR amplification using the domain Bacteria-specific primer set (Fig. 3B), and we detected high copy numbers (5.9 × 106 copies/g [wet weight]) of the pmoA genes as well (Fig. 4). These geochemical and microbiological data strongly suggested that both oxic methane oxidation and anoxic methane oxidation occur in the narrow sulfate reduction zone in sediments associated with Calyptogena colonies.
The MR core offered yet another contrast. In this case little methane was consumed, based on the δ13C(CH4) results, and the abundant microbes present were much more familiar organisms that were previously found in other deep-sea communities. However, it is also worth noting that the archaeal 16S rRNA sequences of Thermoplasmatales relatives were detected in the reference MR core sediments (Fig. 3A and Table 2). The presence of Thermoplasmatales relatives coupled with the ANME-2 assemblage has been demonstrated previously by fingerprint analysis of a water column from the Black Sea (41) and by in situ experiments with a continuous gas bioreactor (5). The data may indicate that these Thermoplasmatales relatives preferentially inhabit environments similar to those inhabited by the AMO community. In addition, we detected the 16S rRNA sequences of the miscellaneous crenarchaeotic group and green nonsulfur bacteria in a deeper zone of the MR sediment core (Fig. 3 and Table 2). These organisms have been detected previously as predominant prokaryotic components of the organic compound-rich subseafloor sediment in the methane hydrate zone of the Nankai Trough (32) and the Sea of Okhotsk (12). Although the physiological characteristics of these organisms remain unknown, there are some interesting common features of their habitats in anoxic organic-compound-rich marine sediments.
In conclusion, the results presented here demonstrate that there are microbial communities that are strongly associated with metabolism of C1 compounds in the deep subseafloor and surface macrofaunal communities in the Kuroshima Knoll methane seep field (Fig. 1). Recent microbial ecology surveys of the sediments coupling methane hydrates and methane seepages have revealed the ecological significance of anoxic methane oxidation in the anoxic zones (14, 17, 19, 25, 27). The great impact of AMO communities on the geochemical processes of methane is evident in the Kuroshima Knoll methane seep field. However, our high-resolution characterization of the pore water methane and the microbial communities in the very shallow sediments also suggests the significant contribution of the oxic methane oxidation by aerobic C1-metabolizing bacterial components and the concomitant methanogenesis to the carbon cycle of the field. The microbial ecosystem found in the Kuroshima Knoll methane seep should provide new insight for understanding the geochemical and microbiological processes in global methane seep systems.
Acknowledgments
We are very grateful to the crews of R/V Yokosuka and the operation team of DSV Shinkai 6500 for helping us collect deep-sea samples.
Geochemical analyses in this study were supported in part by the following grants: MEXT Grant-in-Aid for the 21st Century COE Program “Neo-Science of Natural History” at Hokkaido University, MEXT Special Coordination Fund “Archaean Park” project, and NEDO Proposal Based Research and Development Project ID 02A53002d.
REFERENCES
- 1.Boetius, A., K. Ravenschlag, C. J. Schubert, D. Rickert, F. Widdel, A. Gieseke, R. Amann, B. B. Jorgensen, U. Witte, and O. Pfannkuche. 2000. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407:623-626. [DOI] [PubMed] [Google Scholar]
- 2.Boone, D. R. 2001. Genus V. Methanolobus, p. 283-287. In D. R. Boone, R. W. Castenholz, and G. M. Garrity (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 1. Springer, New York, N.Y. [Google Scholar]
- 3.Boone, D. R., W. B. Whitman, and Y. Koga. 2001. Order III. Methanosarcinales ord. nov., p. 268. In D. R. Boone, R. W. Castenholz, and G. M. Garrity (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 1. Springer, New York, N.Y. [Google Scholar]
- 4.D'Hondt, S., S. Rutherford, and A. J. Spivack. 2002. Metabolic activity of subsurface life in deep-sea sediments. Science 295:2067-2070. [DOI] [PubMed] [Google Scholar]
- 5.Girguis, P. R., V. J. Orphan, S. J. Hallam, and E. F. DeLong. 2003. Growth and methane oxidation rates of anaerobic methanotrophic archaea in a continuous-flow bioreactor. Appl. Environ. Microbiol. 69:5472-5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hales, B. A., C. Edwards, D. A. Ritchie, G. Hall, R. W. Pickup, and J. R. Saunders. 1996. Isolation and identification of methanogen-specific DNA from blanket bog peat by PCR amplification and sequence analysis. Appl. Environ. Microbiol. 62:668-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallam, S. J., P. R. Girguis, C. M. Preston, P. M. Richardson, and E. F. DeLong. 2003. Identification of methyl coenzyme M reductase A (mcrA) genes associated with methane-oxidizing archaea. Appl. Environ. Microbiol. 69:5483-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanson, R. S., and T. E. Hanson. 1996. Methanotrophic bacteria. Microbiol. Rev. 60:439-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinrichs, K.-U., J. M. Hayes, S. P. Sylva, P. G. Brewer, and E. F. DeLong. 1999. Methane-consuming archaebacteria in marine sediments. Nature 398:802-805. [DOI] [PubMed] [Google Scholar]
- 10.Holmes, A. J., A. Costello, M. E. Lidstrom, and J. C. Murrell. 1995. Evidence that particulate methane monooxygenase and ammonia monooxygenase may be evolutionary related. FEMS Microbiol. Lett. 132:203-208. [DOI] [PubMed] [Google Scholar]
- 11.Inagaki, F., Y. Sakihama, A. Inoue, C. Kato, and K. Horikoshi. 2002. Molecular phylogenetic analyses of reverse-transcribed bacterial rRNA obtained from deep-sea cold seep sediments. Environ. Microbiol. 4:277-286. [DOI] [PubMed] [Google Scholar]
- 12.Inagaki, F., M. Suzuki, K. Takai, H. Oida, T. Sakamoto, K. Aoki, K. H. Nealson, and K. Horikoshi. 2003. Microbial communities associated with geological horizons in coastal subseafloor sediments from the Sea of Okhotsk. Appl. Environ. Microbiol. 69:7224-7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janvier, M., and P. A. Grimont. 1995. The genus Methylophaga, a new line of descent within phylogenetic branch gamma of Proteobacteria. Res. Microbiol. 146:543-550. [DOI] [PubMed] [Google Scholar]
- 14.Kallmeyer, J., and A. Boetius. 2004. Effects of temperature and pressure on sulfate reduction and anaerobic oxidation of methane in hydrothermal sediments of Guaymas Basin. Appl. Environ. Microbiol. 70:1231-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karner, M. B., E. F. DeLong, and D. M. Karl. 2001. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409:507-510. [DOI] [PubMed] [Google Scholar]
- 16.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions though comparative studies of nucleotide sequence. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 17.Knittel, K., A. Boetius, A. Lemke, H. Eilers, K. Lochte, O. Pfannkuche, and P. Linke. 2003. Activity, distribution, and diversity of sulfate reducers and other bacteria in sediments above gas hydrate (Cascadia Margin, Oregon). Geomicrobiol. J. 20:269-294. [Google Scholar]
- 18.Krüger, M., A. Meyerdlerks, F. O. Glöckner, R. Amann, F. Widdel, M. Kube, R. Reinhardt, J. Kahnt, R. Böcher, R. K. Thauer, and S. Shima. 2003. A conspicuous nickel protein in microbial mats that oxidize anaerobically. Nature 426:878-881. [DOI] [PubMed] [Google Scholar]
- 19.Lanoil, B. D., R. Sassen, M. T. L. Duc, S. T. Sweet, and K. H. Nealson. 2001. Bacteria and archaea physically associated with Gulf of Mexico gas hydrates. Appl. Environ. Microbiol. 67:5143-5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machiyama, H., T. Matsumoto, R. Matsumoto, M. Hattori, M. Okano, R. Iwase, and H. Tomaru. 2001. Outline of Shinkai 2000 dive surveys on the Kuroshima Knoll, off Ishigaki Island. JAMSTEC J. Deep Sea Res. 19:45-60. [Google Scholar]
- 21.Manheim, F. T. 1968. Disposable syringe techniques for obtaining small quantities of pore water from unconsolidated sediments. J. Sediment. Petrol. 38:666-668. [Google Scholar]
- 22.Martens, C. S., and R. A. Berner. 1974. Methane production. I. The interstitial waters of sulfate-depleted marine sediments. Science 185:1167-1169. [DOI] [PubMed] [Google Scholar]
- 23.McDonald, L. R., E. M. Kenna, and J. C. Murrell. 1995. Detection of methanotrophic bacteria in environmental samples with the PCR. Appl. Environ. Microbiol. 61:116-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald, L. R., and J. C. Murrell. 1997. The methanol dehydrogenase structural gene mxaF and its use as a functional gene probe for methanotrophs and methylotrophs. Appl. Environ. Microbiol. 63:3218-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michaelis, W., R. Seifert, K. Nauhaus, T. Treude, V. Thiel, M. Blumenberg, K. Knittel, A. Gieseke, K. Peterknecht, T. Pape, A. Boetius, R. Amann, B. B. Jørgensen, F. Widdel, J. Peckmann, N. V. Pimenov, and M. B. Gulin. 2002. Microbial reefs in the Black Sea fueled by anaerobic oxidation of methane. Science 297:1013-1015. [DOI] [PubMed] [Google Scholar]
- 26.Murrell, J. C., B. Gilbert, and I. R. McDonald. 2000. Molecular biology and regulation of methane monooxygenase. Arch. Microbiol. 173:325-332. [DOI] [PubMed] [Google Scholar]
- 27.Nauhaus, K., A. Boetius, M. Kruger, and F. Widdel. 2002. In vitro demonstration of anaerobic oxidation of methane coupled to sulphate reduction in sediment from a marine gas hydrate area. Environ. Microbiol. 4:296-305. [DOI] [PubMed] [Google Scholar]
- 28.Ogiwara, S., L. Takeuchi, R. Matsumoto, and H. Machiyama. 2002. The origin of carbonate chimney from Kuroshima Knoll. JAMSTEC J. Deep Sea Res. 21:13-17. [Google Scholar]
- 29.Orphan, V. J., K.-U. Hinrichs, W. Ussler III, C. K. Paull, L. T. Taylor, S. P. Sylva, J. M. Hayes, and E. F. DeLong. 2001. Comparative analysis of methane-oxidizing archaea and sulfate-reducing bacteria in anoxic marine sediments. Appl. Environ. Microbiol. 67:1922-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orphan, V. J., C. H. House, K.-U. Hinrichs, K. D. McKeegan, and E. F. DeLong. 2001. Methane-consuming archaea reveled by directly coupled isotopic and phylogenetic analysis. Science 293:484-487. [DOI] [PubMed] [Google Scholar]
- 31.Orphan, V. J., C. H. House, K.-U. Hinrichs, K. D. McKeegan, and E. F. DeLong. 2002. Multiple archaeal groups mediate methane oxidation in anoxic cold seep sediments. Proc. Natl. Acad. Sci. USA 99:7663-7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed, D. W., Y. Fujita, M. E. Delwiche, D. B. Blackwelder, P. P. Sheridan, T. Uchida, and F. S. Colwell. 2002. Microbial communities from methane hydrate-bearing deep marine sediment in a forearc basin. Appl. Environ. Microbiol. 68:3759-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takai, K., and K. Horikoshi. 2000. Rapid detection and quantification of members of the archaeal community by quantitative PCR using fluorogenic probes. Appl. Environ. Microbiol. 66:5066-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takai, K., H. Oida, Y. Suzuki, H. Hirayama, S. Nakagawa, T. Nunoura, F. Inagaki, K. H. Nealson, and K. Horikoshi. 2004. Spatial distribution of marine Crenarchaeota group I (MGI) in the vicinity of deep-sea hydrothermal systems. Appl. Environ. Microbiol. 70:2404-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teske, A., K.-U. Hinrichs, V. Edgcomb, A. V. Gomez, D. Kysela, S. P. Sylva, M. L. Sogin, and H. W. Jannasch. 2002. Microbial diversity of hydrothermal sediments in the Guaymas Basin: evidence for anaerobic methanotrophic communities. Appl. Environ. Microbiol. 68:1994-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsunogai, U., T. Toki, N. Nakayama, T. Gamo, H. Kato, and S. Kaneko. 2003. WHATS: a new multi-bottle gas-tight sampler for sea-floor vent fluids. Chikyukagaku 37:101-109. [Google Scholar]
- 38.Tsunogai, U., and H. Wakita. 1995. Precursory chemical changes in ground water: Kobe earthquake, Japan. Science 269:61-63. [DOI] [PubMed] [Google Scholar]
- 39.Tsunogai, U., N. Yoshida, and T. Gamo. 2002. Carbon isotopic evidence of methane oxidation through sulfate reduction in sediment beneath seafloor cold seep vents on the seafloor at Nankai Trough. Mar. Geol. 187:145-160. [Google Scholar]
- 40.Tsunogai, U., N. Yoshida, J. Ishibashi, and T. Gamo. 2000. Carbon isotopic distribution of methane in deep-sea hydrothermal plume, Myojin Knoll Caldera, Izu-Bonin arc: implications for microbial methane oxidation in ocean and applications to heat flux estimation. Geochim. Cosmochim. Acta 64:2439-2452. [Google Scholar]
- 41.Vetriani, C., H. V. Tran, and L. J. Kerkhof. 2003. Fingerprinting microbial assemblages from the oxic/anoxic chemocline of the Black Sea. Appl. Environ. Microbiol. 69:6481-6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, P., F. Wang, M. Xu, and X. Xiao. 2003. Molecular phylogeny of methylotrophs in a deep-sea sediment from a tropical west Pacific Warm Pool. FEMS Microbiol. Ecol. 47:77-84. [DOI] [PubMed] [Google Scholar]