Abstract
We have developed a simple method for single-step cloning of any PCR product into a plasmid. A novel selection principle has been applied, in which activation of a drug selection marker is achieved following homologous recombination. In this method a DNA fragment is amplified by PCR with standard oligonucleotides that contain flanking tails derived from the host plasmid and the complete λPR or rrnA1 promoter regions. The resulting PCR product is then electroporated into an Escherichia coli strain harboring both the phage λ Red functions and the host plasmid. Upon homologous recombination of the PCR fragment into the plasmid, expression of a drug selection marker is fully induced due to restoration of its truncated promoter, thus allowing appropriate selection. Recombinant plasmid vectors encoding β-galactosidase and neomycin phosphotransferase were constructed by using this method in two well-known Red systems. This cloning strategy significantly reduces both the time and costs associated with cloning procedures.
Cloning of DNA in Escherichia coli plasmids for both bacterial and eukaryotic studies is an invaluable tool. Current molecular biology techniques for cloning genes into plasmids are mainly based on the generation of gene segments by restriction by endonucleases and subsequent joining to a linearized plasmid DNA vector by DNA ligase. These techniques usually require multiple steps such as preparation and restriction of the vector and the gene of interest, purification of the products, ligation, and electroporation. Often, a desired restriction site is either absent or occurs in an unwanted site, and therefore preliminary manipulations are needed as well.
In recent years, a novel approach for molecular cloning based on bacteriophage-encoded recombination functions has been developed (3, 9, 10). This approach, termed “recombineering” (2), involves the use of PCR products or even synthetic oligonucleotides carrying 35 to 70 nucleotides of flanking homology to a target vector as substrates for recombination (3, 9, 10). The use of homologous recombination for in vivo gene cloning overrides the need for restriction and ligation enzymes with as much precision and efficiency (2). Recombineering is based on either the phage λ Red or the RecET recombination functions (6, 8). The λ genes involved in Red recombination are exo, bet, and gam. The exo gene product has 5′ to 3′ exonuclease activity, and the bet gene product is a single-strand DNA binding protein that promotes annealing. The gam gene product inhibits the RecBCD nuclease, thus preventing the degradation of linear DNA fragments.
Zhang and colleagues (10) developed an elegant use of phage homologous recombination functions for cloning any gene of interest into a plasmid. In their method, a plasmid vector containing a backbone of selectable drug marker and an active origin of replication is PCR amplified. The oligonucleotides for this PCR contain in their 5′ ends homology regions that are chosen to define the exact boundaries of the DNA region to be cloned. The chosen DNA region, which is either present in the bacteria or coelectroporated along with the linear plasmid, is inserted into the plasmid backbone by homologous recombination. Gap repair of the electroporated linear plasmid circularizes the plasmid, thus allowing selection for the drug marker. This cloning strategy is straightforward and works very well in subcloning experiments. It excludes the requirement for tedious DNA purification procedures, the required presence of convenient restriction sites, or the mutational risk of PCR. Yet this method requires purification of genomic DNA for cloning genes that are not already cloned on plasmids or bacterial artificial chromosomes or present on the E. coli chromosome. In such cases, and especially when large genomes are used for cloning, the efficiency, as well as simplicity, is insufficient. Another drawback of this cloning procedure is the high background resulting from self-circularization that occurs when repeats as short as six bases are present in the vector (10).
In this paper, we describe a reliable and simpler strategy for cloning by recombineering any gene of interest that can be amplified by PCR.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. Strain DY378 was described by Yu et al. (9).
TABLE 1.
Compilation of bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5α | F− φ80lacZΔM15Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK− mK+) gal-phoA supE44 λ−thi-1 gyrA96 relA1 | www.invitrogen.com |
| DY378 | W3110 λcI857 Δ(cro-bioA) | 9 |
| Plasmids | ||
| pACYC184 | p15A origin of replication (Tetr Chlr) | 1 |
| pFull-cat | pACYC184 derived; PPrcat (Tetr Chlr) | This study |
| pTrun-cat | pACYC184 derived; PPrtruncatedcat (Tetr Chllow) | This study |
| pKD46 | ParaB γ β exo (Ampr) | 3 |
The plasmid pTrun-cat is a derivative of pACYC184 and was constructed as follows: the pACYC184 plasmid was used as a template in amplification reactions by using Vent polymerase (New England Biolabs, Beverly, Mass.) and the primers Tru-cat-for and NcoI-cat-rev (Table 2) to amplify the chloramphenicol acetyltransferase (cat) gene. The blunt PCR product, encoding the cat gene under the control of a truncated λPR promoter, was cut with NcoI and ligated into an NcoI- and XmnI-linearized pACYC184 plasmid to yield plasmid pTrun-cat. The same cloning steps were repeated with the oligonucleotides Full-cat-for and NcoI-cat-rev to yield the plasmid pFull-cat which encodes cat under the control of the full λPR promoter.
TABLE 2.
List of oligonucleotides used in this study
| Oligonucleotide | Sequencea |
|---|---|
| Tru-cat-for | 5′-tatagaattcttacctctggcggtgataatggttgcatgtactaaggaggttgtatggagaaaaaaatcactgg-3′ |
| Full-cat-for | 5′-tatagaattcgtgttgactattttacctctggcggtgataatggttgcatgtactaaggaggttgtatggagaaaaaatcaactgg-3′ |
| NcoI-cat-rev | 5′-cttaccatggttacgccccgccctgcc-3′ |
| Km-for-full | 5′-ctccatacaacctccttagtacatgcaaccattatcaccgccagaggtaaAATAGTCAACACtgtaggctggagctgctt-3′ |
| Km-for-rrnA1 | 5′-tccagtgatttttttctccatacaacctccttagtacatgcaaccattatAGGGAGTTATTCCGGCCTGACAAGTgtaggctggagctgctt-3′ |
| Km-rev-790 | 5′-ttgccgcggccctctcacttccctgttaagtatcttcctggcatcttccaatgggaattagccatggtc-3′ |
| lacZ-for-full | 5′-ctccatacaacctccttagtacatgcaaccattatcaccgccagaggtaaAATAGTCAACACcgttggccgattcatta-3′ |
| lacZ-rev | 5′-ttgccgcggccctctcacttccctgttaagtatcttcctggcatcttccagagcttgacggggaaag-3′ |
| Seq1-pos670-rev | 5′-CATATTCTGCTGACGCA-3′ |
| seq2-pos900-rev | 5′-gatttgagcgtcagatttcg-3′ |
| seq3-pos161-for | 5′-tctttacgatgccattg-3′ |
| T7 | 5′-gtaatacgactcactatagggc-3′ |
Italics indicate flanking homology to plasmid. Boldface indicates completion of either λPR or rrnA1 promoter.
Induction of recombination functions and preparation of electrocompetent cells.
E. coli DH5α (Invitrogen) electrocompetent cells were prepared as described by Datsenko and Wanner (3) with minor modifications. Briefly, DH5α cells harboring both the pKD46 and pTrun-cat plasmids (DH5α/pKD46/pTrun-cat) were grown overnight at 30°C. Cells were then diluted 20-fold into 1 liter of Luria-Bertani (LB) medium containing 10 mg of tetracycline per liter and 100 mg of ampicillin per liter. When an optical density at 600 nm of ∼0.4 was reached, l-arabinose was added to a final concentration of 1 mM in order to induce recombination functions encoded by plasmid pKD46. Following a 1-h induction, cells were washed three times with ice-cold 12% glycerol and resuspended in 2 ml of ice-cold 12% glycerol.
DY378 electrocompetent cells were prepared as described by Yu et al. (9) with minor alterations. Briefly, DY378 cells harboring the pTrun-cat plasmid were grown overnight at 30°C, diluted 20-fold into a 2-liter flask containing 1 liter of LB medium with 10 mg of tetracycline per liter and further grown to an optical density at 600 nm of ∼0.6. The induction of recombination functions was performed by placing the flask in a water bath at 42°C with shaking for 15 min. Immediately after the induction, the flask was cooled on ice for 10 min. A control culture was cooled on ice without prior heat induction. Cells were then washed three times with ice-cold 12% glycerol and resuspended in 2 ml of ice-cold 12% glycerol. For both DH5α and DY378 electrocompetent cells, aliquots of 50 μl were either immediately used or were frozen and stored at −80°C until use.
PCR preparation of linear cassettes.
PCR conditions were as follows: 5 min at 94°C and 3 cycles of 40 s at 94°C, 40 s at 50°C, and 90 s at 72°C, followed by 30 cycles of 40 s at 94°C, 40 s at 65°C, and 90 s at 72°C. The first three cycles were performed under nonstringent conditions (i.e., low annealing temperature) to allow specific and nonspecific initial amplification of the DNA. Following that, 30 cycles of PCR were performed under more stringent conditions. ReadyMix Taq PCR (Sigma-Aldrich, Rehovot, Israel) was used for all PCRs unless otherwise stated.
All oligonucleotides are listed in Table 2. Oligonucleotides Km-for-full and Km-rev-790 were used to amplify the Tn5 neomycin phosphotransferase (nptII) gene, yielding λPR-nptII. Oligonucleotides Km-for-rrnA1 and Km-rev-790 were also used to amplify nptII, yielding rrnA1-nptII. The template for the nptII gene was a chromosomal DNA to which a pKD4 fragment encoding nptII was integrated. Oligonucleotides lacZ-for-full and lacZ-rev were used to amplify the lacZ gene, yielding λPR-lacZ. The plasmid pBluescript II KS(+) (Stratagene) served as a template for λPR-lacZ. PCR mixtures were purified with a PCR nucleospin extract kit (Macherey-Nagel, Düren, Germany) and eluted in double-distilled water.
Electroporation reactions.
Purified PCR products, corresponding to 50 to 200 ng of DNA in 1 to 5 μl, were mixed in the electroporation cuvette with 50 μl of electrocompetent cells. Electroporation was conducted by using a Bio-Rad pulser in 2-mm cuvettes according to the manufacturer's instructions (2,500 kV, 200 Ω, 25 μF). Following electroporation, 1 ml of SOC medium was added to each cuvette. Cells were incubated for 1 h at 30°C (DY378/pTrun-cat) or 37°C (DH5α/pKD46/pTrun-cat) and then 200 μl from each sample was plated on LB agar plates containing 120 mg of chloramphenicol per liter.
Determination of the percentage of colonies encoding the gene of interest.
The frequency of β-galactosidase-expressing colonies obtained following electroporation of the λPR-lacZ fragment was determined as follows: 200 μl of electroporation reaction mixtures with either λPR-lacZ or λPR-nptII (negative control) was plated on LB agar containing 120 mg of chloramphenicol per liter, 1 mM isopropyl-β-d-thiogalactoside (IPTG) (Sigma, Rehovot, Israel), and 60 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal) (Merck, Darmstadt, Germany) per liter. Plates were incubated overnight at 37°C, and the percentage of blue colonies from total colonies was determined.
The frequency of nptII-expressing colonies following electroporation of either λPR-nptII or rrnA1-nptII fragments was determined as follows: colonies selected on chloramphenicol (120 mg/liter) plates were individually picked and streaked on LB agar plates containing 40 mg of kanamycin per liter. Plates were incubated overnight, and the percentage of resistant colonies from total streaked colonies was determined.
Sequencing.
Plasmids were isolated by standard procedures from representative colonies (DY378 and DH5α/pKD46) obtained after λPR-lacZ electroporation. These plasmids were used as templates for PCRs. PCRs were performed to amplify the upstream region of the λPR-lacZ by using the forward primer seq3-pos161-for corresponding to the plasmid and the reverse primer T7 corresponding to the λPR-lacZ (Table 2). The PCR fragments obtained were purified from 1% agarose gel and sequenced on an ABI Prism genetic analyzer (Applied Biosystems) by using oligonucleotide seq3-pos161-for as a primer (Table 2).
RESULTS
The cloning strategy.
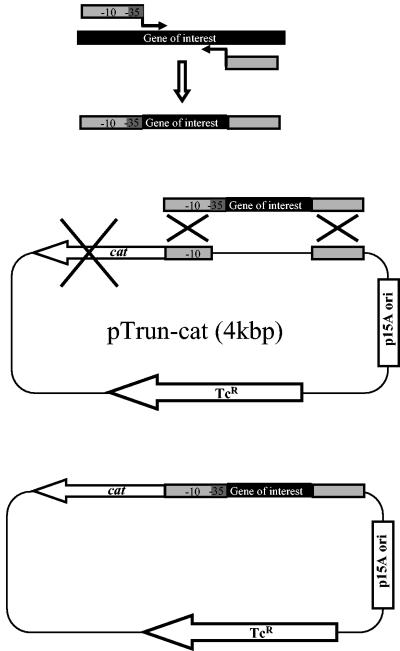
Our aim was to construct a system for rapidly cloning any desired DNA fragment into a plasmid. The system was designed to include an E. coli strain harboring the phage λ Red functions and a target plasmid, which encodes cat under the control of an upstream truncated promoter. The cloning strategy is detailed in Fig. 1. In essence, the gene of interest is amplified with standard oligonucleotides that contain tails derived from the target plasmid. The 5′ end of the upstream oligonucleotide contains the complementing −35 box of the truncated promoter. This box is critical for the binding of RNA polymerase via the σ70 subunit for appropriate transcription (5). Upon targeted homologous recombination of the PCR fragment into the plasmid and complementation of the truncated promoter, expression of the cat gene is greatly enhanced, allowing selection by chloramphenicol of colonies that contain the plasmid with the gene of interest.
FIG. 1.
Diagram of the cloning strategy. (Top) The gene of interest is amplified by PCR by using the indicated upstream (left) and downstream (right) oligonucleotides. The upstream oligonucleotide contains a homology tail to pTrun-cat (light gray) followed by complementation of a −35 box of the λPR or rrnA1 promoter (dark gray) and a PCR primer (right arrow). The downstream oligonucleotide is composed of a pTrun-cat homology tail (light gray) followed by a PCR primer (left arrow). (Middle) Following electroporation and targeted homologous recombination of the PCR fragment, the desired DNA is cloned and complements the truncated promoter that drives cat expression. (Bottom) Upon homologous recombination of the PCR fragment into the plasmid, the cat gene is fully activated and desired colonies are selected on chloramphenicol. The −10 box and the −35 box of the promoter are indicated. Tcr, tetracycline resistance gene; p15A ori, origin of replication.
Determining the optimal chloramphenicol concentration for selection.
To achieve our goal, we chose to use plasmid pACYC184 which encodes the tetracycline resistance gene and the cat gene under constitutive promoters (1). The endogenous cat promoter was replaced with a truncated λPR promoter or with the full λPR promoter. The constructed plasmids were designated pTrun-cat and pFull-cat, respectively. In order to determine the chloramphenicol concentration for use in selection, we compared the resistance of a DH5α strain harboring pFull-cat versus pTrun-cat to elevated concentrations of chloramphenicol. We observed that DH5α/pFull-cat could still grow at a concentration of 400 mg of chloramphenicol per liter in liquid and on solid LB medium after overnight incubation, whereas DH5α/pTrun-cat was already inhibited at 50 mg of chloramphenicol per liter under the same conditions. We chose to use a selective concentration range of 100 to 150 mg of chloramphenicol per liter, which was later found to be optimal for selecting colonies that harbor the target insertion while maintaining a null background of pTrun-cat-harboring bacteria (data not shown). This was probably due to the following reason: a targeted homologous recombination event, prior to chloramphenicol selection, forms a mixture of plasmids containing mostly pTrun-cat and only a minority of plasmids with a fully active cat promoter in the desired bacteria. Therefore, these bacteria are more resistant than the parental DH5α/pTrun-cat bacteria but more sensitive than DH5α bacteria harboring pFull-cat.
In vivo cloning of PCR fragments into the plasmid by using DH5α/pKD46/pTrun-cat.
To enable efficient homologous recombination, we first used the previously described Red helper plasmid pKD46 (3). The Red enzymes encoded by the pKD46 plasmid are under an arabinose-inducible promoter. Transformants carrying both pKD46 and pTrun-cat plasmids were made electrocompetent as described in Materials and Methods. In order to test the cloning system, the Tn5 neomycin phosphotransferase (nptII), which confers kanamycin resistance, and the lacZ reporter gene were amplified with their endogenous promoters. These genes were chosen in order to allow an easy verification of targeted homologous recombination events. Oligonucleotides were designed as depicted in Fig. 1. The 5′ end of the upstream oligonucleotides used for amplifying the gene of interest contained 50 nucleotides (nt) of homologous sequence of both the cat gene and the truncated promoter followed by the −35 box of the λPR promoter or rrnA1 promoter sequence. A 17- to 20-nt primer of the gene of interest was designed at the 3′ end (Table 2, oligonucleotides Km-for-full, Km-for-rrnA1, and lacZ-for-full). The downstream oligonucleotides contained a 50-nt sequence homologous to the pTru-cat plasmid followed by a 17- to 20-nt primer for amplifying the gene of interest (Table 2, oligonucleotides Km-rev-790 and lacZ-rev). The amplified DNA of the nptII gene with the full upstream λPR promoter or an alternative promoter, rrnA1, were named λPR-nptII and rrnA1-nptII, respectively. The lacZ PCR fragment with the full λPR promoter was designated λPR-lacZ. We electroporated 50 to 200 ng of the products amplified by PCR into DH5α/pKD46/pTrun-cat. Following electroporation, cells were spread on LB agar plates containing 120 mg of chloramphenicol per liter and were incubated overnight at 37°C. Incubation at 37°C greatly reduces pKD46 presence as this plasmid has a temperature-sensitive origin of replication (3). The recombination functions are thus eliminated, and construct stability is maintained. Table 3 summarizes the results obtained by using this simple protocol. We obtained 350 chloramphenicol-resistant colonies per λPR-nptII electroporation reaction. Of these colonies, 82% were also kanamycin resistant, thus containing the correct insertion of the nptII gene (Table 3). Similar results were obtained when electroporation of λPR-lacZ was performed. Of 230 colonies that were resistant to chloramphenicol, 91% turned blue on LB plates containing X-Gal, IPTG, and 120 mg of chloramphenicol per ml, as shown in Fig. 2, indicating a very high cloning efficiency. We further tested this cloning system by amplifying the same nptII gene by using a different upstream oligonucleotide, which converted the truncated promoter into a full rrnA1 promoter (rrnA1-nptII). In this case, too, sufficient cloning efficiency was observed, as shown in Table 3. All the above procedures were repeated at least once more with comparable results.
TABLE 3.
Summary of results
| PCR inserta | Red source | Results of electroporation
|
|||
|---|---|---|---|---|---|
| Fresh aliquots
|
Frozen aliquots
|
||||
| No. of colonies obtainedb | % Correct insertionc | No. of colonies obtainedb | % Correct insertionc | ||
| λPR-nptII | DY378 | 550 | 82 | 430 | 86 |
| λPR-nptII | DH5α/pKD46 | 350 | 88 | 315 | 90 |
| rrnA1-nptII | DY378 | 146 | 92 | 172 | 84 |
| rrnA1-nptII | DH5α/pKD46 | 140 | 88 | 133 | 90 |
| λPR-lacZ | DY378 | 224 | ND | 196 | ND |
| λPR-lacZ | DH5α/pKD46 | 230 | 91 | 220 | 88 |
λPR-nptII encodes nptII flanked by pTrun-cat homologies containing complementation of the λPR promoter. rrnA1-nptII encodes nptII flanked by pTrun-cat homologies containing complementation of the rrnA1 promoter. λPR-lacZ encodes β-galactosidase flanked by pTrun-cat homologies containing complementation of the λPR promoter.
One-fifth of each electroporation reaction mixture was plated and counted. Results represent the number of chloramphenicol-resistant colonies obtained per reaction mixture. Standard deviations of multiple platings from the same sample were less than 10% in all cases.
At least 50 obtained colonies electroporated with either λPR-nptII or rrnA1-nptII were tested for kanamycin resistance as described in Materials and Methods. The percentage of kanamycin-resistant colonies was determined accordingly. DH5α/pKD46/pTrun-cat colonies electroporated with λPR-lacZ were plated on LB agar containing chloramphenicol, X-Gal, and IPTG, and the percentage of blue colonies was determined from total colonies (Fig. 2). The percentage of DY378/pTrun-cat blue colonies was not determined (ND) due to endogenous lacZ background activity.
FIG. 2.
About 90% of the colonies selected on chloramphenicol contain the gene of interest. The λPR-lacZ fragment encoding lacZ (left) or λPR-nptII fragment encoding Tn5 neomycin phosphotransferase (right) were electroporated into DH5α/pKD46/pTrun-cat electrocompetent cells. SOC medium was added for 1 h, and bacteria were then spread on LB plates containing chloramphenicol, X-Gal, and IPTG. Representative plates were pictured after overnight incubation at 37°C, and the percentage of blue colonies was determined as described in Material and Methods.
In vivo cloning of PCR fragments into the plasmid by using DY378/pTrun-cat.
The cloning system was also tested by using another well-characterized Red function-containing bacterium, strain DY378 (9). The DY378 strain harbors a prophage encoding the λ Red genes gam, bet, and exo. These genes in the prophage are under the control of a temperature-sensitive repressor and are expressed at 42°C but remain repressed at 32°C. Electroporation was performed as described in Materials and Methods. Results obtained with this strain were similar to those obtained with the DH5α/pKD46 strain (Table 3). Uninduced bacteria did not yield resistant colonies, confirming the involvement of the Red recombination functions in this cloning system. Most importantly, in both DY378 and DH5α strains, no chloramphenicol-resistant colonies were detected in reactions where the PCR fragment was replaced by the corresponding amplifying oligonucleotides or by water.
Sequence analysis of the cloned products.
A primer corresponding to the plasmid backbone and a primer corresponding to the gene of interest were used to amplify the insertion regions. These PCRs that were performed on representative colonies from λPR-nptII, rrnA1-nptII, and λPR-lacZ electroporations of both the DY378 and DH5α strains confirmed the presence of the insert in the plasmid (data not shown). In addition, all (five of five) PCR products that were subjected to sequence analysis confirmed that the intended target DNA was fully inserted into the plasmid without any mutational errors.
Testing efficiency of frozen aliquots.
Frozen electrocompetent cells in glycerol lose some potency compared to fresh cells (7). We wanted to examine whether such stocks harboring recombination proteins can still be used efficiently for our procedures. Therefore, electrocompetent cells harboring pTrun-cat were prepared, and fresh versus frozen (−80°C) stocks were tested for efficiency. The frozen stocks demonstrated only a slight decrease in electrotransformation efficiency and a similar insertion percentage compared to fresh stocks (Table 3). We thus conclude that electrocompetent cells harboring recombination functions can be prepared in advance for routine use with the described cloning procedures.
DISCUSSION
The cloning system that we have developed is based on general principles of recombineering (2). However, we have demonstrated for the first time restoration of gene function by using homologous recombination. We introduced this novel principle for driving the expression of a drug marker gene that is required for efficient selection following targeted homologous recombination. Our cloning system has many advantages over currently used molecular cloning techniques for protein expression. The only components of the system are electrocompetent cells harboring recombination functions and a target plasmid. These cells can be prepared and stored frozen for long periods of time. Designing the oligonucleotides for use in this system is extremely easy as they are based on the presence of fixed sequences at their 5′ ends, joined with the primers used to amplify the gene of interest.
We demonstrated efficient expression of the β-galactosidase gene product and of the nptII gene that were cloned with their endogenous promoters (Fig. 2 and Table 3). Our cloning strategy could be further applied to any parallel expression system simply by transferring the cat gene with its truncated promoter to any expression plasmid (e.g., prokaryotic and eukaryotic expression vectors). Alternatively, a prokaryotic or eukaryotic promoter can be cloned into the pTrun-cat plasmid, converting it to an expression vector. In addition, the principle of using a truncated promoter, which confers very low chloramphenicol resistance (50 mg of chloramphenicol per liter) versus a full promoter, which confers high chloramphenicol resistance (400 mg of chloramphenicol per liter), can be further utilized. For example, constructing a promoterless cat gene (completely sensitive to chloramphenicol) and using the selection principle with a truncated promoter on a low chloramphenicol concentration may offer the advantage of using the obtained construct for a second cloning step on a higher chloramphenicol concentration. This way one can engineer fusion genes, site-directed mutagenesis, and similar constructs by using only two consecutive steps.
The Red recombination functions are known to cause multimerization in colE1-type plasmids in the presence of Gam (2, 4). In addition, when this cloning system with pTrun-cat already established in the bacteria is used, a mixture containing parental and recombinant plasmids is formed despite selection for bacteria highly resistant to chloramphenicol. The parental pTrun-cat plasmids are not eliminated because a negative selection force against them is absent. Both phenomena are acceptable when the cloning system is used for protein expression and for related purposes. Our strategy could also be applied for obtaining pure recombinant plasmids simply by using low-copy-number vectors, like pSC101 derivatives, and/or performing coelectroporation of the target plasmid along with the PCR insert, thus avoiding multimerization (2).
Recombineering is still not accepted as the method of choice for plasmid construction, despite its enormous potential. In this paper we present new principles for utilizing recombineering as a simple and straightforward gene expression methodology.
Acknowledgments
We thank Richard Myers, Ami Tamir, and Motti Gerlic for critical reading of the manuscript and for helpful suggestions.
U.Q. was supported by a Kreitman Foundation Fellowship.
REFERENCES
- 1.Chang, A. C., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Court, D. L., J. A. Sawitzke, and L. C. Thomason. 2002. Genetic engineering using homologous recombination. Annu. Rev. Genet. 36:361-388. [DOI] [PubMed] [Google Scholar]
- 3.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feiss, M., D. A. Siegele, C. F. Rudolph, and S. Frackman. 1982. Cosmid DNA packaging in vivo. Gene 17:123-130. [DOI] [PubMed] [Google Scholar]
- 5.Gralla, J. D. 1991. Transcriptional control—lessons from an E. coli promoter data base. Cell 66:415-418. [DOI] [PubMed] [Google Scholar]
- 6.Kolodner, R., S. D. Hall, and C. Luisi-DeLuca. 1994. Homologous pairing proteins encoded by the Escherichia coli recE and recT genes. Mol. Microbiol. 11:23-30. [DOI] [PubMed] [Google Scholar]
- 7.Michelsen, B. K. 1995. Transformation of Escherichia coli increases 260-fold upon inactivation of T4 DNA ligase. Anal. Biochem. 225:172-174. [DOI] [PubMed] [Google Scholar]
- 8.Poteete, A. R. 2001. What makes the bacteriophage lambda Red system useful for genetic engineering: molecular mechanism and biological function. FEMS Microbiol. Lett. 201:9-14. [DOI] [PubMed] [Google Scholar]
- 9.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang, Y., J. P. Muyrers, G. Testa, and A. F. Stewart. 2000. DNA cloning by homologous recombination in Escherichia coli. Nat. Biotechnol. 18:1314-1317. [DOI] [PubMed] [Google Scholar]