Abstract
Oenococcus oeni is the organism of choice for promoting malolactic fermentation in wine. The population biology of O. oeni is poorly understood and remains unclear. For a better understanding of the mode of genetic variation within this species, we investigated by using multilocus sequence typing (MLST) with the gyrB, pgm, ddl, recP, and mleA genes the genetic diversity and genetic relationships among 18 O. oeni strains isolated in various years from wines of the United States, France, Germany, Spain, and Italy. These strains have also been characterized by ribotyping and restriction fragment length polymorphism (RFLP) analysis of the PCR-amplified 16S-23S rRNA gene intergenic spacer region (ISR). Ribotyping grouped the strains into two groups; however, the RFLP analysis of the ISRs showed no differences in the strains analyzed. In contrast, MLST in oenococci had a good discriminatory ability, and we have found a higher genetic diversity than indicated by ribotyping analysis. All sequence types were represented by a single strain, and all the strains could be distinguished from each other because they had unique combinations of alleles. Strains assumed to be identical showed the same sequence type. Phylogenetic analyses indicated a panmictic population structure in O. oeni. Sequences were analyzed for evidence of recombination by split decomposition analysis and analysis of clustered polymorphisms. All results indicated that recombination plays a major role in creating the genetic heterogeneity of O. oeni. A low standardized index of association value indicated that the O. oeni genes analyzed are close to linkage equilibrium. This study constitutes the first step in the development of an MLST method for O. oeni and the first example of the application of MLST to a nonpathogenic food production bacteria.
Oenococcus oeni, formerly Leuconostoc oenos (7), is the species of lactic acid bacteria (LAB) most frequently associated with malolactic fermentation (MLF) in wine. MLF, which occurs after alcoholic fermentation during wine making, is induced by the growth of LAB. Normally, spontaneous MLF takes place when LAB develop in wine after alcoholic fermentation. However, when fermentation by indigenous bacteria is relied upon, the fitness of the bacteria present to carry out MLF may be highly variable, and consequently their wine-making properties are unpredictable. In order to exercise greater control over wine-making processes, common wine-making practices therefore involve inoculation of wine with either commercially prepared strains or in-house winery strains of malolactic bacteria (4). Therefore, the differentiation of O. oeni strains at the strain level becomes a major concern, since their adaptation to wine and influence on organoleptic quality are strain specific (2). Moreover, manufacturers of malolactic starters need accurate control of their products, and wine-makers have to be able to recognize the inoculated strains during vinification. The need for positive identification of different isolates is also acknowledged by research workers in the field, since many strains from diverse origins are often exchanged between laboratories, and no reliable phenotypic method for certifying their identities is available (20). Because strains of O. oeni are not readily typed with phenotypic tests (20), the study of the genome structure can constitute a useful tool for the taxonomic characterization of strains and their typing. Therefore, unambiguous, discriminatory isolate characterization schemes are essential for population genetic and evolutionary studies.
The genus Oenococcus (7) contains a sole species, O. oeni, with a restricted ecological niche (wine and related habitats); although its dissimilarity to the genus Leuconostoc is generally accepted, there is some controversy regarding both the evolution rate of the species and its diversity (6, 46). Despite the exhaustive phenetic and molecular studies that have been performed on O. oeni, little is known about its population genetics. Studies carried out by using different molecular techniques such as chromosomal DNA-DNA hybridizations (7), 16S and 23S rRNA sequence analysis (39), and 16S-23S rRNA gene (rDNA) intergenic spacer region (ISR) sequencing (30, 46) and also by DNA fingerprinting (29, 44), pulsed-field gel electrophoresis (26), and randomly amplified polymorphic DNA (RAPD) analysis (45) suggest that this species is homogeneous. However, metabolic or physiological criteria, such as lactate dehydrogenases (17), carbohydrate fermentation (18), and cellular fatty acid patterns (42), have shown considerable diversity among strains of O. oeni. Therefore, O. oeni has often been referred to as a highly heterogeneous species, and splitting it into two species (38) or subspecies (41) has been proposed.
Multilocus sequence typing (MLST), a method that is based on partial nucleotide sequences of multiple housekeeping genes, has recently been shown to be a powerful technique for bacterial typing (11). Housekeeping genes are preferred because an analysis of mutations in such genes is more likely to properly reflect the phylogeny of strains. MLST uses variation that accumulates slowly, which is expected to be selectively neutral, and achieves very high resolution by analyzing multiple loci. The MLST method was introduced in 1998 and since then has been used for phylogenetic analysis of many bacterial pathogens such as Neisseria meningitidis (31), Streptococcus pneumoniae (10), Haemophilus influenzae (35), etc. MLST has led to a better understanding of the mode of genetic variation within a bacterial species. Since 1998, the MLST scheme has been applied to important bacterial pathogens including several food-borne human pathogens such as Campylobacter jejuni (8), Vibrio cholerae (14), and Bacillus cereus (23); however, until now, MLST had not been applied to a nonpathogenic food production bacteria used in the food industry, where precise methods for characterizing isolates are required as well.
The present study was undertaken to evaluate the discriminatory power of MLST in O. oeni. The results of our analysis indicate that the O. oeni population exhibits high genetic diversity close to linkage equilibrium with a panmictic population structure. This study also constitutes the first step for the development of an MLST method for O. oeni. The sequence diversity of five genes, possible candidates to be included in a future MLST scheme, was analyzed.
MATERIALS AND METHODS
Strains and DNA preparation.
In this study, we examined a total of 18 O. oeni isolates (Table 1), including reference strains provided by the Spanish Type Culture Collection (CECT) and strains belonging to the bacterial culture collection of the Instituto de Fermentaciones Industriales (BIFI), CSIC, Madrid, Spain (36). Four additional O. oeni strains were isolated from commercial malolactic starter preparations provided by Lallemand Inc. (Montreal, Canada) and Christian Hansen A/S (Horsholm, Denmark). Strain 5001 was kindly provided by E. García-Moruno from the Instituto Sperimentale per l'Enologia, Asti, Italy. Strain O. oeni 51 was isolated from the same wine fermentation and showed a RAPD profile identical to that of O. oeni BIFI-21 (data not shown); therefore, we assumed that the strains are identical. To check the stability of the gene sequences, BIFI-21 was subcultured during approximately 250 generations in culture medium, and its DNA was extracted. Isolates were grown on medium for L. oenos (3) supplemented with 10% tomato juice. Strains were incubated at 30°C in a 5% CO2 atmosphere. Chromosomal DNA was prepared as described previously (46).
TABLE 1.
Properties of O. oeni isolates analyzed and their allele profiles at each locus
| Strain no. | Strain | RTa | ST | Allele no. at locus:
|
Source of isolate
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| gyrB | ddl | pgm | recP | mleA | Country | Year | ||||
| 1 | MCW (PSU-1) | NDb | 1 | 1 | 1 | 1 | 1 | 1 | California, United States | 1958 |
| 2 | CECT 4028 (DSMZ 20255) | B | 2 | 2 | 1 | 2 | 2 | 1 | Bordeaux, France | 1961 |
| 3 | CECT 4029 (DSMZ 20257) | B | 3 | 3 | 2 | 2 | 2 | 1 | CSIRO, Australia | 1958 |
| 4 | CECT 4100T (ATCC 23279) | B | 4 | 4 | 1 | 3 | 2 | 1 | Bordeaux, France | 1961 |
| 5 | CECT 4721 (ATCC 23278) | B | 5 | 4 | 3 | 4 | 2 | 1 | Merbein, Australia | 1958 |
| 6 | CECT 4725 (ATCC 23277) | B | 6 | 4 | 1 | 2 | 3 | 1 | Bordeaux, France | 1961 |
| 7 | CECT 4728 (ML27) | B | 7 | 5 | 1 | 5 | 3 | 1 | California, United States | 1965 |
| 8 | CECT 4758 | A | 8 | 6 | 4 | 6 | 1 | 2 | Valladolid, Spain | 1995 |
| 9 | BIFI-1 | A | 9 | 6 | 5 | 7 | 2 | 1 | Valladolid, Spain | 1999 |
| 10 | BIFI-9 | A | 10 | 6 | 5 | 8 | 1 | 2 | Logron̄o, Spain | 1999 |
| 11 | BIFI-21 | A | 11 | 6 | 1 | 8 | 4 | 2 | Madrid, Spain | 2001 |
| 12 | BIFI-26 | A | 12 | 7 | 7 | 9 | 5 | 1 | Valladolid, Spain | 2001 |
| 13 | BIFI-86 | A | 13 | 6 | 1 | 8 | 6 | 2 | Logroño, Spain | 2003 |
| 14 | 5001 | A | 14 | 8 | 8 | 8 | 7 | 1 | Italy | ND |
| 15 | Uvaferm ALPHA | A | 15 | 6 | 9 | 2 | 2 | 1 | Bordeaux, France | ND |
| 16 | Uvaferm MLD | A | 16 | 6 | 1 | 10 | 1 | 2 | ND | ND |
| 17 | Viniflora OENOS | A | 17 | 6 | 1 | 11 | 8 | 2 | ND | 1993 |
| 18 | Viniflora CH35 | A | 18 | 6 | 10 | 12 | 5 | 2 | Burgundy, France | ND |
RT, ribotype. Ribotypes are as defined by Zavaleta et al. (47).
ND, no data available.
RFLP of the PCR-amplified 16S-23S rDNA ISR.
Restriction fragment length polymorphism (RFLP) analysis of the ISRs was performed as described previously (46). Briefly, the O. oeni ISR PCR-specific reactions were performed by using the primers 16S14f (5′-CTTGTACACACCGCCCGTC) and 23S1R (5′-GGGTTTCCCCATTCGGAAATC) described by Zavaleta et al. (46). These primers amplified a 650-bp fragment in all the O. oeni strains tested. The PCR was performed with approximately 100 ng of total DNA in 0.2-ml microcentrifuge tubes in a total volume of 50 μl as described previously (46). The amplified 16S-23S ISRs from O. oeni strains were digested with the restriction enzymes DdeI, CfoI, NdeI, and TaqI (Roche). The digested products were separated by electrophoresis in 4.5% MS-8 agarose gels (Hispanlab).
Ribotyping.
Chromosomal DNA was digested with HindIII and EcoRI (Roche), and the products were separated by electrophoresis in 0.7% agarose gels in 1× Tris-acetate-EDTA buffer.
Digested DNA was transferred onto positively charged nylon membranes (Roche) by the Southern method. Probe 16S rDNA was obtained from O. oeni CECT 4100T by PCR by using the eubacterial universal pair of primers 63f and 1387r (32). The 16S rDNA probe was digoxigenin labeled and detected by chemiluminescence by using a DIG-High Prime DNA Labeling and Detection Starter Kit (Roche) according to the manufacturer's instructions.
PCR amplification and DNA sequencing.
Since the genome sequence of O. oeni had not been published when this study was performed, we identified a number of candidate loci by searching the O. oeni genome database (http://genome.jgi-psf.org/draft_microbes/oenoe/oenoe.home.html) with gene sequences from other bacteria. The following four loci were chosen for the sequence analysis scheme: gyrB (coding for the B subunit of DNA gyrase and located on contig NZ_AABJ02000001.1), pgm (coding for phosphoglucomutase and located on contig NZ_AABJ02000004.1), ddl (coding for d-alanine-d-alanine ligase, located on contig NZ_AABJ02000021.1), and recP (coding for transketolase and located on contig NZ_AABJ02000005.1). These genes were selected based on the criterion that they are presumptively widely separated on the chromosome. A gene (mleA) coding for the malolactic enzyme, an enzyme of technological interest in O. oeni, was also included in the study (Table 2). The mleA gene is located on contig NZ_AABJ02000005.1 but 85 kb apart from the recP gene in the O. oeni strain MCW chromosome. Primers were designed by using highly conserved DNA regions of these genes for O. oeni obtained from the GenBank database, with the exception of mleA, for which we used primers previously described by Divol et al. (9).
TABLE 2.
Primers used for MLST of O. oeni strains
| Protein | Accession no.a | Gene | Primers | Sequence 5′→3′ | 5′ start positiona | PCR product length (bp) | Accession no. |
|---|---|---|---|---|---|---|---|
| B subunit of DNA gyrase | ZP_00069208 | gyrB | GYRB1 | TGGGCTTCATGGTGTTGGC | 375 | 947 | AJ618989-AJ618996 |
| GYRB2 | CCCTCGACGATAAACAATTC | 1322 | |||||
| d-Alanine-d-alanine ligase | ZP_00069716 | ddl | DDL1 | CGATGTTAGCAAGCGTTCG | 54 | 911 | AJ618997-AJ619006 |
| DDL2 | TTCGTATTTCCCGGTAGTG | 965 | |||||
| Phosphoglucomutase | ZP_00070472 | pgm | PGM1 | CATCCGACTCCGGAATTGAC | 370 | 826 | AJ619007-AJ619018 |
| PGM2 | CCGTAGGATTCTTCAAAACC | 1196 | |||||
| Transketolase | ZP_00069744 | recP | RECP1 | GGCGATGGGGACTTAATGG | 550 | 926 | AJ619666-AJ619673 |
| RECP2 | CCCGTCTTCGCCGACAGC | 1476 | |||||
| Malolactic enzyme | ZP_00069821 | mleA | OO1b | GTGCCGCTTTTTTGGATATTA | 332 | 430 | AJ619674-AJ619675 |
| OO2b | AGCAATTTTATCTTTATAGCT | 762 |
The protein accession number and the indication of the gene position refer to the uncompleted genome sequence of O. oeni MCW (formely, PSU-1) by the DOE Joint Genome Institute (http://genome.jgi-psf.org/draft_microbes/oenoe/oenoe.home.html).
The mleA primers have been described by Divol et al. (9).
PCR was performed to amplify gene fragments from chromosomal DNA of the O. oeni strains. Each 25-μl amplification reaction mixture contained 10 ng of template DNA, 20 mM Tris-HCl (pH 8.0), 50 mM KCl, 2.5 mM MgCl2, a 200 μM concentration of each deoxynucleoside triphosphate, a 1 μM concentration of each primer, and 1 U of AmpliTaq Gold DNA polymerase. The reaction was performed in a GeneAmp PCR System 2400 (Perkin-Elmer) by using the following cycling parameters: 10 min of enzyme activation at 95°C, followed by 30 cycles of 30 sec at 95°C, 1 min at 48°C, and 1 min at 72°C. Amplified products were resolved on a 1.5% agarose gel. The amplification products were purified on QIAquick spin columns (QIAGEN) for direct sequencing with the same PCR primers by the Sanger dideoxynucleotide chain termination method by using an ABI Prism dRhodamine Terminator Cycle Sequencing Ready Reaction Kit (PE Biosystems, Warrington, England), in accordance with the manufacturer's protocol, and an ABI Prism 377 DNA sequencer (Applied Biosystems, Inc.).
Phylogenetic analysis.
For each locus, the sequences obtained for all isolates were compared, and the different sequences were assigned arbitrary allele numbers. For each isolate, the combination of alleles obtained at each locus defined its allelic profile (Table 1). Each isolate was therefore designated by five numbers, constituting an allelic profile or sequence type (ST). We refer to a unique combination of alleles as an ST. The STs were identified by arbitrary numbers assigned in order of description.
Sequence alignments and comparison were done with the program BioEdit, version 4.8.10 (http://jwbrown.mbio.ncsu.edu/BioEdit/bioedit.html) (21), and converted into MEGA and NEXUS files with START (sequence type analysis and recombinatorial tests) (http://outbreak.ceid.ox.ac.uk/software.html). Phylogenetic trees were compiled with MEGA version 2.1 software (http://www.megasoftware.net) by using the unweighted pair group method with arithmetic averages (UPGMA) (28).
The method of split decomposition was used to assess the degree of tree-like structure present in the alleles found for each locus in the complete set of 18 isolates (24). The sequence alignments were converted to NEXUS files, and the split decomposition was performed with SplitsTree 2.0 (http://bibiserv.techfak.uni-bielefeld.de/splits/).
Three types of statistical analysis were applied to the data: the index of association (IA), homoplasy test, and Sawyer's run test. Multilocus linkage disequilibrium was estimated by measuring IA (34). The homoplasy test (33) and the Sawyer's run test (40) were performed according to methods described previously with the START program. Using the START program, we also performed a test to detect selection in our population, and we calculated the dN/dS ratio as described by Nei and Gojobori (37).
Nucleotide sequence accession numbers.
The sequences of all alleles have been deposited in the EMBL and GenBank databases under the accession numbers AJ618989 to AJ618996 (gyrB[r] fragment), AJ618997 to AJ619006 (ddl fragment), AJ619007 to AJ619018 (pgm fragment), AJ619666 to AJ619673 (recP fragment), and AJ61974 to AJ619675 (mleA fragment) (Table 2).
RESULTS
Characterization of O. oeni strains by ribotyping and RFLP analysis of the ISRs.
The O. oeni strains recovered from various years and geographic locations were expected to be diverse. Therefore, PCR products of the 16S-23S rDNA ISRs from all O. oeni strains were digested with endonucleases TaqI, CfoI, DdeI, and NdeI. The 18 strains analyzed showed identical RFLP patterns for each enzyme. The TaqI and CfoI RFLP patterns were coincident with those previously described by Zavaleta et al. (46), with six bands (40, 75, 90, 100, 130, and 220 bp) and three bands (110, 230, and 330 bp), respectively. However, DdeI digestion originates a three-band RFLP pattern (75, 110, and 400 bp), and, unexpectedly, the 16S-23S rDNA ISR PCR fragments remain uncut by NdeI.
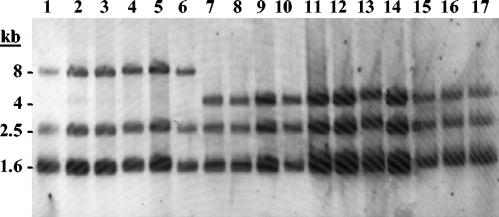
Ribopattern analysis with EcoRI or HindIII revealed three bands for all O. oeni strains. Two different groups of strains were found, corresponding to groups defined previously by Zavaleta et al. (47). Ribotype A showed bands of 1.6, 2.5, and 4 kb for EcoRI and 1, 5, and 12 kb for HindIII; ribotype B showed bands of 1.6, 2.5, and 8 kb for EcoRI and 1, 5, and 6 kb for HindIII. Table 1 lists the assignment of ribotype group A or B to each O. oeni strain. The ribotyping patterns of the EcoRI digest are shown in Fig. 1.
FIG. 1.
Ribotyping patterns of EcoRI digest of chromosomal DNAs from O. oeni strains. Lane 1, CECT 4028; lane 2, CECT 4029; lane 3, CECT 4100; lane 4, CECT 4721; lane 5, CECT 4725; lane 6, CECT 4728; lane 7, CECT 4758; lane 8, BIFI-1; lane 9, BIFI-9; lane 10, BIFI-21; lane 11, BIFI-26; lane 12, BIFI-86; lane 13, 5001; lane 14, Uvaferm ALPHA; lane 15, Uvaferm MLD; lane 16, Viniflora OENOS; lane 17, Viniflora CH35. The molecular sizes (in kilobases) of the labeled fragments are indicated on the left.
Diversity of gene fragments.
We sequenced nucleotide fragments of five genes for each of the 18 O. oeni isolates. Table 3 shows the fragment size for each locus used for this analysis. For all loci, the 18 sequences aligned without gaps or insertions. As shown in Table 3, all loci were polymorphic, and the number of polymorphic nucleotide sites varied between 1 (mleA) and 36 (recP). The proportion of variable sites present in the alleles ranged from 0.3% in the mleA locus to 6.6% in the recP locus, with 1.7, 2.2, and 2.5% for the pgm, ddl, and gyrB loci, respectively. Figure 2 shows the position of the polymorphic sites within sequenced fragments for all loci. Nucleotide substitutions occurring at the third base of a codon ranged from 64% in gyrB to 20% in ddl (Fig. 2). Phylogenetically informative sites (at least two bases present in two or more of the alleles) ranged from 57% in gyrB and pgm to 20% in ddl and are also shown in Fig. 2.
TABLE 3.
Sequence variation at five loci
| Gene | Fragment size (bp) | Mean G+C content (%) | No. of alleles | No. of polymorphic sitesa | No. of nucleotide substitutions per nucleo- tide site | dN/dSb |
|---|---|---|---|---|---|---|
| gyrB | 554 | 40.5 | 8 | 14 (7) | 0.0048 | 8.33 |
| ddl | 444 | 36.8 | 10 | 10 (1) | 0.0020 | 62.01 |
| pgm | 402 | 40.4 | 12 | 7 (2) | 0.0029 | 43.94 |
| recP | 541 | 44.7 | 8 | 36 (18) | 0.0066 | 22.46 |
| mleA | 339 | 39.2 | 2 | 1 (0) | 0.0011 | 0 |
Number of silent polymorphic sites in parentheses.
Calculated by using the START program and expressed as the dN/dS ratio multiplied by 100.
FIG. 2.
Polymorphic sites in each of the five gene fragments studied. Each of the sites where the sequence of one or more of the genes differs from a putative consensus sequence is shown (only sites that differ are shown; sites that are identical to those in the consensus sequence are indicated by periods). The number of strains possessing the allele is indicated in parentheses. Numbering of the polymorphic sites (vertical format) is from the first nucleotide position of the corresponding gene. Asterisks indicate informative polymorphic sites.
Each allele of the five genes analyzed was numbered successively in a ascending order. The number and types of sequence changes have not been taken into account for allele designations. The number of alleles was 8 for gyrB and recP, 10 for ddl, 12 for pgm, and only 2 for mleA (Table 3). This wide range in the number of alleles (2 to 12) suggests that all loci were evolving at different rates. The number of strains possessing each allele is indicated in Fig. 2. In the gyrB and ddl loci only one allele predominated, with the remainder observed in fewer than three strains. However, in the pgm, recP, and mleA loci, two alleles appeared to be predominant.
Table 1 summarizes the allelic profiles of the O. oeni strains used in this study. Each unique combination of allele numbers represents one ST. All STs were represented by a single strain. No strains had identical sequences for all five fragments, and all the strains could be distinguished from each other because they had unique combinations of alleles. Isolates that had been shown to be closely related by using highly discriminatory typing methods, such as O. oeni 51 and BIFI-21, which were isolated from the same wine fermentation and showed identical RAPD patterns, had identical sequences for all the gene fragments analyzed. Moreover, the stability of the gene sequences in O. oeni strains was checked by MLST analysis. This analysis confirmed that the gene sequences analyzed remain unaltered during generations.
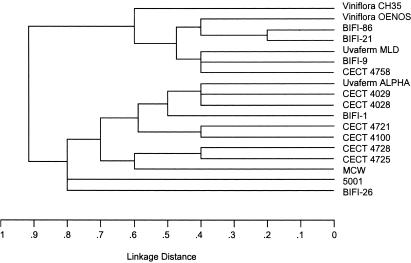
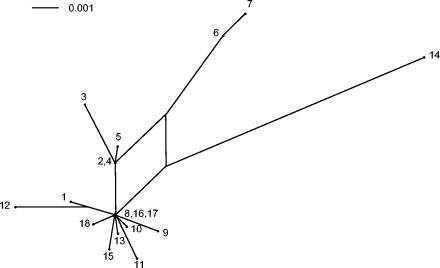
The allelic profiles and a UPGMA tree based on these allelic profiles are shown in Table 1 and Fig. 3, respectively. All STs differed in various loci, except ST-11 and ST-13, which differ only in one locus. There were no significant clusters that correlated with the geographic origin of the strains. Concatenated gyrB, ddl, pgm, recP, and mleA gene sequence fragments were analyzed and graphically displayed with SplitsTree (Fig. 4). The relationship of O. oeni isolates based on this analysis is depicted as a star-like structure with rays of different lengths. This star phylogeny is also consistent with a recombinational population structure and with the observation that each strain carries its own allelic combination. Isolates O. oeni CECT 4028, CECT 4721, CECT 4725, CECT 4728, and 5001 are more closely related than the other strains and their tree branches are interconnected, suggesting recombinational events between them. Consistent with the lineage assignment by BURST, this placed the predicted O. oeni founder strains (CECT 4725, Uvaferm ALPHA, and Viniflora OENOS) at a central position of the split graph. The relationships among other members of the group were assessed by examining the number of nodes between two isolates.
FIG. 3.
UPGMA dendrogram showing the genetic relatedness of the 18 O. oeni strains examined in this study. The dendrogram was constructed from a matrix of the pairwise distances between the allelic profiles of 18 O. oeni strains.
FIG. 4.
Split decomposition analysis based on the allelic profiles of the 18 O. oeni strains examined in this study. The numbering refers to strain numbers.
The dN/dS ratio, where dN indicates the number of nonsynonymous substitutions per nonsynonymous site and dS indicates the number of synonymous substitutions per synonymous site, was calculated as a measure of the degree of selection in the population. Investigating the number of synonymous and nonsynonymous substitutions may, therefore, provide information about the degree of selection operating on a population. The dN/dS ratio was calculated for all five loci, and in gyrB and mleA it was <10% (Table 3). However, the dN/dS ratios of the recP (22.46%), pgm (43.94%), and ddl (62.01%) loci could indicate that the degree of selection is not as strong as in typical housekeeping genes. In the mleA locus, the only nucleotide substitution was synonymous and did not affect the amino acid composition.
Comparative results of ribotyping and RFLP-ISR versus MLST.
Only one RFLP-ISR type was identified among the 18 O. oeni strains analyzed in our study; similarly, the same strains were included in only two ribotypes. However, the number of sequence types (18) was larger (Table 1). This observation suggests that the discriminatory ability of MLST is better than that observed by the use of ribotyping and RFLP analysis of the ISR. A specific ribotype was not restricted to specific STs, and, for example, 11 STs contained isolates with the same ribotype B. These results show clearly that ribotype does not correlate with sequence type (Table 1).
Evidence for recombination in O. oeni.
Bacteria can differ widely in their population structures. While some of them have a clonal population structure, in which all sequence diversity has arisen from the sequential accumulation of point mutations, in many other sequences diversity is greatly increased by intraspecies recombination. The sets of sequences were tested with the homoplasy test (33). The homoplasy test requires a sufficient number of informative sites to yield interpretable results, and if a locus has fewer than 10 informative sites, the analysis will not be performed on it. In all of our data sets, there was insufficient sequence diversity to perform the homoplasy test.
There were two possible examples of a recombinational event in the gyrB and recP genes from unknown sources. The mean divergence between allele 7 of recP (6.66%) is much higher than the mean diversity within the other recP alleles (0.37%), with the exception of allele 3 (3.61%). Alleles 7 and 3 of recP seem to have originated from a different source. In gyrB, allele 3 showed a divergence of 6.42%, and alleles 2, 4, and 5 showed a mean diversity of 4.28%. Therefore, in gyrB alleles 1, 6, 7, and 8 (mean diversity, 1.28%) could have originated from a different source from alleles 3, 2, 4, and 5.
Sawyer's test revealed no detectable cases of intragenic recombination in the sample except in the case of the recP locus, in which there was evidence against the null hypothesis of no recombination (P = 0.04) (Table 4). This evidence disappeared when the maximum condensed fragment value (P = 1.00) was considered.
TABLE 4.
Sawyer's test analysis for evidence of intragenic recombinationa
| Locus | SSCFb (P) | MCFc (P) |
|---|---|---|
| gyrB | 275 (0.61) | 7 (1.00) |
| ddl | 0 (1.00) | 0 (1.00) |
| pgm | 1 (1.00) | 1 (1.00) |
| recP | 5,081 (0.04) | 18 (1.00) |
| mleA | 0 (1.00) | 0 (1.00) |
The Sawyer's test analyses were carried out by using the START program. Results were obtained from 10,000 random resamplings.
SSCF, sum of the square of condensed fragments.
MCF, maximum condensed fragment.
The complete set of isolates was analyzed for linkage disequilibrium. The level of linkage disequilibrium between alleles was assessed by using the IA value (31). In our study, an IA of 0.155 was obtained; this value was not significantly greater than the IA value of 0 expected for a population of linkage equilibrium, implying no departure from the null hypothesis of linkage equilibrium between alleles. To avoid dependence on the number of loci, Hudson described another statistic, the standardized IA (ISA) (22), which does not depend on the number of loci analyzed and is expected to be zero when alleles are in linkage equilibrium (free recombination). That means probably that the distribution of alleles occurs independently from each other. The ISA value was 0.038 for the sample analyzed in this study. This low ISA value is indicative of extensive recombination. The ISA value calculated for O. oeni supports our estimation that the genes investigated are close to linkage equilibrium.
Comparison between gene trees.
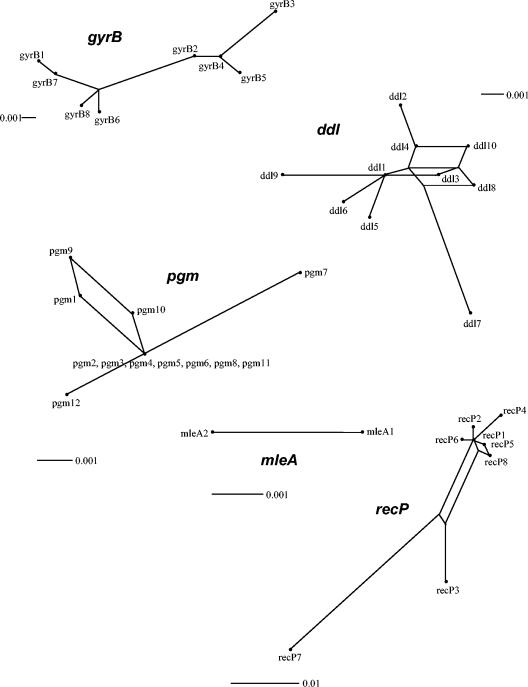
In order to further analyze the population structure of O. oeni, we used split decomposition analysis. The algorithm used in this software is able to display conflicting results in the phylogenetic descent of sequences. A tree-like structure is created when the descent is clonal, but an interconnecting network or a bush-like structure will appear when recombination plays a role in the evolutionary history of O. oeni genes. Figure 5 shows the split graphs for all alleles of the five fragments analyzed. The structure of the split graphs varied substantially between the different loci. The SplitsTree graphs obtained with ddl, pgm, and recP loci present network-like structures. This indicates the presence of homoplasies, probably evolved by intragenic recombination. The split graphs of the other two loci showed no evidence of network-like evolution. The split graph of the gyrB gene displays a star- or bush-like structure consisting of a single origin in the center of the graph, from which single branches radiate. However, in gyrB an additional uncentered edge was observed, suggesting that the evolution of some of the gyrB genes has been initiated by a couple of parallel mutations originating from one ancestor. The split graph of the mleA gene displays a line because only two alleles are analyzed. The fit parameter was 100 for gyrB, ddl, recP, and mleA, indicating that all phylogenetic information in the sequences could be visualized in the graphs. The fit parameter for pgm was lower (40.4), indicating that not all information could be integrated into the graph. The differences in structure among the split graphs obtained for the five loci can be explained by recombination, because recombination can lead to the assembly of genes with different evolutionary histories within one strain.
FIG. 5.
Split decomposition analysis of alleles obtained from 18 O. oeni strains for five loci. All branch lengths are drawn to scale. The observation that in the ddl, pgm, and recP graphs several alleles in the sample are connected to each other by multiple pathways, forming an interconnected network, is suggestive of recombination. The numbering refers to allele numbers.
DISCUSSION
One of the main objectives of this study was to investigate global genomic similarity at the intraspecies level and to develop a sensitive method for strain identification in O. oeni. Therefore, we performed multilocus sequence analysis with five chromosomal genes. We determined the degree of allelic variation in these genes of O. oeni by using a sample of 18 isolates originating in various countries. MLST schemes are based on sequences of multiple (usually seven) loci because the analysis of a single gene provides too little discrimination to be used for molecular typing. As a first step for developing a typing method, we analyzed the sequence diversity of four housekeeping genes and the mleA gene in order to know if they are sufficient to have enough typing discrimination, since the number of loci can be increased to improve resolution, but there will come a point when, for epidemiological purposes, little additional information is obtained for the cost and effort involved (43).
The internal fragments of the five loci that were selected could be amplified from all strains that we examined. The amplified internal fragments were sequenced, and from these sequences we were able to use fragments between 339 and 554 bp for analyses (Table 3). Only two alleles were found in the mleA locus (Tables 1 and 2 and Fig. 2). The lack of diversity among the mleA locus sequences results in less discriminatory power than found in the housekeeping loci. It was previously shown that the partial sequence of the O. oeni ATCC 23279T (CECT 4100) mleA gene was also exactly identical to the mleA gene sequence of O. oeni IOEB 8413. The authors postulated that the malolactic enzyme (MleA) appears to have an identical sequence at species level. They found that the percentage of changes in sequences between O. oeni and other LAB was lower for mleA than for 16S rRNA (19).
The number of alleles per housekeeping locus ranged from 8 to 12 (Table 3). The four loci were highly polymorphic, and all multilocus types were represented by a single strain. The percentage of variable sites (1.7 to 6.6%) in the housekeeping genes was comparable to that seen by Enright et al. (12) in their analysis of a related gram-positive bacteria, Streptococcus pyogenes. The percentage of variable sites was higher than that seen in group B streptococcus (1.2 to 2.5%) (25) and V. cholerae (1.1 to 3.5%) (14) and considerably less than that of C. jejuni (9.2 to 21.7%) (8) or the B. cereus group (9 to 26%) (23).
The percentage of variable sites observed in O. oeni indicates a considerably high degree of genetic diversity, and it is not consistent with some data obtained by other experimental methods. The analysis of ribotyping and RAPD patterns has revealed a high level of genetic homogeneity in O. oeni (47). This homogeneity is consistent with the results of DNA-DNA hybridization (7), total soluble cell protein analysis (6), and 16S-23S ISR sequences (46). The RAPD profiles discerned two main groups of strains coincident with clusters obtained by macrorestriction typing in previous work (41). Ribotyping of the 18 O. oeni strains analyzed in this study indicated that all strains are included in only two ribotypes, A and B, previously described by Zavaleta et al. (47). The study by Zavaleta et al. (47) included 37 culture collection strains, 17 of which fell in group A (which includes CECT 4100T), and 20 fell in group B (represented by CECT 4028 and CECT 4029). The investigators concluded that the two well-defined and consistent groups of strains shown by all these methods are indicative of two distinct main patterns of DNA arrangement in the genome of the species O. oeni. However, by using MLST we could not observe the two well-defined groups of strains previously described.
Although more extensive studies are necessary to assess the population structure of O. oeni, the UPGMA tree based on the allelic profiles (Fig. 3), the split decomposition analysis of concatenated gene sequences (Fig. 4), and the IA values were consistent with the conclusion that O. oeni represents a good example of panmictic structure. Panmictic populations (as found in Neisseria gonorrohoeae) may be so variable that identical strains are only found among isolates from direct contacts. Even panmictic populations can contain clonal groupings or geographical specialization (34). This was not the case in our study, but the number of isolates analyzed here is too low to allow a definitive conclusion. In previous studies based on RAPD, ribotyping, small-plasmid content, and sequencing of RAPD markers, Zavaleta et al. (47) hypothesized that O. oeni follows a closely clonal mode of evolution, similar to the case of some pathogenic bacterial clones such as those of V. cholerae. However, the analysis of the population structure of O. oeni presented here shows a substantial extent of recombination. In Porphyromonas gingivalis, Frandsen et al. (16) and Koehler et al. (27) found ISA values of 0.068 and 0.089, respectively, and suggested a nonclonal population structure characterized by recombination. In our study we calculated a similar ISA value (0.038) that confirms the importance of recombination in O. oeni. Moreover, the ISA value calculated is similar to that reported for N. gonorrohoeae and, therefore, supports our estimation that the genes investigated in O. oeni are close to linkage equilibrium.
The examination of the sequences of housekeeping genes can provide evidence for the significance of recombination, since the variation within these genes is likely to be selectively neutral. The homoplasy test (33) measures the importance of recombination between members of a population. It is only valid when sequences differ by <5% of the nucleotides, and the test requires a sufficient number of alleles and informative sites to yield interpretable results. In our case, all loci analyzed had insufficient numbers of alleles and informative sites to perform this analysis.
Sawyer's test did not detect clear evidence of recombination in any of the loci analyzed (Table 4). In our analysis this test failed to detect recombination, in a similar way as described previously by Farfán et al. (14) for V. cholerae. These authors suggest that the reason for this disagreement may reside in the definition of condensed fragments themselves, which are designed to detect gene conversion in which both source and target sequences are in the sample. From our data gyrB, pgm, and recP showed a net structure in the split graph, suggesting the existence of recombination.
Falush et al. (13) suggested that the panmictic structure in H. pylori may result from frequent recombination during mixed colonization by unrelated strains during chronic colonization of the gastric mucosa. Recombination between O. oeni strains might occur on the fermentation tanks, where several strains of O. oeni may exist simultaneously in a single fermentation and where a favorable environment for horizontal gene transfers could be created. Although it has been shown that O. oeni is able to receive foreign DNA by transformation in vitro (5) and by conjugation (49), there is no evidence for horizontal gene transfer in vivo. The existence of recombination is not unexpected, because the presence of plasmids (1, 46), bacteriophages, and insertion sequences (48) was previously shown in O. oeni. Zé-Zé et al. (48) reported that small differences observed in two O. oeni strains are apparently due to insertion or deletion events related to the presence of insertion sequences.
Recombination can be detected in the aligned sequences by a number of means, the most simple method being the detection either by eye or through statistical analysis of mosaic structure, where different regions of the gene appear to have different evolutionary histories. For example, most of a gene may be identical in sequence for two isolates of a species, whereas a 500-bp region in the middle may differ at 5% of nucleotide sites. Significant mosaic structure is indicative of recombinational exchange, usually among isolates of the same species but occasionally also between closely related species (15). A point mutation will generate a single nucleotide difference, whereas a recombinational exchange is likely to introduce multiple nucleotide differences. Enright and Spratt (10) found in S. pneumoniae a possible example of an interspecies recombinational event in gdh as the mean divergence between an allele and the other pneumococcal alleles was 4.58%, which was much higher than the mean diversity within the other pneumococcal alleles (0.95%) and similar to the mean divergence between these alleles and that of Streptococcus mitis (5.16%). In our study, two possible examples of recombinational events from unknown sources were found. The mean divergence between allele 7 of recP (6.66%) is much higher than the mean diversity within the other recP alleles (0.37%), with the exception of allele 3 (3.61%) (Fig. 2). This divergence is higher than the divergence observed by Enright and Spratt (10) in a possible example of an interspecies recombinational event in the gdh gene between S. pneumoniae and S. mitis (5.16%). In O. oeni, the recP gene of strain 5001 represents a possible example of a recombinational event, but in this case, the possible source remains unknown. As far as we know, this is the first example of a possible recombinational event described in O. oeni. The extension of the analysis to a higher number of strains might offer information about the possible source of this allele.
In summary, our data suggest strongly that the four housekeeping loci chosen were a suitable basis for an MLST scheme, as they could be amplified and sequenced from isolates obtained from a wide variety of sources, were unlinked on the O. oeni chromosome, and exhibited sufficient diversity to provide a high degree of resolution. This study also demonstrates that MLST discriminates among O. oeni isolates effectively and generates data that can be applied to the investigation of the population structure and evolutionary mechanism in this important food organism.
Acknowledgments
This work was supported by grant 07G/0035/2003 from the Comunidad de Madrid and RM03-002 from the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA). B. de las Rivas and A. Marcobal were the respective recipients of a postdoctoral fellowship and a predoctoral fellowship from the Comunidad de Madrid.
We thank R. González, A. V. Carrascosa, and E. García for their critical reading of the manuscript. The help of A. V. Carrascosa in providing the commercial starter strains was greatly appreciated.
REFERENCES
- 1.Brito, L., and H. Pavela. 1999. Presence and analysis of large plasmids in Oenococcus oeni. Plasmid 41:260-267. [DOI] [PubMed] [Google Scholar]
- 2.Britz, T. J., and R. P. Tracey. 1990. The combination effect of pH, SO2, ethanol and temperature on the growth of Leuconostoc oenos. J. Appl. Bacteriol. 68:23-31. [Google Scholar]
- 3.Caspritz, G., and F. Radler. 1983. Malolactic enzyme of Lactobacillus plantarum. J. Biol. Chem. 258:4907-4910. [PubMed] [Google Scholar]
- 4.Delaquis, P., M. Cliff, M. King, B. Girard, J. Hall, and A. Reynolds. 2000. Effect of two commercial malolactic cultures on the chemical and sensory properties of Chancellor wines vinified with different yeasts and fermentation temperatures. Am. J. Enol. Vitic. 51:42-48. [Google Scholar]
- 5.Dicks, L. T. M. 1994. Transformation of Leuconostoc oenos by electroporation. Biotechnol. Techniques 8:901-904. [Google Scholar]
- 6.Dicks, L. T. M., H. J. J. Van Vuuren, and F. Dellaglio. 1990. Taxonomy of Leuconostoc species, particularly Leuconostoc oenos, as revealed by numerical analysis of total soluble cell protein pattern, DNA base composition and DNA-DNA hybridization. Int. J. Syst. Bacteriol. 40:83-91. [Google Scholar]
- 7.Dicks, L. T. M., F. Dellaglio, and M. D. Collins. 1995. Proposal to reclassify Leuconostoc oenos as Oenococcus oeni [corrig.] gen. nov., comb. nov. Int. J. Syst. Bacteriol. 45:395-397. [DOI] [PubMed] [Google Scholar]
- 8.Dingle, K. E., F. M. Colles, D. R. A. Wareing, R. Ure, A. J. Fox, F. E. Bolton, H. J. Bootsma, R. J. L. Willems, R. Urwin, and M. C. J. Maiden. 2001. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 39:14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Divol, B., T. Tonon, S. Morichon, E. Gindreau, and A. Lonvaud-Funel. 2003. Molecular characterization of Oenococcus oeni genes encoding proteins involved in arginine transport. J. Appl. Microbiol. 94:738-746. [DOI] [PubMed] [Google Scholar]
- 10.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049-3060. [DOI] [PubMed] [Google Scholar]
- 11.Enright, M. C., and B. G. Spratt. 1999. Multilocus sequence typing. Trends Microbiol. 7:482-487. [DOI] [PubMed] [Google Scholar]
- 12.Enright, M. C., B. G. Spratt, A. Kalia, J. H. Cross, and D. E. Bessen. 2001. Multilocus sequence typing of Streptococcus pyogenes and the relationships between emm type and clone. Infect. Immun. 69:2416-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falush, D., C. Kraft, N. S. Taylor, P. Correa, J. G. Fox, M. Achtman, and S. Suerbaum. 2001. Recombination and mutation during long-term gastric colonization by Helicobacter pylori: estimates of clock rates, recombination size, and minimal age. Proc. Natl. Acad. Sci. USA 98:15056-15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farfán, M., D. Miñana-Galbis, M. C. Fusté, and J. G. Lorén. 2002. Allelic diversity and population structure in Vibrio cholerae O139 Bengal based on nucleotide sequence analysis. J. Bacteriol. 184:1304-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feil, E. J., M. C. Enright, and B. G. Spratt. 2000. Estimating the relative contributions of mutation and recombination to clonal diversification: a comparison between Neisseria meningitidis and Streptococcus pneumoniae. Res. Microbiol. 151:465-469. [DOI] [PubMed] [Google Scholar]
- 16.Frandsen, E. V., K. Poulsen, M. A. Curtis, and M. Kilian. 2001. Evidence of recombination in Porphyromonas gingivalis and random distribution of putative virulence markers. Infect. Immun. 69:4479-4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garvie, E. I. 1969. Lactic acid dehydrogenases of strains of the genus Leuconostoc. J. Gen. Microbiol. 58:85-94. [DOI] [PubMed] [Google Scholar]
- 18.Garvie, E. I. 1986. Gram-positive cocci: genus Leuconostoc, p. 1071-1075. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. Williams & Wilkins, Baltimore, Md. [Google Scholar]
- 19.Groisillier, A., and A. Lonvaud-Funel. 1999. Comparison of partial malolactic enzyme gene sequences for phylogenetic analysis of some lactic acid bacteria species and relationships with the malic enzyme. Int. J. Syst. Bacteriol. 49:1417-1428. [DOI] [PubMed] [Google Scholar]
- 20.Guerrini, S., A. Bastianini, G. Blaiotta, L. Granchi, G. Moschetti, S. Coppola, P. Romano, and M. Vicenzini. 2003. Phenotypic and genotypic characterization of Oenococcus oeni strains isolated from Italian wines. Int. J. Food Microbiol. 83:1-14. [DOI] [PubMed] [Google Scholar]
- 21.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 22.Haubold, B., and R. R. Hudson. 2000. LIAN 3.0: detecting linkage disequilibrium in multilocus data. Bioinformatics 16:847-848. [DOI] [PubMed] [Google Scholar]
- 23.Helgason, E., N. J. Tourasse, R. Meisal, D. A. Caugant, and A. B. Kolsto. 2004. Multilocus sequence typing scheme for bacteria of the Bacillus cereus group. Appl. Environ. Microbiol. 70:191-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huson, D. H. 1998. SplitsTree: analyzing and visualizing evolutionary data. Bioinformatics 14:68-73. [DOI] [PubMed] [Google Scholar]
- 25.Jones, N., J. F. Bohnsack, S. Takahashi, K. A. Oliver, M. S. Chan, F. Kunst, P. Glaser, C. Rusniok, D. W. M. Crook, R. M. Harding, N. Bisharat, and B. G. Spratt. 2003. Multilocus sequence typing system for group B Streptococcus. J. Clin. Microbiol. 41:2530-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly, W. J., C. M. Huang, and R. V. Asmundson. 1993. Comparison of Leuconostoc oenos strains by pulsed-field gel electrophoresis. Appl. Environ. Microbiol. 59:3969-3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koehler, A., H. Karch, T. Beikler, T. F. Flemmig, S. Suerbaum, and H. Schmidt. 2003. Multilocus sequence analysis of Porphyromonas gingivalis indicates frequent recombination. Microbiology 149:2407-2415. [DOI] [PubMed] [Google Scholar]
- 28.Kumar, S., K. Tamura, and M. Nei. 1994. MEGA: molecular evolutionary genetics analysis software for microcomputers. Comput. Appl. Biosci. 10:189-191. [DOI] [PubMed] [Google Scholar]
- 29.Lamoureux, M., H. Prévost, J. F. Cavin, and C. Diviès. 1993. Recognition of Leuconostoc oenos strains by the use of DNA restriction profiles. Appl. Microbiol. Biotechnol. 39:547-552. [DOI] [PubMed] [Google Scholar]
- 30.Le Jeune, C., and A. Lonvaud-Funel. 1997. Sequence of DNA 16S/23S spacer region of Leuconostoc oenos (Oenococcus oeni): application to strain differentiation. Res. Microbiol. 148:79-86. [DOI] [PubMed] [Google Scholar]
- 31.Maiden, M. C., J. J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchesi, J. R., T. Sato, A. J. Weightman, T. A. Martin, J. C. Fry, S. J. Hiom, and W. G. Wade. 1998. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl. Environ. Microbiol. 64:795-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maynard Smith, J., and N. H. Smith. 1998. Detecting recombination from gene trees. Mol. Biol. Evol. 15:590-599. [DOI] [PubMed] [Google Scholar]
- 34.Maynard Smith, J., N. H. Smith, M. O'Rourke, and B. G. Spratt. 1993. How clonal are bacteria? Proc. Natl. Acad. Sci. USA 90:4384-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meats, E., E. J. Feil, S. Stringer, A. J. Cody, R. Goldstein, J. S. Kroll, T. Popovic, and B. G. Spratt. 2003. Characterization of encapsulated and noncapsulated Haemophilus influenzae and determination of phylogenetic relationships by multilocus sequence typing. J. Clin. Microbiol. 41:1623-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreno-Arribas, M. V., M. C. Polo, F. Jorganes, and R. Muñoz. 2003. Screening of biogenic amine production by lactic acid bacteria isolated from grape must and wine. Int. J. Food Microbiol. 84:117-123. [DOI] [PubMed] [Google Scholar]
- 37.Nei, M., and T. Gojobori. 1986. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 3:418-426. [DOI] [PubMed] [Google Scholar]
- 38.Peynaud, E., and S. Domercq. 1968. Étude de quatre cents souches de coques hétérolactiques isolés de vins. Ann. Inst. Pasteur 19:159-170. [PubMed] [Google Scholar]
- 39.Sato, H., F. Yanagida, T. Shinohara, M. Suzuki, K. Suzuki, and K. Yokotsuka. 2001. Intraspecific diversity of Oenocoocus oeni isolated during red wine-making in Japan. FEMS Microbiol. Lett. 202:109-114. [DOI] [PubMed] [Google Scholar]
- 40.Sawyer, S. 1989. Statistical tests for detecting gene conversion. Mol. Biol. Evol. 6:526-538. [DOI] [PubMed] [Google Scholar]
- 41.Tenreiro, R., M. A. Santos, H. Pavela, and G. Vieira. 1994. Inter-strain relationships among wine leuconostocs and their divergence from other Leuconostoc species, as revealed by low frequency restriction fragment analysis of genomic DNA. J. Appl. Bacteriol. 77:271-280. [DOI] [PubMed] [Google Scholar]
- 42.Tracey, R. P., and T. J. Britz. 1989. Cellular fatty acid composition of Leuconostoc oenos. J. Appl. Bacteriol. 66:445-456. [Google Scholar]
- 43.Urwin, R., and M. C. J. Maiden. 2003. Multi-locus sequence typing: a tool for global epidemiology. Trends Microbiol. 11:479-487. [DOI] [PubMed] [Google Scholar]
- 44.Viti, C., L. Giovannetti, L. Granchi, and S. Ventura. 1996. Species attribution and strain typing of Oenococcus oeni (formerly Leuconostoc oenos) with restriction endonuclease fingerprints. Res. Microbiol. 147:651-660. [DOI] [PubMed] [Google Scholar]
- 45.Zapparoli, G., C. Reguant, A. Bordons, S. Torriani, and F. Dellaglio. 2000. Genomic DNA fingerprinting of Oenococcus oeni strains by pulsed-field gel electrophoresis and randomly amplified polymorphic DNA-PCR. Curr. Microbiol. 40:351-355. [DOI] [PubMed] [Google Scholar]
- 46.Zavaleta, A. I., A. J. Martínez-Murcia, and F. Rodríguez-Varela. 1996. 16S-23S rDNA intergenic sequences indicate that Leuconostoc oenos is phylogenetically homogeneous. Microbiology 142:2105-2114. [DOI] [PubMed] [Google Scholar]
- 47.Zavaleta, A. I., A. J. Martínez-Murcia, and F. Rodríguez-Varela. 1997. Intraspecific genetic diversity of Oenococcus oeni as derived from DNA fingerprinting and sequence analyses. Appl. Environ. Microbiol. 63:1261-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zé-Zé, L., R. Tenreiro, and H. Pavela. 2000. The Oenococcus oeni genome: physical and genetic mapping of a strain GM and comparison with the genome of a “divergent” strain, PSU-1. Microbiology 146:3195-3204. [DOI] [PubMed] [Google Scholar]
- 49.Zúñiga, M., I. Pardo, and S. Ferrer. 1996. Transposons Tn916 and Tn925 can transfer from Enterococcus faecalis to Leuconostoc oenos. FEMS Microbiol. Lett. 135:179-185. [DOI] [PubMed] [Google Scholar]