Abstract
Rhodococcus sp. strain DK17 is able to grow on o-xylene, benzene, toluene, and ethylbenzene. DK17 harbors at least two megaplasmids, and the genes encoding the initial steps in alkylbenzene metabolism are present on the 330-kb pDK2. The genes encoding alkylbenzene degradation were cloned in a cosmid clone and sequenced completely to reveal 35 open reading frames (ORFs). Among the ORFs, we identified two nearly exact copies (one base difference) of genes encoding large and small subunits of an iron sulfur protein terminal oxygenase that are 6 kb apart from each other. Immediately downstream of one copy of the dioxygenase genes (akbA1a and akbA2a) is a gene encoding a dioxygenase ferredoxin component (akbA3), and downstream of the other copy (akbA1b and akbA2b) are genes putatively encoding a meta-cleavage pathway. RT-PCR experiments show that the two copies of the dioxygenase genes are operonic with the downstream putative catabolic genes and that both operons are induced by o-xylene. When expressed in Escherichia coli, AkbA1a-AkbA2a-AkbA3 transformed o-xylene into 2,3- and 3,4-dimethylphenol. These were apparently derived from an unstable o-xylene cis-3,4-dihydrodiol, which readily dehydrates. This indicates a single point of attack of the dioxygenase on the aromatic ring. In contrast, attack of AkbA1a-AkbA2a-AkbA3 on ethylbenzene resulted in the formation of two different cis-dihydrodiols resulting from an oxidation at the 2,3 and the 3,4 positions on the aromatic ring, respectively.
Members of the genus Rhodococcus demonstrate a remarkable ability to utilize a wide variety of natural organic and xenobiotic compounds, including aliphatic, aromatic, and alicyclic hydrocarbons (references 7 and 28; see also the special issue of Antonie Van Leeuwenhoek, volume 74). Besides the ability to degrade a broad spectrum of chemical compounds, many rhodococcal strains are known to catalyze the stereoselective oxidation of structurally different compounds such as indene (25), monoterpene (26), aliphatic alkenes (23), and phenylpropionitrile (10). Accordingly, rhodococci have the great potential to synthesize valuable chemical synthons, and of particular interest is the incorporation of molecular oxygen into the aromatic nucleus to form vicinal arene cis-diols (5, 19). For example, an aromatic dioxygenase from Rhodococcus sp. strain I24 was used for the oxidation of indene to cis-(1S,2R)-dihydroxyindan, which could serve as a precursor for indinavir, a new anti-human immunodeficiency virus drug (25).
To date, several gene clusters involved in the degradation of aromatic compounds have been cloned from Rhodococcus spp. These include degradative genes for biphenyl from Rhodococcus sp. strain M5 (27), Rhodococcus globerulus P6 (3), and Rhodococcus sp. strain RHA1 (21); isopropylbenzene-degrading genes from Rhodococcus erythropolis BD2 (14); and benzoate dioxygenase genes from Rhodococcus sp. strain 19070 (11). However, no in-depth genetic work has been reported regarding the abilities of Rhodococcus strains to degrade o-xylene.
Rhodococcus sp. strain DK17 was originally isolated in Yeochon, Korea, for the ability to grow on o-xylene and was found to also have the capability to grow on benzene, toluene, ethylbenzene, isopropylbenzene, and n-propyl- to n-hexylbenzenes (16). The degradation of o-xylene and toluene in Rhodococcus sp. strain DK17 is initiated by a ring-oxidizing dioxygenase pathway through 3,4-dimethylcatechol and 3- and 4-methylcatechol, respectively (16). This indicates that the o-xylene dioxygenase in strain DK17 can perform unique regioselective hydroxylations depending on the position of the substituent groups on the aromatic ring. This hypothesis is further supported by the finding that the cells of DK17 grown on o-xylene have the ability to oxidize m- and p-xylene to 2,4-dimethylresorcinol and 2,5-dimethylhydroquinone, respectively, although DK17 does not have the capability to grow on the other two xylene isomers (15). We also reported that the genes encoding the initial steps in this alkylbenzene pathway are present on the 330-kb megaplasmid pDK2 (15, 16). Thus, the present work was initiated to identify the genes for the initial ring hydroxylation dioxygenase enzyme and determine whether a single oxygenase is capable of the different regiospecific hydroxylation reactions observed with Rhodococcus sp. strain DK17.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Rhodococcus sp. strain DK17 is the wild-type strain capable of growth on alkylbenzenes. Rhodococcus sp. strains DK176 and DK180, derived from DK17, are unable to grow on alkylbenzenes due to the loss of a 330-kb plasmid (pDK2) and meta-cleavage enzyme activity, respectively (16). Escherichia coli EPI100 [F− mcrA Δ(mrr-hsdRMS-mcrBC) f80dlacZΔM15 ΔlacX74 recA1 endA1 araD139 Δ(ara, leu)7697 galU galK λ− rpsL nupG] (Epicentre, Madison, Wis.) was used as the host strain for the cosmid library construction. Escherichia coli TOP10 [F− mcrA Δ(mrr-hsdRMS-mcrBC) Φ80lacZΔM15 ΔlacX74 recA1 deoR araD139 Δ(ara-leu)7697galU galK rpsL (Strr) endA1 nupG] (Invitrogen, Carlsbad, Calif.) was used as the host for the small-insert library construction.
Rhodococcus sp. strains were grown on mineral salts basal medium (24) containing 20 mM glucose at 30°C. E. coli strains for library construction or cloning were grown on Luria-Bertani (LB) medium at 37°C.
Preparation of cell extracts and SDS-PAGE.
Bacterial cells reaching the exponential phase on 20 mM glucose were harvested and resuspended in 200 ml of fresh mineral salts basal medium containing 5 mM glucose. To induce the alkylbenzene degradative genes, o-xylene was added directly to the suspension at a final concentration of 0.1% (vol/vol) and further incubated at 30°C for 12 h. Cell extracts for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were prepared as described previously (19). SDS-PAGE was performed on 12.5% acrylamide gel in a Hoefer Mighty Small SE245 electrophoresis cell (Amersham Biosciences, Little Chalfont, England). The separated proteins were transferred to a polyvinylidene difluoride membrane. The appropriate Coomassie-stained protein band was excised from the polyvinylidene difluoride membrane and installed into the blot cartridge of a Procise cLC 492 protein sequencer (Applied Biosystems, Foster City, Calif.) for N-terminal sequence analysis.
DNA manipulation.
Total DNA from Rhodococcus sp. strain DK17 was prepared according to the method of Asturias and Timmis (3). Plasmid DNA was purified by a plasmid spin kit (Genenmed, Daejeon, Korea). Agarose gel electrophoresis was performed in Tris-acetate-EDTA buffer. Transfer of DNA from agarose gels to Hybond-N+ membranes (Amersham Biosciences) was carried out using a TurboBlotter transfer system as recommended by the supplier (Schleicher & Schuell, Dassel, Germany). PCR DNA products to be used as probes in colony or Southern blotting experiments were separated by gel electrophoresis and eluted from agarose gels with a gel extraction kit (Genenmed). Colony and Southern hybridizations were performed as recommended by the supplier of a DIG nonradioactive nucleic acid labeling and detection system (Boehringer Mannheim, Mannheim, Germany).
Genomic and small insert DNA library construction and nucleotide sequencing.
A total genomic DNA library was constructed using a pWEB::TNC cosmid cloning kit (Epicentre), as described by the manufacturer. The selected cosmid clone was mechanically sheared with a HydroShear DNA shearing device (Genemachines, San Carlos, Calif.) for the generation of random DNA fragments. Insert fragments were converted to blunt-end DNA with an End-It DNA-Repair kit (Epicentre) and ligated into the vector pCR-Blunt (Invitrogen). The ligation mixture was transformed into TOP10 chemically competent cells (Invitrogen).
An ABI PRISM BigDye Terminator cycle sequencing kit was used to carry out cycle sequencing reactions as recommended by the manufacturer (Applied Biosystems). The cycle sequencing reactions were analyzed using a model 3100 automated DNA sequencer (Applied Biosystems). Custom primers were used for filling in gaps in the assembled sequence. DNA fragments were completely sequenced on both strands. Nucleotide sequences were assembled with DNAstar Lasergene software (DNAstar, Madison, Wis.) and analyzed using BLAST software against the GenBank database (1).
PCR and cloning procedures.
PCR amplification was carried out in a PTC-150 MiniCycler (MJ Research, Watertown, Mass.). Custom primers were supplied by Cosmo Genetech (Seoul, Korea). The PCR was performed in 20 μl of a reaction mixture containing approximately 100 ng of template DNA and 10 pmol of each primer with ReadyMix Taq PCR mix (Sigma, St. Louis, Mo.) according to instructions of the manufacturer. The akbA1a, akbA2a, and akbA3 genes were amplified by PCR (forward primer, 5′-ATGGAGTGGAGCATGTTG-3′; reverse primer, 5′-TCATTGAGACTCGGCGCC-3′) and cloned using a pCR T7 TOPO TA expression kit (Invitrogen) according to the manufacturer's instructions. The thermal cycling program was a 10-min hot start (95°C), 30 cycles of 30 s of denaturation (95°C), 30 s of annealing (55°C), and 1 min of extension (72°C), and a final 10 min of extension (72°C).
The reverse transcription-PCRs (RT-PCRs) were performed in 25 μl of a reaction mixture with 70 ng of total RNA and 25 pmol of each primer with OneStep RT-PCR enzyme mix (QIAGEN, Hilden, Germany). The thermocycler program used for the RT-PCRs was as follows: 50°C for 30 min, 95°C for 15 min, 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min, and 72°C for 10 min. The following primers were designed: to amplify two adjacent akb genes, akbA1b-akbA2b and akbA1a-akbA2a, 5′-ATGGAGTGGAGCATGTTG-3′ (forward) and 5′-TCAGAGGAAGATGTTGAG-3′(reverse); to amplify akbA2b and akbC, 5′-ATGACATCGACCGCGGCG-3′ (forward) and 5′-TTATGCGGGGATGTCGAG-3′ (reverse); to amplify akbCD, 5′-ATGGCAAAAGTGACCGAA-3′ (forward) and 5′-CTATGCCGCGCGGAAATG-3′ (reverse); to amplify akbDE, 5′-ATGGCGAAGACTGTCGAA-3′ (forward) and 5′-CTAACCGAAACGAAATGA-3′ (reverse); to amplify akbEF, 5′-ATGCTTGACGAACAGACG-3′ (forward) and 5′-TCACGAATACGCCACCTG-3′ (reverse); and to amplify akbA2aA3, 5′-ATGACATCGACCGCGGCG-3′ (forward) and 5′-TCATTGAGACTCGGCGCC-3′ (reverse).
Analysis and identification of o-xylene/ethylbenzene metabolites.
A preculture of E. coli BL21(DE3) containing the akbA1a, akbA2a, and akbA3 genes was prepared by inoculating one colony into 50 ml of LB medium supplemented with ampicillin (100 μg/ml) and incubating overnight at 37°C. A total of 4 ml of the overnight culture was transferred to 200 ml of LB medium and incubated under the same conditions. The culture was induced by adding IPTG (isopropyl-β-d-thiogalactopyranoside) to achieve a concentration of 1 mM, when bacterial cells reached an optical density at 600 nm of 0.5 to 0.7, and further incubated for 2 h. Subsequently, the culture was harvested by centrifugation at 10,000 × g for 15 min, washed with 50 mM potassium phosphate buffer (pH 7.4), and resuspended in 30 ml of the same solution supplemented with 20 mM glucose and ampicillin. o-Xylene and ethylbenzene were individually provided in the vapor phase, and the cells were incubated at 30°C for 13 h. The supernatant was extracted with ethyl acetate and dried by a rotary evaporator, and the dried residues were acetylated for stabilization as described previously (16). o-Xylene or ethylbenzene metabolites formed by the meta-cleavage dioxygenase mutant strain DK180 were prepared as described previously (16).
Gas chromatography-mass spectrometry (GC-MS) analysis of metabolites was carried out with a Hewlett-Packard 5973 mass spectrometer (electron impact ionization, 70 eV) connected to a 6890 gas chromatogram fitted with a fused silica capillary column (HP-5) (0.25 by 30 m; 0.25 μm film thickness). The following conditions were used for the GC: 1 ml of He/min; on-column injection mode; oven temperature, 60°C for 2 min; thermal gradient, 5°C/min to 220°C and then held at 220°C.
Chemicals.
Aromatic compounds used in this study were obtained from Sigma-Aldrich Korea (Seoul, Korea). (+)-cis-3-Ethyl-3,5-cyclohexadiene-1,2-diol (cis-2,3-ethylbenzenedihydrodiol) and 3-ethylcatechol were prepared from ethylbenzene by use of Pseudomonas putida 39/D (8) and P. putida PpF107 (30), respectively, following published procedures. All solvents were purchased from Mallinckrodt Baker, Inc. (Phillipsburg, New Jersey). All chemicals were analytical-grade purity or above.
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study have been deposited in the GenBank database under accession number AY502075.
RESULTS
Cloning the genes for o-xylene degradation.
Rhodococcus sp. strain DK17 harbors a large catabolic megaplasmid, designated pDK2, carrying the genes for o-xylene degradation (16). Plasmid-cured derivatives such as DK176 lose the ability to grow not only on o-xylene but also on benzene, toluene, and ethylbenzene. To identify enzymes involved in o-xylene degradation, the total cellular protein patterns were compared between DK17 and DK176 following growth on glucose and o-xylene. Several new and very obvious protein bands were present in the DK17 extract that were not present in the DK176 extract when the soluble cell extracts were separated on one-dimensional SDS-PAGE (data not shown). These proteins appear to be encoded by the plasmid pDK2 and are specifically induced upon exposure of DK17 to o-xylene. Many oxygenase components of ring hydroxylation dioxygenase enzymes are comprised of a large and small subunit of approximately 50 and 25 kDa (12). The N-terminal amino acid sequence of a protein band with an approximate size of 50 kDa, which is differentially produced by DK17 in comparison to DK176, was determined to be MLRSERFSPGEDFGQ. A search of the GenBank database revealed that this 15-amino-acid N-terminal sequence is identical to the N-terminal sequence of the large subunit of a ring hydroxylation dioxygenase from the polychlorinated biphenyl-degrading Rhodococcus sp. strain RHA1 (17).
Since the N-terminal sequence of the DK17 o-xylene oxygenase matched that of an oxygenase in RHA1 it is likely that the genes share significant identity as well. In their initial analysis of genes encoding oxygenases in RHA1, Kitagawa et al. (17) described a set of slightly degenerate primers designed to amplify oxygenase genes. Application of the primers (forward, 5′-TGCASSTWTCACGGSTGG-3′; reverse, 5′-CTCGACTCCGAGCTTCCAGTT-3′) in a PCR with total genomic DNA from DK17 amplified a ∼300-bp fragment, as expected from a potential ring hydroxylation oxygenase gene target. With the PCR fragment used as a probe, 1,000 cosmid colonies were screened, with only one clone, designated pKEB2002, showing a strong hybridization signal.
A Southern hybridization experiment was performed with the cosmid clone pKEB2002 as the probe for PFGE-separated total genomic DNA from the wild-type strain DK17 and the pDK2-minus strain DK176. The probe hybridized to pDK2 in the DK17 lane, while no hybridization was seen for the plasmid-minus strain DK176. This observation confirms that the insert in pKEB2002 is derived from the 330-kb megaplasmid pDK2 as expected, since the genes for o-xylene degradation were previously located on pDK2 (16).
Identification and o-xylene-mediated induction of the degradative genes.
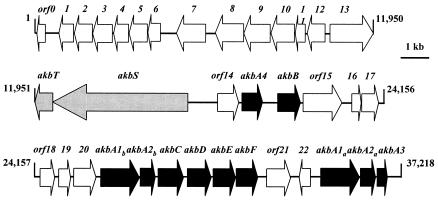
The entire pKEB2002 cosmid clone containing the gene encoding an o-xylene dioxygenase oxygenase component was sequenced as described in the Materials and Methods. Analysis of the 37,218-bp sequenced region (Fig. 1) identified two genes (designated akbA1a and akbA1b, approximately 6 kb apart from each other) encoding an oxygenase component large subunit with a deduced N-terminal sequence identical to the sequence of 15 N-terminal amino acids determined for the putative o-xylene oxygenase component large subunit described above. Immediately following each of the two genes encoding a large subunit are genes (designated akbA2a and akbA2b) encoding a small subunit for an oxygenase component. It is interesting that the 1,963-bp akbA1a-akbA2a and akbA1b-akbA2b gene regions are identical to each other except for one base pair. The region of identity starts 18 bp in front of the two akbA1 genes (just in front of the ribosome binding site) and continues to 3 bp after the two akbA2 stop codons. The single base difference (T versus C) between the two regions does not affect the amino acid sequence of the encoded AkbA1a and AkbA2a proteins, as akbA1a has an ATT Ile codon for the 32nd amino acid in the protein whereas akbA1b has an ATC Ile codon. Also, deduced amino acid sequence alignment of AkbA1a and AkbA1b with the large subunits of different Rieske dioxygenases reveals the presence of motifs for a Rieske-type (2Fe-2S) iron-sulfur center and one mononuclear nonheme iron (12, 20).
FIG. 1.
Gene organization of a 37,218-bp region from Rhodococcus sp. strain DK17. Black arrows indicate structural genes for alkylbenzene metabolism. Two-component regulatory genes are shown as gray-shaded arrows. Genes for miscellaneous or unknown functions are shown as white arrows. The directions of transcription are indicated by arrowheads. The nucleotide numbers are marked on the beginning and the end of each line.
In addition to the duplicated akbA1A2 genes, other genes putatively involved in o-xylene degradation can be identified in the sequence of the pKEB2002 cosmid clone. The akbA1a and akbA1b genes on the 3′ edge of the cosmid clone are followed by a gene, designated akbA3, encoding a ferredoxin component of a multicomponent dioxygenase. The 5′ end of a gene encoding a putative meta-cleavage product hydrolase is at the end of the sequenced region. On the other hand, the akbA1b and akbA2b genes are followed by genes, designated akbCDEF, putatively encoding the proteins for a complete meta-cleavage pathway, namely, a meta-cleavage dioxygenase (AkbC), a meta-cleavage hydrolase product (AkbD), a hydratase (AkbE), and an aldolase (AkbF). More than 5 kb upstream of the akbA1a and akbA1b genes are two genes, designated akbA4 and akbB, encoding a putative reductase component of a dioxygenase and a cis-dihydrodiol dehydrogenase. Similarities between the open reading frames (ORFs) and representative homologs are summarized in Table 1.
TABLE 1.
Predicted ORFs identified on a 37,218 base pair region from the 330-kb megaplasmid pDK2
| ORF | Start-stop codons | Representative homolog (gene) | Identity (%)a | Organism | NCBI protein database access no.b |
|---|---|---|---|---|---|
| orf0 | 312-7 | Hypothetical protein | No match | ||
| orf1 | 1,285-761 | Outer-membrane protein (orf6) | 44 | Rhodococcus sp. NCIMB12038 | AAQ98851 |
| orf2 | 1,985-1,338 | Transmembrane protein (orf5) | 87 | Rhodococcus sp. NCIMB12038 | AAQ98850 |
| orf3 | 2,689-1,982 | Transmembrane protein (orf4) | 86 | Rhodococcus sp. NCIMB12038 | AAQ98849 |
| orf4 | 3,271-2,738 | Unknown protein (orf3) | 76 | Rhodococcus sp. NCIMB12038 | AAQ98848 |
| orf5 | 3,935-3,282 | Unknown protein (orf2) | 83 | Rhodococcus sp. NCIMB12038 | AAQ98847 |
| orf6 | 4,387-3,932 | Unknown protein (orf1) | 88 | Rhodococcus sp. NCIMB12038 | AAQ98846 |
| orf7 | 5,982-4,936 | Hypothetical protein | No match | ||
| orf8 | 7,313-6,303 | 4-Hydroxy-2-oxovalerate aldolase (xylK) | 61 | Novosphingobium aromaticivorans F199 | AAD03993 |
| orf9 | 8,257-7,310 | Acetaldehyde dehydrogenase (cmtH) | 67 | Pseudomonas putida F1 | 2209341L |
| orf10 | 9,102-8,254 | 2-Hydroxypenta-2,4-dienoate hydratase(tobG) | 54 | Pseudomonas putida PB4071 | AAG09414 |
| orf11 | 9,475-9,110 | Hypothetical protein | No match | ||
| orf12 | 10,171-9,527 | Putative enoyl-CoA-hydratase | 92 | Rhodococcus erythropolis BD2 | AAP74050 |
| orf13 | 10,385-11,923 | Putative medium-chain acyl-CoA ligase (alkK) | 99 | Rhodococcus erythropolis BD2 | AAP74049 |
| akbT | 12,580-11,951 | Response regulator (bphT) | 96 | Rhodococcus sp. RHA1 | BAC75412 |
| akbS | 17,367-12,577 | Sensor kinase (bphS) | 83 | Rhodococcus sp. RHA1 | BAC75411 |
| orf14 | 18,396-19,154 | Hypothetical protein | No match | ||
| akbA4 | 19,271-20,500 | Ferredoxin reductase (etbA4) | 100 | Rhodococcus sp. RHA1 | BAD03966 |
| akbB | 20,533-21,345 | Dihydrodiol dehydrogenase (bphB2) | 100 | Rhodococcus sp. RHA1 | BAD03967 |
| orf15 | 21,453-22,826 | 6-Oxohexanoate dehydrogenase (chnE) | 62 | Rhodococcus sp. Phi2 | AAN37492 |
| orf16 | 23,213-23,548 | Hypothetical protein | No match | ||
| orf17 | 23,566-24,156 | Hypothetical protein | No match | ||
| orf18 | 24,348-24,860 | Hypothetical protein | No match | ||
| orf19 | 24,995-25,414 | Hypothetical protein | No match | ||
| orf20 | 26,307-25,510 | Hypothetical protein | No match | ||
| akbA1b | 26,466-27,848 | Ethylbenzene dioxygenase large subunit (etbA1) | 100 | Rhodococcus sp. RHA1 | BAC92712 |
| akbA2b | 27,871-28,419 | Ethylbenzene dioxygenase small subunit (etbA2) | 100 | Rhodococcus sp. RHA1 | BAC92713 |
| akbC | 28,479-29,396 | 2,3-Dihydroxybiphenyl 1,2-dioxygenase (etbC) | 100 | Rhodococcus sp. RHA1 | BAC92714 |
| akbD | 29,517-30,374 | 2-Hydroxy-6-oxo-6-phenylhexa-2,4-dienoate hydrolase (bphD) | 100 | Rhodococcus sp. RHA1 | BAC92715 |
| akbE | 30,425-31,228 | 2-Hydroxypenta-2,4-dienoate hydratase (bphE2) | 100 | Rhodococcus sp. RHA1 | BAC92716 |
| akbF | 31,240-32,016 | 4-Hydroxy-2-oxovalerate aldolase (bphF2) | 100 | Rhodococcus sp. RHA1 | BAC92717 |
| orf21 | 32,562-33,413 | Putative N5,N10-methylenetetrahydromethanopterin reductase-related protein | 55 | Streptomyces avermitilis MA-4680 | BAC75195 |
| orf22 | 34,129-33,722 | Hypothetical protein | No match | ||
| akbA1a | 34,461-35,843 | Ethylbenzene dioxygenase large subunit (ebdA1) | 100 | Rhodococcus sp. RHA1 | BAC92718 |
| akbA2a | 35,866-36,414 | Ethylbenzene dioxygenase small subunit (ebdA2) | 100 | Rhodococcus sp. RHA1 | BAC92719 |
| akbA3 | 36,444-36,809 | Ethylbenzene dioxygenase ferredoxin (ebdA3) | 100 | Rhodococcus sp. RHA1 | BAC92720 |
Percentages of identity were obtained by aligning the deduced amino acid sequences by use of Blastp.
NCBI, National Center for Biotechnology Information.
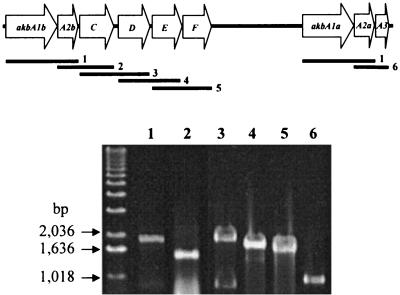
To confirm that the identified akb genes are actually expressed in response to o-xylene and to confirm the operonic nature of the akb genes, RT-PCR experiments were performed with total RNA extracted from the o-xylene-induced cells of DK17 as described in Materials and Methods. The PCR primers were designed to generate PCR products as follows: akbA1a to akbA2a and akbA1b to akbA2b, 1,954 bp; akbA2b to akbC, 1,526 bp; akbC to akbD, 1,896 bp; akbD to akbE, 1,712 bp; akbE to akbF, 1,592 bp; and akbA2a to akbA3, 944 bp. Whereas PCR without RT did not show any PCR product, the RT-PCR showed PCR products of the expected size (Fig. 2). These data show that the akbA1a, akbA2a, and akbA3 genes and akbA2a, akbA2b, and akbCDEF genes are transcribed as operons. In addition, since no RT-PCR product was detectable for any of the akb genes following growth of DK17 on glucose (data not shown), identified akb genes are specifically induced by growth on o-xylene.
FIG. 2.
Operonic nature of the akb genes in Rhodococcus sp. strain DK17 and agarose gel electrophoresis of RT-PCR products. The expected PCR products for each well are indicated in the gene map. The first lane was loaded with a molecular weight marker.
Heterologous expression of akbA1a, akbA2a, and akbA3.
The protein production and gene expression data indicate that the akbA1A2 genes encode a corresponding putative oxygenase large subunit produced in response to growth of Rhodococcus sp. strain DK17 on o-xylene. To functionally confirm the role of this putative oxygenase in alkylbenzene degradation, the akbA1a, akbA2a, and akbA3 genes were cloned and expressed in E. coli. Dioxygenase enzymes often require a short electron transfer chain to shuttle electrons from NAD(P)H to the oxygenase component that performs the catalytic reaction (20, 29). In many cases of heterologous expression of genes for dioxygenases in E. coli, the native reductase component may be left out, as E. coli reductases may substitute for them (11, 18). This being the case, the contiguous akbA1a, akbA2a, and akbA3 genes encoding the oxygenase and ferredoxin components were PCR amplified and cloned into the expression vector pCRT7/CT-TOPO to construct the recombinant plasmid pKEB051.
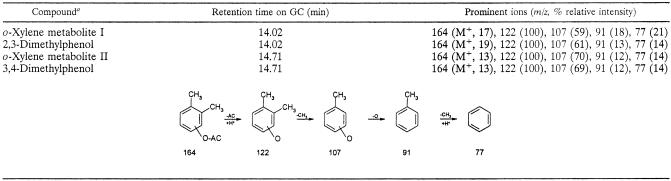
The resting induced cells of E. coli BL21(DE3) harboring pKEB051 were incubated in the presence of o-xylene to allow for conversion of o-xylene to an oxidized product by the expressed o-xylene oxygenase. The potential oxidized products were extracted and stabilized by acetylation for GC-MS analysis. Two peaks were detected at 14.02 min (o-xylene metabolite I) and 14.71 min (o-xylene metabolite II) on the total ion chromatogram. Both metabolites have the same molecular ion at m/z 164 and a prominent ion due to fission of acetate at m/z 122 (Table 2). Since this result indicates that the original metabolites are monohydroxylated o-xylenes, the acetylated derivatives of authentic 2,3- and 3,4-dimethylphenol were analyzed for comparison and found to have the same mass spectra and GC retention times as those of o-xylene metabolites I and II, respectively. As determined on the basis of previous work with DK17 (16), the expected product of the o-xylene dioxygenase biotransformation is o-xylene cis-3,4-dihydrodiol. However, this compound is likely to be unstable due to the electron-donating nature of the two adjacent methyl groups and would readily dehydrate to 2,3- and 3,4-dimethylphenol (3- and 4-hydroxy-o-xylene, respectively).
TABLE 2.
GC-MS data for 2,3- and 3,4-dimethylphenol and o-xylene metabolites formed by E. coli carrying pKEB051 and for the fragmentation patterns of each metabolite
Each sample was analyzed as an acetatylated derivative.
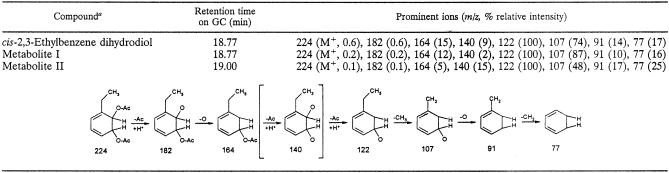
Previous work with DK17 implicated the o-xylene catabolic pathway in the degradation of a number of other alkylbenzenes (15, 16). This being the case, the ability of o-xylene dioxygenase to oxidize other alkyl-substituted benzenes was tested. Ethylbenzene was chosen as an alternative substrate due to its representative nature of alkylbenzenes in general, the availability of cis-dihydrodiol standards, and the fact that growth of DK17 on ethylbenzene is comparable to that on o-xylene. Following incubation of induced BL21(DE3)(pKEB051) resting cells in the presence of ethylbenzene, the extracted metabolites were acetylated and analyzed by GC-MS. Two peaks for ethylbenzene metabolites are seen at 18.77 min (major) and 19.00 min (minor) on the total ion chromatogram which have the same molecular ion at m/z 224 (Table 3). This suggests that the metabolites have two additional hydroxyls compared to ethylbenzene and thus are most likely cis-dihydrodiols. Two possible cis-dihydrodiols could be synthesized from ethylbenzene: cis-2,3 and cis-3,4. Authentic acetylated cis-2,3-ethylbenzene dihydrodiol shows an retention time (18.77 min) and mass spectrum identical to those of the major metabolite formed from ethylbenzene by DK17 o-xylene dioxygenase. Since the minor product at 19.00 min has a molecular weight the same as and a mass spectrum similar to those of cis-2,3-ethylbenzene dihydrodiol, it is most likely cis-3,4-ethylbenzene dihydrodiol.
TABLE 3.
GC-MS data for cis-2,3-ethylbenzene dihydrodiol and ethylbenzene metabolites formed by E. coli carrying pKEB051 and for the fragmentation patterns of each metabolite
Each sample was analyzed as an acetatylated derivative.
To corroborate the above assessment, the mutant strain Rhodococcus sp. strain DK180 was chosen for additional biotransformation experiments. This mutant is blocked in the meta-cleavage step and accumulates 3,4-dimethylcatechol from o-xylene and both 3- and 4-methylcatechol from toluene (16). DK180 was grown on glucose in the presence of ethylbenzene, the culture supernatant extracted with ethyl acetate and potential metabolites derivatized with N-methyl-N-trimethylsilyltrifluoroacetamide. Analysis by capillary GC-MS revealed two peaks for ethylbenzene metabolites at 19.80 min (major) and 19.92 min (minor) on the total ion chromatogram. GC-MS comparison with authentic standards of 3- and 4-ethylcatechol reveal that the major metabolite at 19.80 min is 3-ethylcatechol whereas the minor metabolite at 19.92 min is 4-ethylcatechol. These data suggest that the minor cis-ethylbenzene dihydrodiol metabolite produced from ethylbenzene by the o-xylene oxygenase expressed in E. coli is most likely cis-3,4-ethylbenzene dihydrodiol.
DISCUSSION
The present study utilized a combination of whole-cell protein analysis, gene cloning and sequencing, and heterologous gene expression to identify the genes encoding a three- component (sulfur protein terminal oxygenase, ferredoxin, and reductase) o-xylene dioxygenase. Using the N-terminal sequence of an o-xylene-induced iron sulfur protein large subunit, we identified a cosmid clone containing two nearly exact copies (one base difference) of genes encoding large and small subunits of an iron sulfur protein terminal oxygenase 6 kb apart from each other. The Rhodococcus sp. strain DK17 genes encoding an o-xylene dioxygenase iron sulfur protein large subunit show a remarkable degree of identity with genes encoding a large subunit identified in Rhodococcus sp. strain RHA1, which are genes proposed to be involved in the degradation of biphenyl or ethylbenzene (17). Although no definitive function was assigned to the genes identified in strain RHA1, the high (over 99%) degree of identity to the genes encoding an o-xylene/alkylbenzene dioxygenase large subunit in DK17 suggests that the RHA1 oxygenase genes are involved in the degradation of alkylbenzenes. This hypothesis is also in good agreement with the fact that RHA1 grows on o-xylene as well as on toluene, isopropylbenzene, and ethylbenzene (17). In fact, inspection of the unfinished RHA1 genome sequence (http://www.bcgsc.ca/gc/rhodococcus) reveals a high degree of identity along the entire sequenced cosmid clone from DK17. One end of RHA1 contig 520 (107,903 bp) is over 99% identical to bases 1 to 11,401 of the DK17 sequence presented here, with the exception of a possible insertion sequence in the RHA1 sequence at position 7,486 in the DK17 sequence. RHA1 contig 399 (11,892 bp) is over 99% identical to bases 15,418 to 27,286 of the DK17 sequence. One end of RHA1 contig 479 (21,198 bp) is over 99% identical to bases 28,026 to 37,218 (the end of the cloned DK17 region) of the DK17 sequence. Since the cloned DK17 genes encoding the o-xylene catabolic pathway are located on a large catabolic plasmid (16), it is quite possible that this has promoted spread of the catabolic genes throughout the Rhodococcus population and thus shows up in the RHA1 genome sequence.
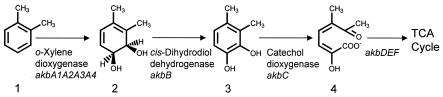
Since the focus of the present paper is on the identification of the genes for the initial dioxygenase iron sulfur protein, the genes (akbA1a, akbA2a, and akbA3) encoding the iron sulfur protein and ferredoxin components of o-xylene dioxygenase were expressed in E. coli to determine whether this enzyme is able to perform regioselective hydroxylations depending on the position of the substituent groups on the aromatic ring. With o-xylene as the substrate, two phenolic compounds were detected as products: 2,3- and 3,4-dimethylphenol (3- and 4-hydroxy-o-xylene). These mostly likely were derived from an initial o-xylene cis-3,4-dihydrodiol, a relatively unstable compound that would dehydrate readily. Also, a mutation in the akbC gene causing a nonsense codon at the 41st amino acid in the deduced protein was identified in the mutant strain DK180 by PCR amplification and sequencing (data not shown). DK180 was previously shown to be blocked at the meta-cleavage dioxygenase step for the degradation of a wide variety of alkyl-substituted hydrocarbons, including o-xylene, toluene, ethylbenzene, isopropylbenzene, and n-propyl- through n-hexylbenzene (16), implicating akbC as an essential enzyme in the degradation of alkylbenzenes by DK17. These results led us to postulate a dioxygenase-initiated pathway for o-xylene degradation in DK17 (Fig. 3). In fact, previous to the present work, a monooxygenase-initiated pathway was predominantly known for o-xylene (2, 4, 6). Although it has been proposed that a Nocardia sp. strain (9) and Rhodococcus sp. strain C125 (22) metabolize o-xylene through an initial aromatic dioxygenase to form a cis-dihydrodiol, there is no direct evidence for the presence of a dioxygenase in either strain.
FIG. 3.
Proposed pathway for early steps in the catabolism of o-xylene by Rhodococcus sp. strain DK17. The enzyme and gene designations for each step in the pathway are shown. The pathway intermediates are indicated as follows: compound 1, o-xylene; compound 2, 1,2-dihydroxy-3,4-dimethylcyclohexa-3,5-diene; compound 3, 3,4-dimethylcatechol; compound 4, 2-hydroxy-5-methyl-6-oxo-hepta-2,4-dienoate.
We previously observed that DK17 oxidized toluene at the 2,3- and 3,4 positions on the aromatic ring, resulting in two different cis-dihydrodiols and 3- and 4-methylcatechol, respectively (16). In this work we showed that the cloned o-xylene dioxygenase expressed in E. coli is capable of oxidizing ethylbenzene to 2,3- and 3,4-cis-dihydrodiols. Similar results were obtained with toluene as the substrate (data not shown). This confirms that a single dioxygenase is capable of hydroxylating aromatic compounds such as toluene and ethylbenzene at two different positions on the aromatic ring. These data suggest that o-xylene, with two alkyl side chains, fits into the active site of the enzyme only one way whereas substrates with only a single alkyl side chain can fit into the active site of the enzyme two different ways, allowing oxidation of the aromatic ring at two possible positions. The novel ability of the o-xylene dioxygenase to oxidize unusual positions on the aromatic ring of alkyl-substituted benzenes is also seen in the oxidization of m-xylene to 2,4-dimethylresorcinol and p-xylene to 2,5-dimethylhydroquinone (15). Rhodococcus sp. strain DK17 o-xylene dioxygenase thus catalyzes unique oxidations of aromatic compounds, producing products that are not seen in oxidations by other dioxygenases (13).
Acknowledgments
This work was substantially supported by a grant from the Ministry of Science and Technology, Republic of Korea, to E.K. (MG02-0301-005-1-0-0) through the 21C Frontier Microbial Genomics and Applications Center Program. Scientific exchange visits were supported in part by an International Cooperative Research Program grant (F01-2002-000-10006-0) from KOSEF. G.J.Z. acknowledges the support of the National Science Foundation through grants MCB-0078465 and CHE-9810248.
We thank C. Alan Kachel for excellent DNA sequencing assistance.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myera, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Arenghi, F. L., M. Pinti, E. Galli, and P. Barbieri. 1999. Identification of the Pseudomonas stutzeri OX1 toluene-o-xylene monooxygenase regulatory gene (touR) and of its cognate promoter. Appl. Environ. Microbiol. 65:4057-4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asturias, J. A., and K. N. Timmis. 1993. Three different 2,3-dihydroxy biphenyl-1,2-dioxygenase genes in the gram-positive polychlorobiphenyl-degrading bacterium Rhodococcus globerulus P6. J. Bacteriol. 175:4631-4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baggi, G., P. Barbieri, E. Galli, and S. Tollari. 1987. Isolation of a Pseudomonas stutzeri strain that degrades o-xylene. Appl. Environ. Microbiol. 53:2129-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell, K. S., J. C. Philp, D. W. Aw, and N. Christofi. 1998. The genus Rhodococcus. J. Appl. Microbiol. 85:195-210. [DOI] [PubMed] [Google Scholar]
- 6.Bertoni, G., M. Martino, E. Galli, and P. Barbieri. 1998. Analysis of the gene cluster encoding toluene/o-xylene monooxygenase from Pseudomonas stutzeri OX1. Appl. Environ. Microbiol. 64:3626-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finnerty, W. R. 1992. The biology and genetics of the genus Rhodococcus. Annu. Rev. Microbiol. 46:193-218. [DOI] [PubMed] [Google Scholar]
- 8.Gibson, D. T., M. Hensley, H. Yoshioka, and T. J. Mabry. 1970. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry 9:1626-1630. [DOI] [PubMed] [Google Scholar]
- 9.Gibson, D. T., V. Mahadevan, and J. F. Davey. 1974. Bacterial metabolism of para- and meta-xylene: oxidation of the aromatic ring. J. Bacteriol. 119:930-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilligan, T., H. Yamada, and T. Nagasawa. 1993. Production of S-(+)-2-phenylpropionic acid from (R,S)-2-phenylpropionitrile by the combination of nitrile hydratase and stereoselective amidase in Rhodococcus equi TG328. Appl. Microbiol. Biotechnol. 39:720-725. [DOI] [PubMed] [Google Scholar]
- 11.Haddad, S., D. M. Eby, and E. L. Neidle. 2001. Cloning and expression of the benzoate dioxygenase genes from Rhodococcus sp. strain 19070. Appl. Environ. Microbiol. 67:2507-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harayama, S., M. Kok, and E. L. Neidle. 1992. Functional and evolutionary relationships among diverse oxygenases. Annu. Rev. Microbiol. 46:565-601. [DOI] [PubMed] [Google Scholar]
- 13.Hudlicky, T., D. Gonzalez, and D. T. Gibson. 1999. Enzymatic dihydroxylation of aromatics in enantioselective synthesis: expanding asymmetric methodology. Aldrichimica Acta 32:35-62. [Google Scholar]
- 14.Kesseler, M., E. R. Dabbs, B. Averhoff, and G. Gottschalk. 1996. Studies on the isopropylbenzene 2,3-dioxygenase and the 3-isopropylcatechol 2,3-dioxygenase genes encoded by the linear plasmid of Rhodococcus erythropolis BD2. Microbiology 142:3241-3251. [DOI] [PubMed] [Google Scholar]
- 15.Kim, D., Y. Kim, J. W. Jung, G. J. Zylstra, Y. M. Kim, S.-K. Kim, and E. Kim. 2003. Regioselective oxidation of xylene isomers by Rhodococcus sp. strain DK17. FEMS Microbiol. Lett. 223:211-214. [DOI] [PubMed] [Google Scholar]
- 16.Kim, D., Y.-S. Kim, S.-K. Kim, S. W. Kim, G. J. Zylstra, Y. M. Kim, and E. Kim. 2002. Monocyclic aromatic hydrocarbon degradation by Rhodococcus sp. strain DK17. Appl. Environ. Microbiol. 68:3270-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitagawa, W., A. Suzuki, T. Hoaki, E. Masai, and M. Fukuda. 2001. Multiplicity of aromatic ring hydroxylation dioxygenase genes in a strong PCB degrader, Rhodococcus sp. strain RHA1 demonstrated by denaturing gradient gel electrophoresis. Biosci. Biotechnol. Biochem. 65:1907-1911. [DOI] [PubMed] [Google Scholar]
- 18.Martin, V. J., and W. W. Mohn. 1999. A novel aromatic-ring-hydroxylating dioxygenase from the diterpenoid-degrading bacterium Pseudomonas abietaniphila BKME-9. J. Bacteriol. 181:2675-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien, X. M., J. A. Parker, P. A. Lessard, and A. J. Sinskey. 2002. Engineering an indene bioconversion process for the production of cis-aminoindanol: a model system for the production of chiral synthons. Appl. Microbiol. Biotechnol. 59:389-399. [DOI] [PubMed] [Google Scholar]
- 20.Parales, R. E. 2003. The role of active-site residues in naphthalene dioxygenase. J. Ind. Microbiol. Biotechnol. 30:271-278. [DOI] [PubMed] [Google Scholar]
- 21.Sakai, M., K. Miyauchi, N. Kato, E. Masai, and M. Fukuda. 2003. 2-Hydroxypenta-2,4-dienoate metabolic pathway genes in a strong polychlorinated biphenyl degrader, Rhodococcus sp. strain RHA1. Appl. Environ. Microbiol. 69:427-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schraa, G., B. M. Bethe, A. R. W. vanNeerven, W. J. Van den Tweel, E. Van der Wende, and A. J. B. Zehnder. 1987. Degradation 1,2-dimethylbenzene by Corynebacterium strain C125. Antonie Leeuwenhoek 53:159-170. [DOI] [PubMed]
- 23.Smith, T. J., J. S. Lloyd, S. C. Gallagher, W. L. Fosdike, J. C. Murrell, and H. Dalton. 1999. Heterologous expression of alkene monooxygenase from Rhodococcus rhodochrous B-276. Eur. J. Biochem. 260:446-452. [DOI] [PubMed] [Google Scholar]
- 24.Stanier, R. Y., N. J. Palleroni, and M. Duodoroff. 1966. The aerobic pseudomonads: a taxonomic study. J. Gen. Microbiol. 43:159-271. [DOI] [PubMed] [Google Scholar]
- 25.Treadway, S. L., K. S. Yanagimachi, E. Lankenau, P. A. Lessard, G. Stephanopoulos, and A. J. Sinskey. 1999. Isolation and characterization of indene bioconversion genes from Rhodococcus strain I24. Appl. Microbiol. Biotechnol. 51:786-793. [DOI] [PubMed] [Google Scholar]
- 26.van der Werf, M. J., C. van der Ven, F. Barbirato, M. H. Eppink, J. A. de Bont, and W. J. van Berkel. 1999. Stereoselective carveol dehydrogenase from Rhodococcus erythropolis DCL14. A novel nicotinoprotein belonging to the short chain dehydrogenase/reductase superfamily. J. Biol. Chem. 274:26296-26304. [DOI] [PubMed] [Google Scholar]
- 27.Wang, Y., J. Garnon, D. Labbe, H. Bergeron, and P. C. Lau. 1995. Sequence and expression of the bpdC1C2BADE genes involved in the initial steps of biphenyl/chlorobiphenyl degradation by Rhodococcus sp. M5. Gene 164:117-122. [DOI] [PubMed] [Google Scholar]
- 28.Warhurst, A. M., and C. A. Fewson. 1994. Biotransformations catalyzed by the genus Rhodococcus. Crit. Rev. Biotechnol. 14:29-73. [DOI] [PubMed] [Google Scholar]
- 29.Zylstra, G. J. 1994. Molecular analysis of aromatic hydrocarbon degradation, p. 83-115. In S. J. Garte (ed.), Molecular environmental biology. Lewis Publishers, Boca Raton, Fla.
- 30.Zylstra, G. J., W. R. McCombie, D. T. Gibson, and B. A. Finette. 1988. Toluene degradation by Pseudomonas putida: genetic organization of the tod operon. Appl. Environ. Microbiol. 54:1498-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]