Abstract
Therapeutic application of cardiac resident stem/progenitor cells (CSC/CPCs) is limited due to decline of their regenerative potential with donor age. A variety of studies have shown that the cardiac aging was the problem of the stem cells, but little is known about the impact of age on the subgroups CSC/CPCs, the relationship between subgroups CSC/CPCs ageing and age-related dysfunction. Here, we studied Sca-1+CD31− subgroups of CSCs from younger(2~3months) and older(22~24months) age mice, biological differentiation was realized using specific mediums for 14 days to induce cardiomyocyte, smooth muscle cells or endothelial cells and immunostain analysis of differentiated cell resulting were done. Proliferation and cell cycle were measured by flow cytometry assay, then used microarray to dissect variability from younger and older mice. Although the number of CSCs was higher in older mice, the advanced age significantly reduced the differentiation ability into cardiac cell lineages and the proliferation ability. Transcriptional changes in Sca-1+CD31− subgroups of CSCs during aging are related to Vitamin B6 metabolism, circadian rhythm, Tyrosine metabolism, Complement and coagulation cascades. Taking together these results indicate that Cardiac resident stem/progenitor cells have significant differences in their proliferative, pluripotency and gene profiles and those differences are age depending.
Keywords: cardiac resident stem/progenitor cells, aging, differentiation, proliferation, Gerotarget
INTRODUCTION
Aging is an universal phenomenon in living beings, and many geriatric diseases (such as cardiovascular disease and metabolic dysfunction) as results of aging, numerous studies have shown that the stem cell problem primarily results in aging, all ageing phenomena can be interpreted at the level of somatic stem cells [1–3]. Cardiac resident stem/progenitor cells (CSC/CPCs) represent an ideal source of autologous cell-based therapy for heart diseases by maintaining myocardial cell homeostasis. Several populations of CSC/CPCs have been identified based on expression of different stem cell-associated antigens. Sca-1+ cells in cardiac tissue may be the most common CSC/CPCs and be relatively easy to isolate [4], Sca-1+CD31− cells show cardiomyogenic differentiation and cell transplantation for myocardial repair [5, 6]. Changes in the properties of CSC/CPCs with age involving increased symmetric division, decreased cell proliferation and differentiation, loss of self-renewing capacity, partial depletion of the primitive pool, decreased expression of stem cell markers, impaired the migration ability, and changed the cell cycle into an irreversible quiescent state [7–9]. However, some other reports suggested that the impact of age on the quantity and quality of human cardiac stem cells is quite limited [10]. Clearly, many aspects about stem cells and aging remain to be understood. The influence of age on CSC/CPCs activity is critically important for an organism during development and senescence. We have analyzed the phenotypic, genotypic features and biological-related changes in Sca-1+CD31− CSC/CPCs of mice heart from different ages, this will establish a framework for future comparative and functional studies.
RESULTS
CSCs isolation and phenotype
We have used FACS to isolate CSCs using Lineage, CD45, Sca-1, and CD31 markers whose expression is conserved across mouse strains and during aging [11]. The Lin−CD45−Sca-1+CD31−cells were isolated from younger or older C57BL/6 mice (Figure 1A-B). The percentage of cells was calculated based on a mean of twenty mice, consistent with previous works and the number of CSCs was higher from older mice heart than younger (P < 0.05). Analysis of CD31 and Sca-1 expression by immune fluorescence staining revealed only express Sca-1in CSCs (Figure 1C).
Figure 1. sorting strategy for isolating Lin−CD45− Sca−1+CD31−cells from young (A) and old (B) C57BL/6 mice.
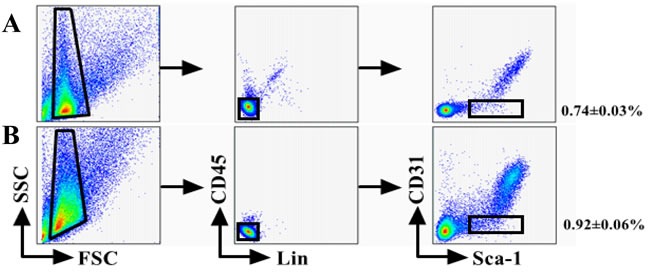
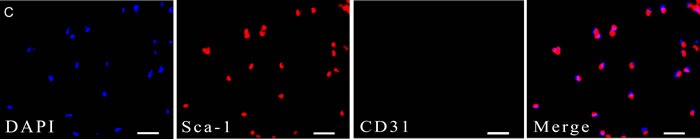
The percentage of cells was calculated based on a mean of twenty mice. Differences were analyzed with Student's t-test (P < 0.05). C. Analysis of CD31 and Sca-1 expression by IF staining. All cells were triple-stained for Sca-1 (red), CD31 (green), and DAPI (blue). Sca-1 expression in Sca-1+CD31− cells with no CD31 expression in Sca-1+CD31−cells.Merging fluorescent signals showed no heterogenous populations after FACS-based sorting. Scale bars, 10 μm.
In vitro differentiation of Sca-1+CD31− CSCs from younger and older C57BL/6 mice
Differentiation is an important feature of stem and progenitor cells. To assess the differentiation activity of CSCs into cardiomyocyte cells, endothelial lineages cells and smooth muscle cells, the same number of Sca-1+CD31− cells from younger and older mice were cultured in differentiation medium and control medium (without growth factors). After induction cultivation, specific markers were analyzed by immune fluorescence. After 2 weeks of induction, the cells morphology have changed, Cardiac Troponin I(cTnI)-positive cells, α-Smooth Muscle Actin(α-SMA)-positive cells and Von Willebrand Factor(VWF)-positive cells were detected by IF in younger and older groups (Figure 2-A,C,E). However, the differentiation efficiency results indicated that CSCs from groups of younger had a statistically significant higher (p < 0.01) for cTnI (73.40±10.64% VS 40.10±7.84%), α-SMA(81.24±11.23% VS 54.28±9.07%) and VWF(64.85±10.68% VS 37.89±7.47%) differentiation efficiency compared to older(Figure 2-B,D,F); No Cardiac Troponin I-positive, α-Smooth Muscle Actin-positive and Von Willebrand Factor-positive cells were observed in control groups (data not shown).
Figure 2. In vitro differentiation of CSCs from young and old C57BL/6 mice.
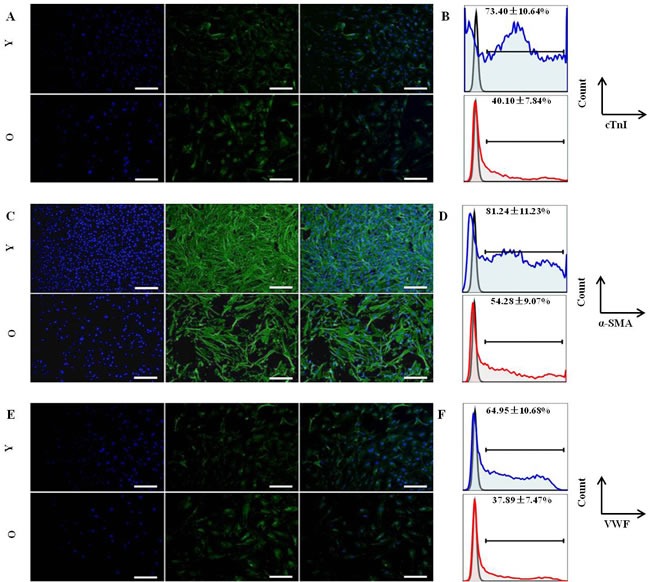
Cells were stained for specific markers after induction. A. Immunofluorescence staining (green) of cardiac-specific marker-cTnI; Nuclei (blue) counterstained with DAPI; then merged images. Y:young mice; O:old mice; B. The differentiation efficiency of CSCs for cardiomyocyte from young and old C57BL/6 mice, the value is the percentage of cTnI marker cells among total Sca-1+-labeled cells, blue: young mice; red:old mice; C. Immunofluorescence staining (green) of smooth muscle marker-α-SMA; Nuclei (blue) counterstained with DAPI; then merged images. Y:young mice; O:old mice; D. The differentiation efficiency of CSCs for smooth muscle cells from young and old C57BL/6 mice, the value is the percentage of α-SMA marker cells among total Sca-1+-labeled cells, blue: young mice; red:old mice; E. Immunofluorescence staining (green) of enodthelial cell marker-VWF; Nuclei (blue) counterstained with DAPI; then merged images. Y:young mice; O:old mice; F. The differentiation efficiency of CSCs for endothelial cells from young and old C57BL/6 mice, the value is the percentage of VWF marker cells among total Sca-1+-labeled cells, blue: young mice; red:old mice; Every sample was performed in triplicate, differences were analyzed with Student's t-test (P < 0.01). Scale bars, 50 μm.
Overall, these data indicated that younger mice Sca-1+CD31−cells have a stronger ability differentiation into cardiac cell lineages.
Cell proliferation of CSCs from younger and older C57BL/6 mice
Characterization of populations of CSCs from different ageing group by flow cytometry reveled that no statistical significant differences exist between group respects levels of stem cell markers Lin and CD45 used for that, positive cells for CD45 and Lin were less 1%, but the significant differences between younger and older group respects for CD31 and Sca-1 (Figure 3A) indicated that the number of CSCs was higher in older mice.
Figure 3. Proliferation profile from mice cardiac stem cells at different age.
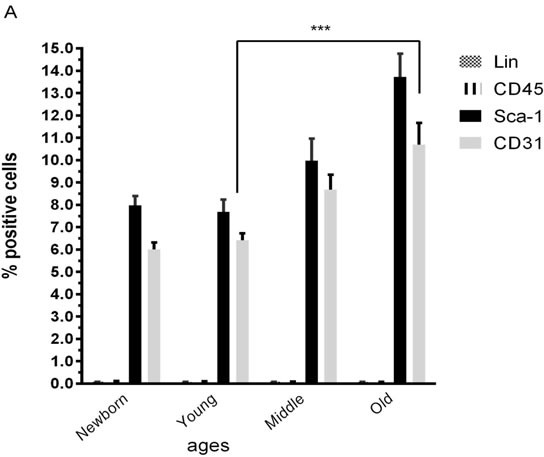
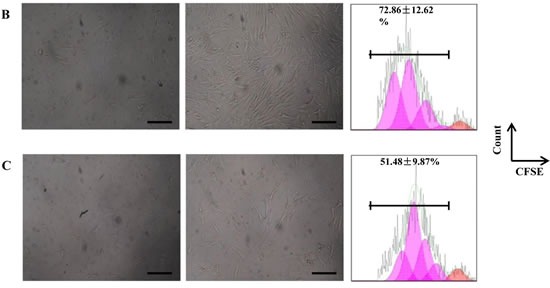
A. Characterization by flow cytometry assay of percentage of positives cardiac stem cells marker (Sca-1), endothelial marker (CD31) and hematopoietic markers (Lin and CD45) Newborn(1-3 days), Young(2-3months), Middle(6-8months),Old(22-24months). B. Proliferation assay of CSCs from young mice for 6 days. C. Proliferation assay of CSCs from old mice for 6 days. One representative experiment is shown. Differences were analyzed with Student's t-test (P < 0.05). Scale bars, 50 μm.
Proliferation assays results indicated that CSCs from groups of younger (72.86 ±12.62%) had a statistically significant higher (p < 0.05) proliferation ability compared to older (51.48 ± 9.87%) (Figure 3B-C).
Cell cycle of CSCs from younger and older C57BL/6 mice
The percentage of CSCs in G0/G1, S, and G2/M phase of the cell cycle was evaluated from younger and older mice heart CSC cells. We found that 90.55±4.01% of younger mice heart CSC cells were in G0/G1 phase, 7.72±2.64% in S phase and 1.73±1.41% in G2/M phase; 85.45±3.54% of older mice heart CSC cells were in G0/G1 phase, 11.86±2.71% in S phase and 2.69±1.74% in G2/M phase; the percentage of cell in S phase was significantly increase (p<0.05) of older mice heart CSC cells. Conversely, the percentage of cells in G0/G1 and G2/M phase was no significantly change between older and younger mice (Figure 4A). These changes were associated with the expression of genes involved in cell cycle. No significant differences were observed for the expression of p19, p27, Cyclin B1 and Cyclin D1 between younger and older mice heart CSC cells (Figure 4B).
Figure 4.
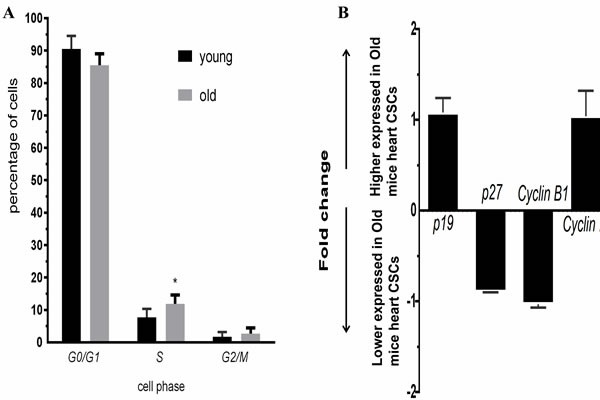
A.Cell cycle of CSCs from young and old C57BL/6 mice, blue: young; red:old; B. Expression profiles of cell cycle genes detected by QRT-PCR. Each bar represents the fold change of genes expression in old mice heart CSCs cells VS young mice heart CSCs cells. Expression levels were nomalized to that of gene GAPDH and the expression level of young mice heart CSCs cells was used as calibrator to calculate the fold change. Calculated difference change based on a mean of three biological replicates. The ΔΔCT values obtained were subjected to unpaired Student's t test implemented in Prism software. Bars above and below the x-axis show genes that are up- or down-regulated, respectively, in old mice heart CSCs cells. The asterisks indicate samples whose values are statistically significantly, *P < 0.05, **P < 0.01, ***P < 0.001.
MRNA Array Analysis
Expression profiling of undifferentiated Sca1+CD31− cells from younger or older mice. A total of 35 genes were differentially expressed between younger and older mice (false discovery rate < 0.05 and folder-change ≥ 2, Figure 5A, Table 1). There existed 25 up-regulated and 10 down-regulated mRNAs in older mice when compared to younger. Furthermore, after GO function and KEGG pathway analysis, these differential expression genes were significantly enriched function in microsatellite binding, aldehyde oxidase activity and et al. (Figure 5C); enriched pathways in Vitamin B6 metabolism, circadian rhythm, Tyrosine metabolism, Complement and coagulation cascades and et al (Figure 5D).
Figure 5. The mRNA expression signatures in old mice CSCs versus young mice CSCs.
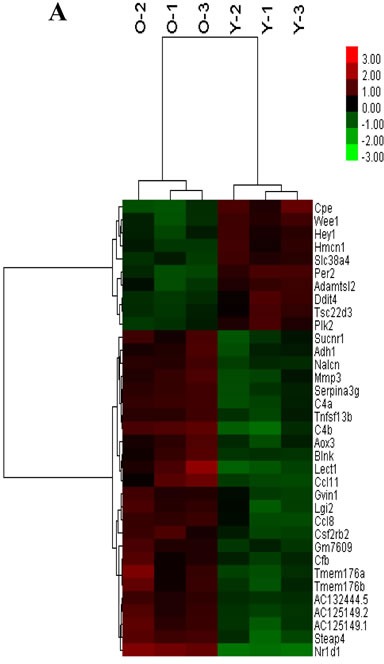
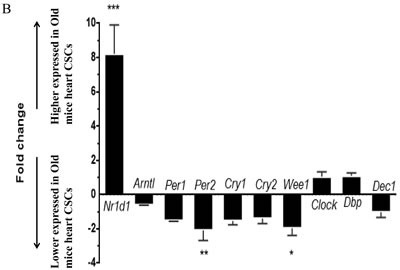
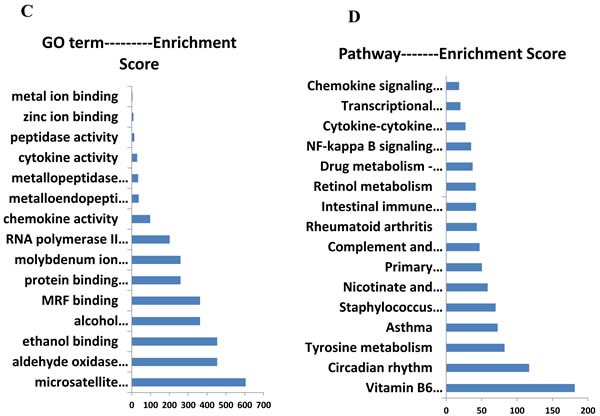
A. The tree was based on the log2 transformation of the normalized probe signal intensity using hierarchical clustering. O-old mice CSCs; Y-young mice CSCs; Red: up expression genes; Green: down expression genes. Every sample was performed in triplicate. B. Expression profiles of circadian clock genes detected by real time RT-PCR. Each bar represents the fold change of genes expression in old mice heart CSCs cells VS young mice heart CSCs cells. Expression levels were nomalized to that of gene GAPDH and the expression level of young mice heart CSCs cells was used as calibrator to calculate the fold change. Calculated difference change based on a mean of three biological replicates. The ΔΔCT values obtained were subjected to unpaired Student's t test implemented in Prism software. Bars above and below the x-axis show genes that are up- or down-regulated, respectively, in old mice heart CSCs cells. The asterisks indicate samples whose values are statistically significantly, *P < 0.05, **P < 0.01, ***P < 0.001.C. Functional categories of differentially expressed genes. D. Pathway enrichment of differentially expressed genes.
Table 1. Differentially expressed mRNAs.
| Gene Symbol | Gene Description | Fold Change | p-value | Gene Feature |
|---|---|---|---|---|
| Cpe | carboxypeptidase E | −3.13329 | 0.000122 | down |
| Per2 | period circadian clock 2 | −2.76163 | 0.000073 | down |
| Wee1 | WEE 1 homolog 1 | −2.42462 | 0.00014 | down |
| Slc38a4 | solute carrier family 38, member 4 | −2.08692 | 0.000149 | down |
| Hmcn1 | hemicentin 1 | −2.06809 | 0.000318 | down |
| Plk2 | polo-like kinase 2 | −2.0679 | 0.000327 | down |
| Ddit4 | DNA-damage-inducible transcript 4 | −2.22285 | 0.000425 | down |
| Adamtsl2 | ADAMTS-like 2 | −2.19011 | 0.000408 | down |
| Hey1 | Hairy enhancer-of-split related with YRPW motif 1 | −2.00755 | 0.000452 | down |
| Tsc22d3 | TSC22 domain family, member 3 | −2.02136 | 0.000612 | down |
| Nr1d1 | nuclear receptor subfamily 1, group D, member 1 | 6.598208 | 0.000047 | up |
| C4b | complement component 4B | 3.865675 | 0.000051 | up |
| Lect1 | leukocyte cell derived chemotaxin 1 | 3.839932 | 0.000252 | up |
| Steap4 | STEAP family member 4 | 3.274248 | 0.000065 | up |
| Ccl11 | chemokine (C-C motif) ligand 11 | 3.03196 | 0.000354 | up |
| AC125149.1 | 2.857723 | 0.000109 | up | |
| C4a | complement component 4A | 2.596466 | 0.000078 | up |
| AC125149.2 | 2.482776 | 0.000105 | up | |
| Mmp3 | matrix metallopeptidase 3 | 2.435376 | 0.000283 | up |
| Ccl8 | chemokine (C-C motif) ligand 8 | 2.410426 | 0.000292 | up |
| Serpina3g | serine peptidase inhibitor, clade A,member 3G | 2.397298 | 0.000096 | up |
| Csf2rb2 | colony stimulating factor 2 receptor, beta 2, low-affinity (granulocyte-macrophage) | 2.396598 | 0.000225 | up |
| Blnk | B cell linker | 2.303156 | 0.000287 | up |
| Tnfsf13b | tumor necrosis factor (ligand) superfamily, member 13b | 2.215785 | 0.000145 | up |
| Nalcn | sodium leak channel | 2.214615 | 0.000091 | up |
| Gm7609 | predicted pseudogene 7609 | 2.140701 | 0.000203 | up |
| AC132444.5 | 2.125757 | 0.000056 | up | |
| Tmem176a | transmembrane protein 176A | 2.848028 | 0.000702 | up |
| Tmem176b | transmembrane protein 176B | 2.52496 | 0.000559 | up |
| Lgi2 | leucine-rich repeat LGI family, member 2 | 2.40872 | 0.000608 | up |
| Sucnr1 | succinate receptor 1 | 2.330601 | 0.000639 | up |
| Aox3 | aldehyde oxidase 3 | 2.239808 | 0.000483 | up |
| Gvin1 | GTPase, very large interferon inducible 1 | 2.060591 | 0.000519 | up |
| Adh1 | alcohol dehydrogenase 1 (class I) | 2.08528 | 0.001018 | up |
| Cfb | complement factor B | 2.034334 | 0.001178 | up |
The mRNA expression level in old mice CSCs versus young mice CSCs. All genes had p-value < 0.05 and fold change ≥2. Down: down expression genes in old mice CSCs; Up: up expression genes in old mice CSCs.
The circadian rhythms genes were significantly changed with aging [12]. To validate the microarray data, 10 circadian clock genes were chosen for analysis by real time RT-PCR: Per1,Per2,Cry1,Cry2,Clock, Arntl, Nr1d1,Dbp,Wee1andDec1. Among these genes, with higher expression of Nr1d1 (P<0.001) and lower expression of Per2 and Wee1 (P<0.05) in older mice CSCs as compared to younger, and real time RT-PCR results confirmed the mRNA microarray results (Figure 5B).
DISCUSSION
The characteristics of stem cells such as cell cycle, self-renewability, proliferation and lineage composition are altered with aging [2]. Senescent cells accumulate in tissues with advancing age. Tissue-specific stem cells, for example those of the hematopoietic and musculoskeletal system are also known to undergo degenerative changes with age [13], but impact of age on the quantity and quality of human cardiosphere-derived cells (CDCs, as one of cardiac stem cells subgroups) is quite limited [10]. However, few studies have focused on the influence of donor age on other subgroups of CSC. Here, we tested whether the regulation of stem-cell activity of Sca-1+CD31−CSCs were impaired as donor age increased. The current study compared Sca-1+CD31−CSCs from younger (2–3 months) and older (22–24 months) C57BL/6 mice as biological age. Although the number of CSCs was higher in older mice, the advanced age significantly reduced the differentiation ability into cardiac cell lineages (Figure 2) and the proliferation ability (Figure 3B-C). The cell cycle stages G1, S, G2 and M are time-dependent, and the expression of some key cell cycle genes play an important role in the process of the cell cycle changed. The Clock genes such as Per1, Per2, Clock and Bmal play an important role in regulating cell proliferation through some cell cycle-related genes, such as Wee1, c-Myc and Ccnd1 [14]. The circadian clock affects the cell proliferation through the protein WEE1, to regulate the cell cycle, and the wee1 expression was directly regulated by the circadian clockwork [15, 16]. In our results, Wee1 expression with a 2.4 folder change down expression in older mice heart CSC, however no significant differences were observed for the expression of p19, p27, Cyclin B1 and Cyclin D1 between younger and older mice heart CSC cells. Per2 regulated the cell cycle transition from G1 to S stages [17], the lower expression of Per2 and Wee1 (P<0.05) at older mice CSCs as compared to younger mice CSCs related with the percentage of cell in S phase was significantly increase (p<0.05) of older mice heart CSC cells (Figure 4A).
For determining the molecular basis for differences between younger and older Sca-1+CD31−CSCs in differentiation, proliferation and cell cycle, analysis of mRNA profiling in younger and older Sca-1+CD31−CSCs. We found that the expression profile showed function in microsatellite binding, aldehyde oxidase activity and et al. The differently expressed genes showed pathway in Vitamin B6 metabolism, circadian rhythm, Tyrosine metabolism, Complement and coagulation cascades et al. The heart as peripheral oscillator for 13% of genes expressed exhibit significant circadian rhythmicity [18]. The circadian clock regulate the biological rhythm through transcription-translation feedback [19]. As a clock gene, Rev-erbα focused more on its
effects on adipogenesis and as a key mediator between clockwork and inflammation [20]. Rev-erbα expression gradually decreased during BMSC osteogenesis, and overexpression of Rev-erbα in BMSCs inhibited cell proliferation and osteogenesis, suggested age-related BMSC changes may be associated with the expression of Rev-erba [21]. Some research compared the Rev-erbα expression levels in different tissues of rats at the ages of 1 month, 3 months, and 6 months, the results showed a decreasing trend during aging, suggested Rev-erbα levels may be associated with tissue aging [22]. However, in our results, the Rev-erbα expression was higher in older mice heart CSC (Table 1; Figure 5), the increasing Rev-erbα levels may play an important role in activity of CSC during aging. Among clock genes,Per2 as mediator of vascular senescence, immune response, angiogenesis and endothelial function,plays an important role in cell cycle progression, the balance of cell proliferation and apoptosis [23], the pathophysiology of the cardiovascular [24] and aging [25]. Some reports showed that no significant differences in the rhythm of expression ofPer2andBmal1, as well as that ofDbpwas found in the heart of agedvsyounger mice [26]. However, in our results, the Per2expression was lower in olderer mice heart CSC, may be relative with the decreasing differentiation ability of older mice CSC. Per1 and Per2 expression pattern with aging in other reports [12] was consistent with our results. With aging, we suggested differently express in several rhythmic genes, with the effects somewhat relative with CSC differentiation. The reason for this difference is not clear. We next sought to research the biological significant circadian clock mechanism in CSCs. Thus, differences about this clock genes expression over CSCs on aging may reflect distinct functions.
The functions of the immune system also altered with aging, so that it no longer resemble the immune system of the younger individuals [27]. The complement system played an important role in both innate and acquired immunogenicity. The complement pathway has been implicated in the pathogenesis of many age-related diseases, e.g macular degeneration [28], arrhythmogenic cardiomyopathy (ACM) [29, 30]. The complement component C4 which has the isotypes C4A and C4B is an effector protein of the immune system [31], the C4A protein is more relevant in immunoclearance, whereas C4B is more important in the defense against microbes [32]. Expression of complement component C1q, C3, C4, C5 and factor B up expressed with aging in C57BL/6 mice [33]. Inhibiting C5aR (CD88) signaling improves cardiac function, suggested that complement modulation could be a new therapeutic strategy for ACM [29, 30]. In our results, the expression of complement component C4a, C4b and inflammation genes Ccl11, Ccl8 were up expressed in older CSCs, indicated that targeting the complement system for the management of cardiac disease maybe a new therapy tool.
As the number of CSCs was higher in older mice, but the older mice Sca-1+CD31−cells have weaker differentiation ability into cardiac cell lineages and weaker proliferation ability in vitro. The gene profile findings indicate that the molecular clockwork is mostly preserved in the aged heart. These findings suggest that Rev-erbα, Per2 and Wee1 are directly or indirectly affected the circadian rhythm-dependent changes in aging-induced CSC properties. However, the mechanism for a circadian rhythm-dependent change in Rev-erbα, Per2 and Wee1 expression by aging remains to be determined. The next objective of our work was to investigate whether these differently expressed circadian rhythm genes control CSC differentiation capacities, regulate cell cycle, and play a role in cell proliferation abilities with aging.
CONCLUSIONS
The stem-cell activity of CSCs assessed by cell proliferation, cell cycle and cell differentiation ability varied among CSCs and was obviously associated with donor ages. This suggests that CSCs from older mice are more prone to undergo senescence than those isolated from younger and age is a critical determinant of the activity of CSCs. The importance of our study lies in the fact that age from the CSCs source directly influences their differentiation, proliferative and gene profiles, also it is the first time where is shown the direct molecular difference of circadian rhythm genes Rev-erbα, Per2 and Wee1, immune response genes C4b, Ccl11, and Cfb on participated in aging of CSCs. Summary we affirm that younger group of mice has the most proliferative and pluripotent CSCs be able to future functional studies.
MATERIALS AND METHODS
Experimental animals and ethics statement
Younger(2~3months) and older(22~24months)C57BL/6J mice (Experimental Animal Center of Guilin Medical College, China) were used for cell isolation. All procedures involving animals were approved by the Institutional Animal Care and Use Committee.
Cell isolation and fluorescence-activated cell sorting
Whole hearts were extracted from either male or female of younger and older C57BL/6J mice, split, and washed several times with ice-colder PBS to remove residual blood, and then incubated with indicated combinations of monoclonal antibodies against mouse antigens Sca-1-PE-Cy7, CD31-APC, CD45-FITC, Lin-PE (eBioscience) or with isotype-matched control antibodies before analysis by fluorescence-activated cell sorting (FACS). Freshly sorted CSC (Lin−CD45−Sca-1+CD31−) were used for experiments, except as indicated. The methods were expanded as described [34].
Cell culture and in vitro differentiation
The same number of Lin−CD45−Sca-1+CD31−cells from younger or older mice heart were initially seeded in normal medium. For cardiomyocyte, smooth muscle cell and endothelial cell induction, cells were cultured in DMEM/F12 supplemented with growth factors for 14 days. The methods were expanded as described [34].When induction was complete, lineage-specific markers were analyzed by IF staining.
The differentiation efficiency was applied to indicate the differentiation ability of Sca-1+CD31−cells from younger or older mice heart, and the value is the percentage of lineage-specific marker cells among total Sca-1+-labeled cells, and was assessed using FACSverse (BD Biosciences).
The cells in vitro proliferation
Cells proliferation assays were carried out as described previously [35].The cells proliferation was assessed using FACSverse (BD Biosciences). Proliferation index was applied to indicate the proliferative status of Sca-1+CD31−cells from younger or older mice heart, and the value (percentage of CFSE-diluted cells among total CFSE-labeled CSC cells) was designed as 1 for cultures with medium only, and those for other culture conditions were normalized to the medium only group.
Cell cycle analysis
Cells cycle analysis were using Propidium Iodide Flow Cytometry Assay Kit (Abcam, USA), Briefly, about 1 × 105 cells/ml of CSC cells from younger and older mice were seeded into 24 well plates and kept under 37°C, 5% CO2overnight. Fixation of the cells was carried out using 66% ethanol for at least 2 h at 4°C. After centrifugation at 500 × g for 5 min, the cells were washed with PBS prior to staining. About 200 μl of staining solution composed of 1 × PI and RNase was added to the cell suspension and incubated in dark at 37°C for 20–30 min. The signals of at least 1×104single cells were recorded on a FACS Canto II flow cytometer (BD Biosciences, Heidelberg, Germany), and DNA histograms were analyzed with the WinCycle software (Phoenix Flow Systems, San Diego, CA, USA) after doublet exclusion.
RNA preparation and quality control
Total cellular RNA was extracted from 1× 105 Lin−CD45−Sca-1+ CD31− cells from younger or older mice heart, for three samples, isolated using FACS, 10 mice as a pool for per sample. RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. RNA concentrations were determined photometrically using a NanoDrop 1000 (Peqlab, Erlangen, Germany). Overall RNA quality was assessed by 1% agarose gels with 1 kb molecular weight marker separated in parallel.
Quantitative real-time RT-PCR
Total RNA was isolated and 1 μg of it was reverse transcribed with oligo dT primers using a first strand cDNA synthesis kit (Promega) according to manufacturer's instructions. cDNAs were PCR amplified (40 cycles at 95°C for 10 s, 60°C for 30 s and 72°C for 30 s) using SYBR green master mix (Takara) in 96 wells, followed by a melting curve analysis. Fluorescence intensities were analyzed in real time ABI/Prism 7700 Sequences Detector System (Applied Biosystems), and the relative quantitation (ΔΔCt) method was used to evaluate gene expression between the younger and older mice CSC cells for each gene examined. The expressions of the genes were normalized to a housekeeping gene, GAPDH.
Whole genome gene expression microarrays and Bioinformatical analysis of data
The methods were expanded as described [34].
Immune fluorescence (IF)
The methods were expanded as described [34].
Statistical analysis
Signal intensity and mRNA expression levels were analyzed by Student's t-test or one-wayANOVA. Data are expressed as mean±standard deviations for each parameter measured in each group. Statistical analyses for QRT-PCR were performed with GraphPad Prism 6 software (GraphPad software, CA, USA). Unpaired t-test was used for two-groups comparisons, Two sided p-value was calculated. P<0.05 was considered statistically significant.
Acknowledgments
We thank Donglin Chen, Wenjing Zhao from Gene-Cloud Biotechnology Information Center for excellent data handling.
Footnotes
CONFLICTS OF INTEREST
The authors have no competing financial interests.
GRANT SUPPORT
This study was supported by grants from National Natural Science Foundation of China (31660344, to Qiong Wu; 31260285, to Zuping Zhou), Natural Science Foundation of Guangxi (2016GXNSFAA380169, 2015GXNSFBA139154, to QiongWu; 2014GXNSFCB118002, to Zuping Zhou), key project of Guangxi Normal University (2015ZD001, to Qiong Wu), Basic Capabilities Building Project of Guangxi University for Young Teachers (KY2016YB067, to Qiong Wu) and Guangxi Bagui Scholars Program (to Zuping Zhou).
REFERENCES
- 1.Anversa P, Rota M, Urbanek K, Hosoda T, Sonnenblick EH, Leri A, Kajstura J, Bolli R. Myocardial aging--a stem cell problem. Basic Res Cardiol. 2005;100:482–93. doi: 10.1007/s00395-005-0554-3. [DOI] [PubMed] [Google Scholar]
- 2.Hariharan N, Sussman MA. Cardiac aging - Getting to the stem of the problem. J Mol Cell Cardiol. 2015;83:32–6. doi: 10.1016/j.yjmcc.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho AD, Wagner W, Mahlknecht U. Stem cells and ageing. The potential of stem cells to overcome age-related deteriorations of the body in regenerative medicine. EMBO Rep. 2005;6:S35–8. doi: 10.1038/sj.embor.7400436. Spec No. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagai T, Matsuura K, Komuro I. Cardiac side population cells and Sca-1-positive cells. Methods Mol Biol. 2013;1036:63–74. doi: 10.1007/978-1-62703-511-8_5. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Chen H, Feng B, Wang X, He X, Hu R, Yin M, Wang W, Fu W, Xu Z. Isolation and characterization of a Sca-1+/CD31- progenitor cell lineage derived from mouse heart tissue. BMC Biotechnol. 2014;14:75. doi: 10.1186/1472-6750-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Li Q, Hu Q, Suntharalingam P, From AH, Zhang J. Intra-myocardial injection of both growth factors and heart derived Sca-1+/CD31- cells attenuates post-MI LV remodeling more than does cell transplantation alone: neither intervention enhances functionally significant cardiomyocyte regeneration. PLoS ONE. 2014;9:e95247. doi: 10.1371/journal.pone.0095247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rota M, Goichberg P, Anversa P, Leri A. Aging Effects on Cardiac Progenitor Cell Physiology. Compr Physiol. 2015;5:1705–50. doi: 10.1002/cphy.c140082. [DOI] [PubMed] [Google Scholar]
- 8.Cesselli D, Beltrami AP, D'Aurizio F, Marcon P, Bergamin N, Toffoletto B, Pandolfi M, Puppato E, Marino L, Signore S, Livi U, Verardo R, Piazza S, et al. Effects of age and heart failure on human cardiac stem cell function. Am J Pathol. 2011;179:349–66. doi: 10.1016/j.ajpath.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsiao LC, Perbellini F, Gomes RS, Tan JJ, Vieira S, Faggian G, Clarke K, Carr CA. Murine cardiosphere-derived cells are impaired by age but not by cardiac dystrophic dysfunction. Stem Cells Dev. 2014;23:1027–36. doi: 10.1089/scd.2013.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura T, Hosoyama T, Kawamura D, Takeuchi Y, Tanaka Y, Samura M, Ueno K, Nishimoto A, Kurazumi H, Suzuki R, Ito H, Sakata K, Mikamo A, et al. Influence of aging on the quantity and quality of human cardiac stem cells. Sci Rep. 2016;6:22781. doi: 10.1038/srep22781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yilmaz OH, Kiel MJ, Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107:924–30. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CY, Logan RW, Ma T, Lewis DA, Tseng GC, Sibille E, McClung CA. Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2016;113:206–11. doi: 10.1073/pnas.1508249112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fafian-Labora J, Fernandez-Pernas P, Fuentes I, De Toro J, Oreiro N, Sangiao-Alvarellos S, Mateos J, Arufe MC. Influence of age on rat bone-marrow mesenchymal stem cells potential. Sci Rep. 2015;5:16765. doi: 10.1038/srep16765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weigl Y, Ashkenazi IE, Peleg L. Rhythmic profiles of cell cycle and circadian clock gene transcripts in mice: a possible association between two periodic systems. J Exp Biol. 2013;216:2276–82. doi: 10.1242/jeb.081729. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302:255–9. doi: 10.1126/science.1086271. [DOI] [PubMed] [Google Scholar]
- 16.El Cheikh R, Bernard S, El Khatib N. Modeling circadian clock-cell cycle interaction effects on cell population growth rates. J Theor Biol. 2014;363:318–31. doi: 10.1016/j.jtbi.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Johnson CH. Circadian clocks and cell division: what's the pacemaker? Cell Cycle. 2010;9:3864–73. doi: 10.4161/cc.9.19.13205. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martino T, Arab S, Straume M, Belsham DD, Tata N, Cai F, Liu P, Trivieri M, Ralph M, Sole MJ. Day/night rhythms in gene expression of the normal murine heart. J Mol Med (Berl) 2004;82:256–64. doi: 10.1007/s00109-003-0520-1. [DOI] [PubMed] [Google Scholar]
- 19.Lavebratt C, Sjöholm LK, Partonen T, Schalling M, Forsell Y. PER2 variantion is associated with depression vulnerability. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:570–81. doi: 10.1002/ajmg.b.31021. [DOI] [PubMed] [Google Scholar]
- 20.Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, Taniguchi N, Ohno H, Kizaki T. A circadian clock gene, Rev-erbalpha, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol. 2014;192:407–17. doi: 10.4049/jimmunol.1301982. [DOI] [PubMed] [Google Scholar]
- 21.He Y, Lin F, Chen Y, Tan Z, Bai D, Zhao Q. Overexpression of the Circadian Clock Gene Rev-erbalpha Affects Murine Bone Mesenchymal Stem Cell Proliferation and Osteogenesis. Stem Cells Dev. 2015;24:1194–204. doi: 10.1089/scd.2014.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang R, Yan C, Zhou X, Qian B, Li F, Sun Y, Shi C, Li B, Saito S, Horimoto K, Zhou H. Association of Rev-erbα in adipose tissues with Type 2 diabetes mellitus amelioration after gastric bypass surgery in Goto-Kakizaki rats. Am J Physiol Regul Integr Comp Physiol. 2013;305:R134–46. doi: 10.1152/ajpregu.00520.2012. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Ao Y, Yang K, Tang H, Chen D. Circadian clock gene Per2 plays an important role in cell proliferation, apoptosis and cell cycle progression in human oral squamous cell carcinoma. Oncol Rep. 2016 doi: 10.3892/or.2016.4724. [DOI] [PubMed] [Google Scholar]
- 24.Wang CY, Wen MS, Wang HW, Hsieh IC, Li Y, Liu PY, Lin FC, Liao JK. Increased vascular senescence and impaired endothelial progenitor cell function mediated by mutation of circadian gene Per2. Circulation. 2008;118:2166–73. doi: 10.1161/circulationaha.108.790469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Morita Y, Han B, Niemann S, Loffler B, Rudolph KL. Per2 induction limits lymphoid-biased haematopoietic stem cells and lymphopoiesis in the context of DNA damage and ageing. Nat Cell Biol. 2016;18:480–90. doi: 10.1038/ncb3342. [DOI] [PubMed] [Google Scholar]
- 26.Durgan DJ, Tsai JY, Grenett MH, Pat BM, Ratcliffe WF, Villegas-Montoya C, Garvey ME, Nagendran J, Dyck JR, Bray MS, Gamble KL, Gimble JM, Young ME. Evidence suggesting that the cardiomyocyte circadian clock modulates responsiveness of the heart to hypertrophic stimuli in mice. Chronobiol Int. 2011;28:187–203. doi: 10.3109/07420528.2010.550406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. 2016;73:1765–86. doi: 10.1007/s00018-016-2147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kubicka-Trząska A, Karska-Basta I, Dziedzina S, Sanak M. [The genetic variability of complement system in pathogenesis of age-related macular degeneration] Klin Oczna. 2015;117:130–5. doi. [PubMed] [Google Scholar]
- 29.Mavroidis M, Davos CH, Psarras S, Varela A, N CA, Katsimpoulas M, Kostavasili I, Maasch C, Vater A, van Tintelen JP, Capetanaki Y. Complement system modulation as a target for treatment of arrhythmogenic cardiomyopathy. Basic Res Cardiol. 2015;110:27. doi: 10.1007/s00395-015-0485-6. [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Chen M. Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur J Pharmacol. 2016 doi: 10.1016/j.ejphar.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flachsbart F, Caliebe A, Heinsen FA, Hemming-Karlsen T, Schreiber S, Franke A, Nebel A. Investigation of complement component C4 copy number variation in human longevity. PLoS One. 2014;9:e86188. doi: 10.1371/journal.pone.0086188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Chung EK, Wu YL, Savelli SL, Nagaraja HN, Zhou B, Hebert M, Jones KN, Shu Y, Kitzmiller K, Blanchong CA, McBride KL, Higgins GC, et al. Gene copy-number variation and associated polymorphisms of complement component C4 in human systemic lupus erythematosus (SLE): low copy number is a risk factor for and high copy number is a protective factor against SLE susceptibility in European Americans. Am J Hum Genet. 2007;80:1037–54. doi: 10.1086/518257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reichwald J, Danner S, Wiederhold KH, Staufenbiel M. Expression of complement system components during aging and amyloid deposition in APP transgenic mice. J Neuroinflammation. 2009;6:35. doi: 10.1186/1742-2094-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Q, Zhan J, Li Y, Wang X, Xu L, Yu J, Pu S, Zhou Z. Differentiation-Associated MicroRNA Alterations in Mouse Heart-Derived Sca-1(+)CD31(-) and Sca-1(+)CD31(+) Cells. Stem Cells Int. 2016;2016:9586751. doi: 10.1155/2016/9586751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Z, French DL, Ma G, Eisenstein S, Chen Y, Divino CM, Keller G, Chen SH, Pan PY. Development and function of myeloid-derived suppressor cells generated from mouse embryonic and hematopoietic stem cells. Stem Cells. 2010;28:620–32. doi: 10.1002/stem.301. [DOI] [PMC free article] [PubMed] [Google Scholar]


