Abstract
Campylobacter jejuni is prevalent in poultry, but the effect of combined refrigerated and frozen storage on its survival, conditions relevant to poultry processing and storage, has not been evaluated. Therefore, the effects of refrigeration at 4°C, freezing at −20°C, and a combination of refrigeration and freezing on the survival of C. jejuni in ground chicken and on chicken skin were examined. Samples were enumerated using tryptic soy agar containing sheep's blood and modified cefoperazone charcoal deoxycholate agar. Refrigerated storage alone for 3 to 7 days produced a reduction in cell counts of 0.34 to 0.81 log10 CFU/g in ground chicken and a reduction in cell counts of 0.31 to 0.63 log10 CFU/g on chicken skin. Declines were comparable for each sample type using either plating medium. Frozen storage, alone and with prerefrigeration, produced a reduction in cell counts of 0.56 to 1.57 log10 CFU/g in ground chicken and a reduction in cell counts of 1.38 to 3.39 log10 CFU/g on chicken skin over a 2-week period. The recovery of C. jejuni following freezing was similar on both plating media. The survival following frozen storage was greater in ground chicken than on chicken skin with or without prerefrigeration. Cell counts after freezing were lower on chicken skin samples that had been prerefrigerated for 7 days than in those that had been prerefrigerated for 0, 1, or 3 days. This was not observed for ground chicken samples, possibly due to their composition. C. jejuni survived storage at 4 and −20°C with either sample type. This study indicates that, individually or in combination, refrigeration and freezing are not a substitute for safe handling and proper cooking of poultry.
Campylobacter species are found in the intestinal tracts of many birds and mammals used for food production, including sheep, cattle, pigs, and poultry (23), and are among the most frequently reported causes of bacterial gastroenteritis in humans in many developed (7) and developing (19) countries around the world. Most Campylobacter infections in developed countries are sporadic and may result from contact with pets, the consumption of untreated water, or the consumption of raw milk or raw or undercooked poultry (7). Due to their fastidious growth requirements and absence of growth below 30°C, Campylobacter spp. do not multiply in foods held at room temperature, and infection by Campylobacter spp. most often results from consuming foods cross-contaminated in the kitchen by raw meats (23).
An estimated 2.5 million cases of Campylobacter infection occur each year in the United States, and 80% of these cases have been found to be the result of foodborne transmission (16). Epidemiological studies indicate a significant association between Campylobacter infection in humans and the handling and eating of raw or undercooked poultry (14). A U.S. study determined that exposure to contaminated poultry constituted 50% of all cases of Campylobacter infection (7). Broiler chickens contaminated with Campylobacter may have a contamination rate as high as 60% at levels as high as 1.5 × 106 CFU/fresh bird (23), and Campylobacter contamination as high as 103 to 106 CFU/g on fresh chicken has been documented (7). A survey of raw product pathogen prevalence indicated that 88.2% of broiler chickens and 59.8% of ground chicken samples harbor Campylobacter jejuni and/or Campylobacter coli (22). In another study, 89% of chicken neck skin samples and 75% of chicken subcutaneous samples were shown to contain Campylobacter (14). A large percentage of fresh and frozen poultry have been found to harbor Campylobacter, generally (14).
Refrigeration and freezing are interventions used in the control of bacterial growth in foods. Campylobacters have an optimal growth temperature range of 37 to 42°C and do not grow below 30°C (15), but C. jejuni has been shown to display physiological activity at 4°C (10) and Campylobacter can survive in water for several weeks at 4°C (23). Counts of C. jejuni on poultry carcasses have been shown to decline during refrigerated storage (29), but studies have shown that a portion of the C. jejuni population survives on raw or cooked poultry samples during refrigeration (1, 3, 5, 6, 15, 26). Freezing also decreases counts of C. jejuni and reductions of 1 to 3 log10 in flesh foods stored at −15 to −20°C have been observed (29), but C. jejuni does survive during frozen storage in poultry samples (2, 15, 26). Thus, although microbial growth is arrested during freezing due to reduced water activity and lowered temperature (27) and a portion of the microflora may be killed due to these factors, a fraction may survive or be sublethally injured.
The presence of viable C. jejuni after refrigerated and frozen storage is significant, given that ingestion of only 500 C. jejuni cells has resulted in illness in human experimental infections (24). The survival of C. jejuni after refrigerated or frozen storage in an ambient atmosphere has been studied by using various chicken skin and chicken meat preparations. Chicken skin preparations studied include nonsterile raw skin stored at 4°C (5), gamma-irradiated raw skin stored at 4 and −20°C (26), and UV-irradiated raw skin stored at 4 and −20°C (15). Chicken meat preparations studied include chicken mince sterilized by autoclaving and stored at 5°C (6), ground chicken meat sterilized by autoclaving and stored at 4°C (3), and nonsterile comminuted chicken meat stored at 5°C (1) and −20°C (2).
The survival of C. jejuni during storage under either refrigeration or freezing conditions has been evaluated using various samples, but the effect of combined refrigeration and freezing, conditions to which poultry products would be exposed during processing and subsequent storage by the consumer, is poorly understood and quantitative data are lacking. In addition, the type of sample tested under various cold storage conditions has varied among studies. The survival of C. jejuni inoculated on raw chicken skin and in raw ground chicken sterilized by gamma irradiation and stored under the conditions presented in this study has not been previously evaluated.
During processing, transport, and storage by the consumer, poultry products are held at refrigeration and freezing temperatures for time periods of various durations. Therefore, this study examines the effect of refrigeration, freezing, and combined refrigeration and freezing on the survival of C. jejuni on gamma-irradiated raw chicken skin and in raw ground chicken samples. The samples were stored for various time intervals under the following conditions: (i) refrigeration at 4°C, (ii) freezing at −20°C, and (iii) refrigeration at 4°C followed by freezing at −20°C. The information obtained will help to evaluate the effect of these storage conditions on the survival of C. jejuni in poultry products.
MATERIALS AND METHODS
Preparation of bacterial cultures and media.
The three strains used in this study were obtained from the culture collection of the Microbial Food Safety Research Unit of the USDA Eastern Regional Research Center (ERRC), Wyndmoor, Pa. The C. jejuni strains used were MFS #CJ1 (ovine), MFS #CJ2 (bovine), and MFS #CJ3 (poultry). Frozen stock cultures were prepared by culturing in brain heart infusion broth (Beckton Dickinson Microbiology Systems, Sparks, Md.) under microaerobic conditions for 24 h in a 42°C incubator (model 317512; Hotpack Corp., Philadelphia, Pa.). Under microscopic observation, all strains showed high motility and spiral rod morphology (11, 13, 21, 25). The strains were further confirmed as Campylobacter by biochemical tests (catalase and oxidase) and growth under microaerophilic conditions at 37 to 42°C but not at 25°C (11, 13, 21, 25). The strains were confirmed as the C. jejuni species by hippurate hydrolysis (9, 25). The cells were harvested, and the pellet was resuspended in 10 ml of brain heart infusion broth containing 10% glycerol. Then, 200-μl portions were aliquoted in cryogenic vials and stored frozen at −70°C. Microaerobic conditions were achieved using BBL Gas Pak 100 or BBL Gas Pak 150 anaerobic jars (Becton Dickinson Microbiology Systems) with BBL CampyPak Plus microaerophilic system envelopes. The frozen stock cultures were plated onto tryptic soy agar containing 5% sheep's blood (TSAB; Gibson Laboratories Inc., Lexington, Ky.), and the plates were incubated at 42°C for 24 h under microaerobic conditions. Modified cefoperazone charcoal deoxycholate agar (mCCDA; Oxoid, Hampshire, England) was prepared according to the manufacturer's instructions. Peptone water was prepared as a 0.1% solution of Bacto-Peptone (Beckton Dickinson Microbiology Systems).
Preparation of ground chicken samples and chicken skin medallions.
Two batches of boneless skinless chicken breasts and chicken leg-thigh pieces with skin from the same processor were purchased at a local retail market over a 1-year period at intervals of 6 months. Each batch was processed as follows: the chicken breast was ground at ERRC with a commercial meat grinder (model MG8912; Univex, Salem, N.H.) and divided into 10-g portions. The chicken skin was removed from the leg-thigh pieces, and 10-cm2 medallions weighing approximately 2.5 g were cut using a stainless steel coring tool. The ground chicken portions and chicken skin medallions were double sealed in vacuum packaging bags and irradiated at −30°C (42-kGy dose) in a self-contained cesium irradiator (Lockheed Georgia Company, Marietta, Ga.) located at the ERRC. The samples from each batch were stored at −20°C in a constant-temperature freezer for a maximum period of 6 months. The sterility of the samples was tested during the course of the experiments by dilution in peptone water and plating on TSAB.
Determination of pH of samples.
Both sample types were thawed following storage at −20°C, and the pH was determined prior to inoculation by using a flat-surface electrode (model OR8135BN; Orion Research, Beverly, Mass.).
Inoculation of ground chicken samples and chicken skin medallions.
Cells of each strain of C. jejuni were removed from surface-plated TSAB plates using Pur-Wraps sterile wood cotton-tipped applicator swabs (Hardwood Products Company LLC, Guilford, Maine) wetted with sterile 0.1% peptone water. The bacteria were suspended in 10 ml of 0.1% peptone water, and the optical density at 600 nm was determined for each suspension. Based on the optical density, portions of each suspension were combined to give a cocktail containing equivalent numbers of CFU for each strain at a final concentration of approximately 109 CFU/ml. This cocktail was used to inoculate the ground chicken and chicken skin samples. Irradiated samples were thawed and placed in sterile petri dishes. The weight of each ground chicken and chicken skin sample was determined and recorded. The ground chicken samples and chicken skin medallions were then placed into Whirl Pak (Nasco, Fort Atkinson, Wis.) puncture-proof bags (55 oz [B01195] and 18 oz [B1065], respectively). The samples were inoculated with an aliquot of the three-strain cocktail of C. jejuni to give a final concentration of approximately 108 CFU/g, based on the weight of the sample. The inoculation was performed in the sampling bag. The inoculum (approximately 200 μl for the chicken skin samples and 800 μl for the ground chicken samples) was added to the samples with the aid of a pipette. The ground chicken samples were mixed by hand to fully incorporate the bacterial culture, and the 10-cm2 chicken skin medallions were immersed in the bacterial culture. The full quantity of the inoculum was retained in the sampling bags for both sample types. Each sample bag was closed using successive folds across the width of the bag so that excess air was removed, and the bag was held closed with the integral wire fastener to prevent drying of the sample. A separate ground chicken sample and a chicken skin medallion were inoculated for testing at each sampling time point. For each storage condition, sets of the ground chicken samples and chicken skin medallions were inoculated and processed simultaneously. The samples were refrigerated at 4°C and/or frozen at −20°C in a constant-temperature freezer prior to sampling.
Survival of C. jejuni in ground chicken and on chicken skin.
The following treatment and sampling protocols were used: (i) freezing at −20°C followed by sampling at 1, 3, 7, and 14 days postfreezing (freezing with no prerefrigeration), or (ii) refrigeration at 4°C for 1, 3, or 7 days with subsequent freezing at −20°C and sampling at 1, 3, 7, and 14 days postfreezing (freezing with prerefrigeration) (Table 1). At the given time intervals, an individual chicken skin sample and ground chicken sample from a set of inoculated samples was removed to determine the survival of C. jejuni. Initial inoculum levels prior to refrigerated and frozen storage (prerefrigeration and prefreezing samples, respectively [Table 1]) were determined and compared to evaluate the effect of 1, 3, and 7 days of refrigerated storage alone. Prefreezing counts were compared with postfreezing counts to evaluate the effect of 1, 3, 7, and 14 days of frozen storage (Table 1). After the different storage periods, the samples were diluted 1:10 in 0.1% peptone water based on the previously determined weight of the sample and pummeled for 2 min using a Stomacher 400 lab blender (Seward Medical, London, United Kingdom). The homogenate was serially diluted in 0.1% peptone water and plated onto both TSAB and mCCDA using an Autoplate 4000 spiral plater (Exotech, Inc., Gaithersburg, Md.). An uninoculated irradiated control sample was processed for each replicate under each experimental condition to test sterility. The plates were incubated for 48 h at 42°C under microaerobic conditions as above.
TABLE 1.
Sampling schedules for freezing and freezing following refrigeration of ground chicken samples and chicken skin medallions inoculated with C. jejuni
| No. of days at 4°C | Prerefrigeration (4°C)a | Prefreezing (−20°C)b | Postfreezing (days at −20°C)c |
|---|---|---|---|
| 0 | NAd | P −20 | 1, 3, 7, and 14 |
| 1 | P 4 | P −20 | 1, 3, 7, and 14 |
| 3 | P 4 | P −20 | 1, 3, 7, and 14 |
| 7 | P 4 | P −20 | 1, 3, 7, and 14 |
P 4, enumeration of C. jejuni organisms from chicken samples prior to refrigeration.
P −20, enumeration of C. jejuni organisms from chicken samples prior to freezing or postrefrigeration and prior to freezing.
Enumeration of C. jejuni organisms from chicken samples after freezing for 1, 3, 7, or 14 days.
NA, not applicable.
Calculation of data points.
Experiments evaluating each storage condition were replicated three times, and each sample in each experiment was plated in triplicate onto TSAB and mCCDA. The plates were counted manually, and each plate count was multiplied by the appropriate dilution factor to estimate the total CFU per gram for each sample. The following formula was used to calculate survival: (log10 Nt − log10 N0), where N0 is the total count in CFU per gram at time zero (prerefrigeration and prefreezing counts) and Nt is the total count in CFU per gram at time t (prefreezing counts and postfreezing counts). Thus, differences in initial inoculum levels were normalized. The data points in each graph represent the means of the survival values derived from three replicate experiments under each storage condition. All data points were subjected to analysis of variance to determine the effects and interactions of time, refrigeration treatment, sample type, and plating medium on the survival values. All comparisons were examined by the Bonferroni least significant difference mean separation technique at the P = 0.05 significance level (17). The values plotted were means (n = 3) ± the standard deviations (SD). The figures show the change in cell numbers (survival) after refrigeration only (see Fig. 1, below), freezing only (see Fig. 2), and freezing following prerefrigeration (see Fig. 3 to 5).
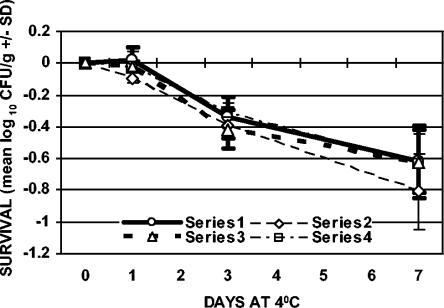
FIG. 1.
Survival of C. jejuni with refrigeration only (4°C). Series 1, ground chicken plated on TSAB; series 2, ground chicken plated on mCCDA; series 3, chicken skin plated on TSAB; series 4, chicken skin plated on mCCDA. The values plotted are means ± SD (n = 3).
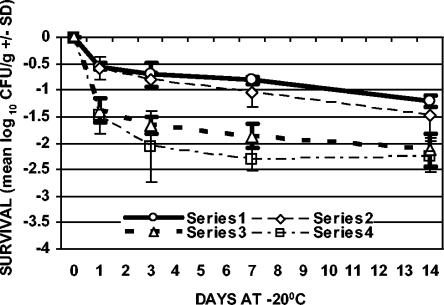
FIG. 2.
Survival of C. jejuni with freezing only (−20°C). Series 1, ground chicken plated on TSAB; series 2, ground chicken plated on mCCDA; series 3, chicken skin plated on TSAB; series 4, chicken skin plated on mCCDA. The values plotted are means ± SD (n = 3).
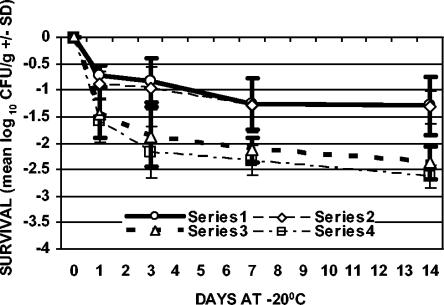
FIG. 3.
Survival of C. jejuni with freezing (−20°C) after 1 day of refrigeration (4°C). Series 1, ground chicken plated on TSAB; series 2, ground chicken plated on mCCDA; series 3, chicken skin plated on TSAB; series 4, chicken skin plated on mCCDA. The values plotted are means ± SD (n = 3).
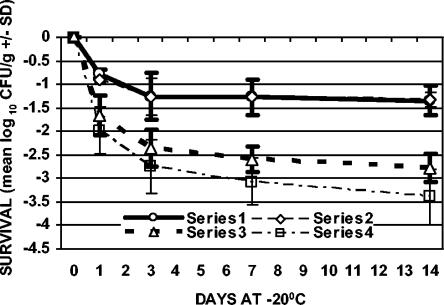
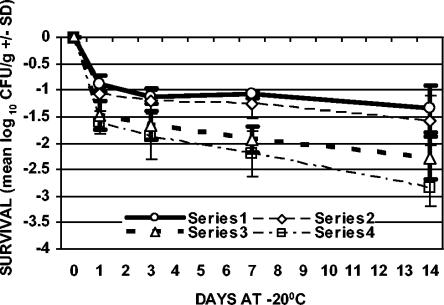
FIG. 5.
Survival of C. jejuni with freezing (−20°C) after 7 days of refrigeration (4°C). Series 1, ground chicken plated on TSAB; series 2, ground chicken plated on mCCDA; series 3, chicken skin plated on TSAB; series 4, chicken skin plated on mCCDA. The values plotted are means ± SD (n = 3).
RESULTS AND DISCUSSION
During commercial processing and during storage by the consumer, poultry products are most typically held at refrigeration and freezing temperatures for time periods of various durations. In the present study, the ability of C. jejuni to survive refrigeration at 4°C, freezing at −20°C, and freezing at −20°C following refrigeration at 4°C was evaluated by using artificially contaminated, gamma-irradiated raw chicken skin and raw ground chicken samples. Since stressed pathogens are often sensitive to components in the medium used in their recovery (12, 20), nonselective TSAB and selective mCCDA media were used for comparison of quantitative recovery of C. jejuni. TSAB and mCCDA are commonly used in the recovery of Campylobacter, and a similar media combination (Columbia blood agar and mCCDA) has been used for comparison of quantitative recovery of C. jejuni following freezing in beef (18).
Refrigerated storage alone resulted in small decreases in viable counts after 3 and 7 days (Fig. 1). The minimum reduction in viable counts of C. jejuni after 3 days of refrigeration was 0.34 (±0.13) log10 CFU/g in ground chicken and 0.31 (±0.04) log10 CFU/g on chicken skin. Maximum reductions in viable counts of 0.81 (±0.24) log10 CFU/g in ground chicken and 0.63 (±0.19) log10 CFU/g on chicken skin after 7 days of exposure were observed. The recovery of C. jejuni from ground chicken or chicken skin after refrigeration was similar using TSAB and mCCDA media. When examined for each sample type and sampling day combination, statistical analysis indicated that there was no significant media effect.
In addition to refrigeration alone, the effect of freezing on the survival of C. jejuni, both with and without prerefrigeration, was evaluated. Samples were stored at −20°C for 1, 3, 7, and 14 days (freezing with no prerefrigeration) or held at 4°C for 1, 3, or 7 days and then stored at −20°C for 1, 3, 7, and 14 days (freezing with prerefrigeration) (Table 1). For both sample types, the greatest rate of decline in cell counts with respect to time occurred during the first day of frozen storage with and without prerefrigeration (Fig. 2 to 5). The results from samples that were not refrigerated prior to freezing showed that viable counts of C. jejuni declined 0.56 (±0.07) to 1.48 (±0.42) log10 CFU/g in ground chicken and 1.38 (±0.23) to 2.26 (±0.30) log10 CFU/g on chicken skin over the 14-day period of storage (Fig. 2). The recovery of C. jejuni from samples that were refrigerated for 1, 3, or 7 days prior to freezing showed that viable counts declined 0.73 (±0.20) to 1.57 (±0.48) log10 CFU/g in ground chicken and 1.44 (±0.23) to 3.39 (±0.60) log10 CFU/g on chicken skin over the 14-day period of storage (Fig. 3 to 5). The greatest absolute declines in cell counts following freezing were observed for chicken skin samples that were prerefrigerated for 7 days, stored at −20°C for 14 days, and plated on TSAB and mCCDA media (2.77 [±0.30] and 3.39 [±0.60] log10 CFU/g, respectively) (Fig. 5). Declines in cell counts were greater in chicken skin than in ground chicken (using either plating medium) following 0, 3, 7, and 0 days of freezing for the 0-, 1-, 3-, and 7-day prerefrigeration treatments, respectively (Table 1; Fig. 2 to 5). Thus, the survival of C. jejuni was significantly higher in ground chicken than in chicken skin at the end of the 2-week freezing period, with or without prerefrigeration. For both sample types, recovery following freezing, with or without prerefrigeration, was similar using either TSAB or mCCDA media. When examined for each prerefrigeration treatment, sample type, and sampling day combination, statistical analysis indicated that there was no significant medium effect on recovery following refrigeration or freezing. All uninoculated control samples processed during the course of the experiments were confirmed to be sterile.
The statistical analysis presented here suggests that the recovery of C. jejuni from each of the sample types following storage under the conditions described is similar using selective and nonselective media. The original study that evaluated CCD agar (a blood-free C. jejuni-selective agar) for the isolation of C. jejuni found that the recovery of some C. jejuni strains (not cold stressed) was significantly lower on CCD agar than on a nonselective agar (agar with nutrient broth no. 2 [Oxoid] and 5% lysed horse blood) (4). A study using nonselective Columbia blood agar and selective mCCDA to recover C. jejuni from beef trimmings stored at −18°C reported a significant difference in recovery from the two types of agar both before and after freezing (18). This difference did not change over time and was interpreted as an indication that sublethal injury was not occurring. In the present study, the recovery of C. jejuni on mCCDA and TSAB was similar for a given sample type, but this similarity did not show a statistical change during the course of the experiment. This may suggest that C. jejuni is not being sublethally injured under the conditions evaluated here, but further study would be needed to definitively determine the presence or absence of injury.
There was statistical evidence that cell count declines increased with time following freezing of chicken skin samples that were prerefrigerated for 3 and 7 days and, to a lesser extent, for ground chicken samples that were prerefrigerated for 3 and 7 days (Fig. 4 and 5). Refrigeration of chicken skin samples before freezing produced a significant change in C. jejuni survival compared to freezing alone (Fig. 2 to 5). The declines in cell counts following freezing averaged over both types of plating medium and all sampling days for chicken skin samples that were prerefrigerated for 0, 1, 3, and 7 days prior to freezing were 1.52, 1.65, 1.59, and 2.06 log10 CFU/g, respectively. There was a significantly greater decline in cell counts (averaged over both types of plating media and all sampling days) for frozen chicken skin samples that were prerefrigerated for 7 days (2.06 log10 CFU/g) than for frozen chicken skin samples that had not been prerefrigerated or that were prerefrigerated for 1 or 3 days.
FIG. 4.
Survival of C. jejuni with freezing (−20°C) after 3 days of refrigeration (4°C). Series 1, ground chicken plated on TSAB; series 2, ground chicken plated on mCCDA; series 3, chicken skin plated on TSAB; series 4, chicken skin plated on mCCDA. The values plotted are means ± SD (n = 3).
Refrigeration of ground chicken samples before freezing did not produce a significant change in C. jejuni survival compared to freezing alone (Fig. 2 to 5). The declines in cell counts following freezing averaged over both types of plating media and all sampling days for ground chicken samples that were prerefrigerated for 0, 1, 3, and 7 days prior to freezing were 0.72, 0.86, 0.96, and 0.94 log10 CFU/g, respectively. Statistical analysis indicated that there was no significant difference in survival with respect to prerefrigeration for ground chicken. The difference in cell count declines that were averaged over both types of plating medium and all sampling days for chicken skin samples as opposed to ground chicken samples (0.80, 0.79, 0.63, and 1.12 log10 CFU/g, respectively, for the 0-, 1-, 3-, and 7-day prerefrigeration periods) was significant for each refrigeration period but was significantly larger for 7 days of prerefrigeration than for 0, 1, or 3 days of prerefrigeration.
The surface location of C. jejuni on chicken skin (as opposed to the ground chicken matrix) may account for the effect of extended prerefrigeration described above and, ultimately, for the lower recovery from chicken skin samples than from ground chicken samples following freezing both with and without prerefrigeration over the 14-day sampling period. The chemical composition and microenvironments characteristic of chicken skin and ground chicken are likely significant factors in the varying recoveries from these two sample types. The specific chemical components of a food can increase or reduce the damage to bacterial cells resulting from freezing. The presence of proteins, simple and complex carbohydrates, and triglycerides and the degree of food viscosity can contribute to the resistance of bacteria to freezing, while the presence of ions, inorganic salts, acids, surface-active components, or enzymes is associated with decreased tolerance to freezing (27).
Protective chemical components, such as proteins, fatty acids, and oils, that may be contained within the surface structures of chicken skin (including follicles) may enhance the survival of contaminating bacteria by inhibiting the formation of ice crystals (15). Poultry are colonized by high levels of Campylobacter on their feathers, skin, and in their intestinal tract, and defeathering and evisceration lead to the contamination of carcasses (14). Cooling of chicken carcasses by immersion in liquid during processing can contribute to the inoculation of Campylobacter, and survival on the skin may be enhanced because drying is limited (23). Campylobacter can be protected by attachment to chicken skin (14), and skin swelling due to water can increase the surface area available for contamination by bacteria, generally (5). Active and inactive C. jejuni cells have been located at the bottom of feather follicles and deep channels in chicken skin during storage at 4°C for 72 h (5). These sites can provide a suitable microenvironment for the survival of Campylobacter, although the majority of live and inactive cells remained on the skin surface (5).
Although chicken skin may provide a protective microenvironment for C. jejuni, the degree of protection afforded by chicken skin during frozen storage under the conditions of this study may not be equivalent to that provided by ground chicken. This may account for the difference in recovery of C. jejuni from these sample types following freezing, as the results presented suggest. It is likely that C. jejuni is more sensitive to freezing when inoculated on a food surface (chicken skin) than when inoculated in the interior of a food matrix (ground chicken), and the difference in chemical composition and the microenvironments specific to ground chicken and chicken skin may play a role. In addition, the surface inoculation of C. jejuni on chicken skin in the protocol described certainly exposes a larger proportion of the bacteria to the effects of the surrounding atmospheric conditions, which may also be an important factor under food processing and packaging conditions.
It has been reported that the pH of a food may play a critical role in the injury and survival of bacteria following frozen storage (20). Campylobacter is inactivated at pH values below 5.0 and above 9.0 (23). The pH values of the ground chicken and chicken skin samples in this study were 5.96 and 6.41, respectively, at the time of inoculation. Therefore, C. jejuni in the ground chicken and chicken skin samples was not inactivated as a result of the sample pH at the time of inoculation. The survival of Campylobacter fetus subsp. jejuni on meat having a pH of 6.4 (higher pH) has been shown to be greater than the survival on meat having a pH of 5.8 (lower pH) under freezing conditions (8). In the present study, the pH values for each sample type were probably not the most significant factor contributing to the difference in survival of C. jejuni organisms on chicken skin and in ground chicken following freezing. This conclusion is suggested by the observation that the higher-pH sample (chicken skin, pH 6.41) showed a greater decline in viable counts than the lower-pH sample (ground chicken, pH 5.96).
Prior studies addressing the survival of C. jejuni during refrigerated and frozen storage on various chicken skin preparations and in various chicken meat preparations have reported varied results. Studies of C. jejuni survival during refrigeration on chicken skin have demonstrated maintenance of counts. C. jejuni survived when inoculated on UV-irradiated raw chicken breast skins in an ambient atmosphere in a heat-sealed bag at 4°C (15). Counts increased, or remained near the initial inoculum level, and never declined below the initial inoculum level during 7 days of storage. There was no significant change in counts when C. jejuni was inoculated on gamma-irradiated raw chicken skin and stored in air in a sealed petri dish at 4°C for 48 h (26). No significant change in counts of C. jejuni (green fluorescent protein reporter strain) was seen when samples were inoculated on nonsterile raw chicken skin and stored in an ambient atmosphere in an inverted container at 4°C for 72 h (5). In the present study, the minimum decline in C. jejuni counts on sterile raw chicken skin samples after 3 days of storage at 4°C in an ambient atmosphere in closed bags (with residual air removed) was 0.31 (±0.04) log10 CFU/g. A maximum decline of 0.63 (±0.19) log10 CFU/g on chicken skin following 7 days of refrigeration was observed. The results of the present study for the survival of C. jejuni on chicken skin during refrigeration agree with the observation of prior studies that C. jejuni does survive following 2, 3, and 7 days of refrigerated storage on chicken skin (references 5, 15, and 26, respectively). Differences in sample packaging conditions, among other factors, may account for the differences in recovery following refrigeration on chicken skin observed in the present study and in the prior studies mentioned. In the present study, the samples were stored in closed bags with the residual air removed. The samples were packaged so as to eliminate or minimize the surrounding air space (not vacuum sealed), and the initial packaging condition was retained during the course of the experiments. The samples showed no visible evidence of drying during refrigeration. Campylobacter has been shown to decline to nondetectable levels on fresh broiler carcasses following 10 days of storage at 4°C in some cases (14).
Previous studies of C. jejuni survival during refrigeration in chicken meat have demonstrated small declines after 1 week of storage and recovery after long-term storage. C. jejuni inoculated in sterile autoclaved ground chicken meat and stored at 4°C in an ambient atmosphere in a covered beaker, or C. jejuni (nalidixic acid-resistant strain) inoculated on raw nonsterile chicken drumsticks in an ambient atmosphere in a tied bag, showed declines of less than 1 log10 CFU/g and 1 log10 CFU/cm2, respectively, after 7 days of storage (3). C. jejuni inoculated in sterile autoclaved chicken mince declined approximately 1 log10 CFU/g over 7 days during aerobic storage in a bag at 5°C (6). C. jejuni has been recovered after 21 days in nonsterile comminuted raw chicken meat stored under ambient conditions in sealed bags stored at 5°C (1). To our knowledge, no studies evaluating the survival of C. jejuni in sterile skinless raw ground chicken meat during refrigeration have been conducted. In the present study, the minimum decline in C. jejuni counts in sterile raw ground chicken samples after 3 days of storage at 4°C in an ambient atmosphere in closed bags (with residual air removed) was 0.34 (±0.13) log10 CFU/g. A maximum decline of 0.81 (±0.24) log10 CFU/g in ground chicken following 7 days of refrigeration was observed. The results of the present study for the survival of C. jejuni following refrigeration in ground chicken show general agreement with the observation of the prior studies mentioned (3, 6) that C. jejuni survives during refrigerated storage in chicken meat samples.
Counts of C. jejuni during frozen storage on various chicken skin preparations were shown to decline to various degrees in prior studies. During frozen storage at −20°C in air in sealed petri dishes, C. jejuni inoculated on gamma-irradiated raw chicken skin showed a steady decline in viable counts of 2 to 3 log CFU/cm2 over 48 h (26). C. jejuni inoculated on UV-irradiated raw chicken breast skins stored at −20°C in a normal atmosphere in heat-sealed bags declined approximately 3 log10 CFU/ml/cm2 over a 14-day period (15). In the present study, viable counts of C. jejuni on sterile raw chicken skin declined 1.38 (±0.23) to 3.39 (±0.60) log10 CFU/g during 14 days of frozen storage in an ambient atmosphere in a closed bag (residual air removed), with and without prerefrigeration. A maximum decline of approximately 3.39 (±0.60) log10 CFU/g after 14 days of freezing when preceded by 7 days of refrigeration was observed. Viable C. jejuni organisms were recovered from chicken skin on all sampling days after freezing, with and without prerefrigeration. The results of the present study for the survival of C. jejuni on chicken skin during frozen storage agree with the observation of the prior studies mentioned (15, 26) that significant declines in C. jejuni counts on chicken skin occur during frozen storage. The survival of C. jejuni inoculated in frozen sterile raw ground chicken has not been evaluated under the combined cold storage conditions presented in this study. The survival of C. jejuni in nonsterile comminuted chicken meat stored in an ambient atmosphere in a sealed bag at −18°C for a minimum of 1 month has been demonstrated (2). A study of C. jejuni inoculated in nonsterile raw ground beef demonstrated a decline of 2 log10 CFU/g following 14 days of storage in a normal atmosphere in a bag at −15°C (28). In the present study, viable counts of C. jejuni in sterile raw ground chicken declined 0.56 (±0.07) to1.57 (±0.48) log10 CFU/g during 14 days while frozen in an ambient atmosphere in closed bags (residual air removed), with and without prerefrigeration. Viable C. jejuni was recovered from ground chicken on all sampling days following freezing, with and without prerefrigeration. This result is in general agreement with the findings of the previous studies mentioned that demonstrated the long-term survival of C. jejuni in chicken meat (comminuted) following freezing at −18°C (2) and that demonstrated the survival of C. jejuni in raw ground meat (beef) during extended frozen storage (following significant initial declines) (28).
In addition to individual refrigeration and freezing treatments (Fig. 1 and 2, respectively), the present study examined the effects of refrigeration on the subsequent survival of C. jejuni following freezing (Fig. 3 to 5). The effect of combined cold storage conditions presented here has not been previously evaluated. The results of this combined treatment show that the susceptibility of C. jejuni to freezing in ground chicken is not significantly affected by prerefrigeration under the conditions tested, but the susceptibility of C. jejuni to freezing on chicken skin is significantly affected by 7 days of prerefrigeration at 4°C. Viable C. jejuni organisms were recovered from both sample types on all sampling days for all combined refrigeration and freezing treatments. The present study also addresses the effect of sample type (ground chicken versus chicken skin) and the effect of sample preparation (gamma-irradiated raw samples) on the survival of C. jejuni under these combined cold storage conditions.
In conclusion, a significant portion of C. jejuni on the poultry samples studied survived during refrigerated, frozen, and combined refrigerated and frozen storage. Hence, the present study indicates that these treatments alone will not add a significant margin of safety with respect to this pathogen and cannot replace sanitary production and handling. Poultry contaminated with C. jejuni may lead to infection if not properly handled and sufficiently cooked. Further studies are in progress to evaluate the survival of cold-stressed C. jejuni in chicken samples following heat treatment.
Acknowledgments
We thank Benne Marmer of the Microbial Food Safety Research Unit at ERRC for irradiating the ground chicken and chicken skin samples used in this study. We also thank John Phillips, statistician at the USDA Agricultural Research Service and North Atlantic Area, who performed the statistical calculations and assisted in the analyses of the data.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
REFERENCES
- 1.Beuchat, L. R. 1985. Efficacy of media and methods for detecting and enumerating Campylobacter jejuni in refrigerated chicken meat. Appl. Environ. Microbiol. 50:934-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beuchat, L. R. 1987. Efficacy of some methods and media for detecting and enumerating Campylobacter jejuni in frozen chicken meat. J. Appl. Bacteriol. 62:217-221. [DOI] [PubMed] [Google Scholar]
- 3.Blankenship, L. C., and S. E. Craven. 1982. Campylobacter jejuni survival in chicken meat as a function of temperature. Appl. Environ. Microbiol. 44:88-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolton, F. J., D. N. Hutchinson, and D. Coates. 1984. Blood-free selective medium for the isolation of Campylobacter jejuni from feces. J. Clin. Microbiol. 19:169-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chantarapanont, W., M. Berrang, and J. F. Frank. 2003. Direct microscopic observation and viability determination of Campylobacter jejuni on chicken skin. J. Food Prot. 6:2222-2230. [DOI] [PubMed] [Google Scholar]
- 6.Chynoweth, R. W., J. A. Hudson, and K. Thom. 1998. Aerobic growth and survival of Campylobacter jejuni in food and stream water. Lett. Appl. Microbiol. 27:341-344. [DOI] [PubMed] [Google Scholar]
- 7.Freidman, C. R., J. Neimann, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 121-138. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 8.Gill, C. O., and L. M. Harris. 1982. Survival and growth of Campylobacter fetus subsp. jejuni on meat and in cooked foods. Appl. Environ. Microbiol. 44:259-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hani, E. K., and V. L. Chan. 1995. Expression and characterization of Campylobacter jejuni benzoylglycine aminohydrolase (hippuricaase) gene in Escherichia coli. J. Bacteriol. 177:2396-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazeleger, W. C., J. A. Wouters, F. M. Rombouts, and T. Abee. 1998. Physiological activity of Campylobacter jejuni far below the minimal growth temperature. Appl. Environ. Microbiol. 64:3917-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holt, J. G., N. R. Krieg, P. H. A. Sneath, J. T. Staley, and S. T. Williams. 1994. Bergey's manual of determinative bacteriology, 9th ed. Williams and Wilkins, Baltimore, Md.
- 12.Humphrey, T. J. 1986. Injury and recovery in freeze- or heat-damaged Campylobacter jejuni. Lett. Appl. Microbiol. 3:81-84. [Google Scholar]
- 13.Hunt, J. M., C. Abeyta, and T. Tran. 1998. Campylobacter, p. 7.01-7.27. In Food and Drug Administration bacteriological analytical manual, 8th ed. AOAC International, Gaithersburg, Md.
- 14.Jacobs-Reitsma, W. 2000. Campylobacter in the food supply, p. 467-481. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 15.Lee, A., S. C. Smith, and P. J. Coloe. 1998. Survival and growth of Campylobacter jejuni after artificial inoculation onto chicken skin as a function of temperature and packaging conditions. J. Food Prot. 61:1609-1614. [DOI] [PubMed] [Google Scholar]
- 16.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller, R. G., Jr. 1981. Simultaneous statistical inference, 2nd ed., p. 67-70. Springer-Verlag, New York, N.Y.
- 18.Moorhead, S. M., and G. A. Dykes. 2002. Survival of Campylobacter jejuni on beef trimmings during freezing frozen storage. Lett. Appl. Microbiol. 34:72-76. [DOI] [PubMed] [Google Scholar]
- 19.Oberhelman, R. A., and D. N. Taylor. 2000. Campylobacter infections in developing countries, p. 139-153. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 20.Palumbo, S. A., and A. C. Williams. 1991. Resistance of Listeria monocytogenes to freezing in foods. Food Microbiol. 8:63-68. [Google Scholar]
- 21.Ransom, G. M., and B. E. Rose. 1998. Isolation, identification, and enumeration of Campylobacter jejuni/coli from meat and poultry products, p. 6-1-6-10. In B. P. Dey and C. P. Lattuada (ed.), Microbiology laboratory guidebook, 3rd ed., vol. 1. U.S. Government Printing Office, Washington, D.C. [Google Scholar]
- 22.Ransom, G. M., B. Kaplan, A. M. McNamara, and I. K. Wachsmuth. 2000. Campylobacter prevention and control: the USDA Food Safety and Inspection Service role and new food safety approaches, p. 511-528. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 23.Skirrow, M. B., and M. J. Blaser. 1995. Campylobacter jejuni, p. 825-848. In M. J. Blaser, P. D. Smith, J. I. Ravdin, H. B. Greenburg, and R. L. Guerrant (ed.), Infections of the gastrointestinal tract. Raven Press, Ltd., New York, N.Y.
- 24.Skirrow, M. B., and M. J. Blaser. 2000. Clinical aspects of Campylobacter infection, p. 69-88. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 25.Smibert, R. M. 1984. Campylobacter, p. 111-118. In J. G. Holt and N. R. Krieg (ed.), Bergey's manual of systematic bacteriology, vol. 1. Williams and Wilkins, Baltimore, Md. [Google Scholar]
- 26.Solow, B. T., O. M. Cloak, and P. M. Fratamico. 2003. Effect of temperature on viability of Campylobacter jejuni and Campylobacter coli on raw chicken or pork skin. J. Food Prot. 66:2023-2031. [DOI] [PubMed] [Google Scholar]
- 27.Speck, M. L., and B. Ray. 1977. Effects of freezing and storage on microorganisms in frozen foods: a review. J. Food Prot. 40:333-336. [DOI] [PubMed] [Google Scholar]
- 28.Stern, N. J., and A. W. Kotula. 1982. Survival of Campylobacter jejuni inoculated into ground beef. Appl. Environ. Microbiol. 44:1150-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stern, N. J., and S. U. Kazmi. 1989. Campylobacter jejuni, p. 71-110. In M. P. Doyle (ed.), Foodborne bacterial pathogens. Marcel Dekker, Inc., New York, N.Y.