Abstract
We have developed two multiplex PCR assays that detect typical and atypical enteropathogenic Escherichia coli (EPEC) isolates, enteroaggregative E. coli (EAEC) isolates, enterotoxigenic E. coli (ETEC) isolates, enteroinvasive E. coli (EIEC) isolates, Shiga toxin-producing E. coli (STEC) isolates, and Shigella spp. The targets selected for each group were eae and bfpA for EPEC isolates, the target of probe CVD432 for EAEC isolates, the genes encoding heat-labile and heat-stable toxins for ETEC isolates, stx1 and stx2 for STEC isolates, and ipaH for EIEC isolates and Shigella spp. These PCRs were specific and sensitive for rapid detection of target isolates in stools. Among 150 stool specimens from the acute diarrhea tested, 9 samples (6%) had atypical EPEC, 9 (6%) had typical EPEC, 7 (4.7%) had EAEC, 3 (2%) had EIEC, 3 (2%) had Shigella spp., and 1 (0.7%) had an O26 STEC strain; we also detected mixed infections, 2 (1.3%) with EAEC and Shigella spp., 1 (0.7%) with atypical and typical EPEC strains, and another with atypical EPEC and EAEC strains. One of the multiplex PCRs directly applied to 36 stool specimens correctly identified 100% of EPEC and EAEC isolates.
Five categories of Escherichia coli have been well associated with diarrhea in several epidemiological studies (9): enteropathogenic E. coli (EPEC), enteroaggregative E. coli (EAEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), and Shiga toxin-producing E. coli (STEC). The virulence mechanisms that characterize these categories of E. coli are genetically encoded by chromosomal, plasmid, and bacteriophage DNAs and are represented by the following genes: eae (attaching and effacing lesions), bfpA (localized adherence), the gene encoding enteroaggregative adherence, ipaH (enteroinvasive mechanism), the genes encoding heat-labile toxin (LT) and heat-stable toxin (ST), and stx1 and stx2 (Shiga toxins). To correctly identify diarrheagenic E. coli strains, these organisms must be differentiated from nonpathogenic members of the normal flora. Serotypic markers correlate, sometimes very closely, with specific categories of diarrheagenic E. coli; however, these markers are rarely sufficient in and of themselves to reliably identify a strain as diarrheagenic. Thus, the detection of diarrheagenic E. coli has focused increasingly on the identification of certain characteristics which themselves determine the virulence of these organisms. This identification process may include HEp-2 cell adherence, DNA hybridization, and PCR assays to detect the presence of specific virulence traits or the genes encoding these traits. The first two types of assays require special expertise, employ cell culture and radioactive material, and are time-consuming.
We developed two multiplex PCR assays to detect the five categories of diarrheagenic E. coli organisms and Shigella spp. and assessed the direct application of those assays to human diarrheal stool samples.
The targets selected for each category were eae and bfpA for EPEC isolates, the target of probe CVD432 for EAEC isolates, the LT and ST genes for ETEC isolates, stx1 and stx2 for STEC isolates, and ipaH for EIEC isolates and Shigella spp. For each target gene, a different pair of primers was selected from the literature (Table 1). Multiplex PCR assay 1 utilizes three primer pairs and detects the presence of eae, bfpA, and the target of CVD432, generating amplification products of 917, 326, and 630 bp, respectively. Detection of eae confirms the presence of typical and/or atypical EPEC strains, while testing for bfpA confirms the presence of the bundle-forming pilus major subunit that is found only in typical EPEC strains (4, 5, 12). To include the identification of EAEC strains in the multiplex PCR, we selected a primer pair complementary to the EAEC probe sequence that detects 90% of EAEC strains (15). PCR assay 2 uses five primer pairs and detects the presence of the LT and ST genes, stx1, stx2, and ipaH, generating PCR products of distinct sizes which are easily distinguished after agarose gel electrophoresis. The primers detect the genes encoding LT and porcine and human ST in order to detect all types of ETEC in a single multiplex reaction (18). The stx1 and stx2 primers were designed to amplify Stx1 and all Stx2 variants (11). The ipaH sequences are present at multiple sites on both the large invasive plasmid and the chromosomes in Shigella spp. and EIEC strains (16).
TABLE 1.
PCR primers used in this study
| Primer designation | Primers (5′ to 3′) (reference)a | Target gene or probe | Amplicon size (bp) | Primer concn. (pmol) |
|---|---|---|---|---|
| eae1 | CTGAACGGCGATTACGCGAA | eae | 917 | 5 |
| eae2 | CCAGACGATACGATCCAG (12) | |||
| BFP1 | AATGGTGCTTGCGCTTGCTGC | bfpA | 326 | 5 |
| BFP2 | GCCGCTTTATCCAACCTGGTA (5) | |||
| EAEC1 | CTGGCGAAAGACTGTATCAT | CVD432 | 630 | 5 |
| EAEC2 | CAATGTATAGAAATCCGCTGTT (15) | |||
| LTf | GGCGACAGATTATACCGTGC | LT gene | 450 | 5 |
| LTr | CGGTCTCTATATTCCCTGTT (18) | |||
| STf | ATTTTTMTTTCTGTATTRTCTT | ST gene | 190 | 6.47 |
| STr | CACCCGGTACARGCAGGATT (18) | |||
| IpaH1 | GTTCCTTGACCGCCTTTCCGATACCGTC | ipaH | 600 | 10 |
| IpaH2 | GCCGGTCAGCCACCCTCTGAGAGTAC (16) | |||
| Stx1f | ATAAATCGCCATTCGTTGACTAC | stx1 | 180 | 3.88 |
| Stx1r | AGAACGCCCACTGAGATCATC (11) | |||
| Stx2f | GGCACTGTCTGAAACTGCTCC | stx2 | 255 | 2.5 |
| Stx2r | TCGCCAGTTATCTGACATTCTG (11) |
M, A/C; R, A/G.
All strains examined by PCR were grown on MacConkey agar plates at 37°C. DNA was extracted from bacteria by resuspending one bacterial colony in 50 μl of deionized water, boiling the suspension for 5 min, and centrifuging it at 10,000 × g for 1 min. The supernatant was then used as the DNA template for PCR. Having confirmed the specificity of each primer set by monoplex PCR, we combined the primer sets in different ratios and tested the reference strains with several PCR cycling protocols. The optimized protocol was carried out with a 50-μl mixture containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, a 2 mM concentration of each deoxynucleoside triphosphate, 1.5 U of AccuPrime Taq DNA polymerase (Invitrogen), 2 μl of the DNA template, and the PCR primers. The optimal concentration of each primer pair in the reaction mixture was determined empirically. Each primer pair concentration was varied independently until the PCR products exhibited equal intensities on 2% agarose gels when a DNA mixture of the five prototype E. coli strains was used as the PCR template. The concentration for each primer pair used in the final reactions is given in Table 1. The PCR mixtures were then subjected to the following cycling conditions: for assay 1, 50°C (2 min, 1 cycle); 95°C (5 min, 1 cycle); 40 cycles of 95°C (40 s), 58°C (1 min), and 72°C (2 min); and a final extension step at 72°C (7 min, 1 cycle); and for assay 2, 50°C (2 min, 1 cycle); 95°C (5 min, 1 cycle); 40 cycles of 95°C (45 s), 50°C (1 min), and 72°C (1 min); and 72°C (7 min, 1 cycle) in a thermal cycler (model system 2400; Perkin-Elmer Corporation, Norwalk, Conn.). PCR products (10 μl) were visualized after electrophoresis in 2% agarose gels in Tris-borate-EDTA buffer and ethidium bromide staining. In all experiments, the DNA mixture from the prototype EPEC E2348/69, EAEC O42, ETEC H10407, EIEC EDL1284, and STEC EDL931 strains served as the positive control (9), while E. coli K-12 DH5α was the negative control.
The two multiplex PCRs were further validated with 270 additional reference strains. One hundred EPEC and 50 EAEC reference strains were identified in a previous case-control study by their reactivity with the eae, EPEC adherence factor, and EAEC probes (4, 14). Fifty ETEC, 20 EIEC, and 50 Shigella species reference strains were also identified by DNA hybridization by other laboratories. The strains were subjected to both multiplex PCRs, and the results were compared with those obtained by monoplex PCR. Both multiplex PCR assays showed 100% specificity in identifying the reference strains; most importantly, nonspecific bands were not visualized. The same results were seen when DNA from the reference E. coli strains was mixed and used in the multiplex PCR assays (Fig. 1 and 2).
FIG. 1.
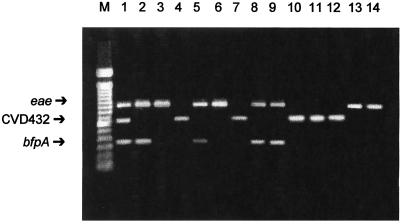
Multiplex PCR assay 1 of reference strains and clinical and stool samples. Lane 1, EPEC E2348/69 (eae and bfpA) and EAEC 042 (CVD432); lane 2, EPEC HSP43-1 (eae and bfpA); lane 3, atypical EPEC MA343-4 (eae); lane 4, EAEC MA233-1 (CVD432); lane 5, patient sample 5 (eae and bfpA); lane 6, patient sample 6 (eae); lane 7, patient sample 29 (CVD432); lane 8, stool sample 2 (eae and bfpA); lane 9, stool sample 8 (eae and bfpA); lane 10, stool sample 3 (CVD432); lane 11, stool sample 5 (CVD432); lane 12, stool sample 12 (CVD432); lane 13, stool sample 33 (eae); lane 14, stool sample 34 (eae); lane M, DNA molecular size markers (100-bp ladder).
FIG. 2.
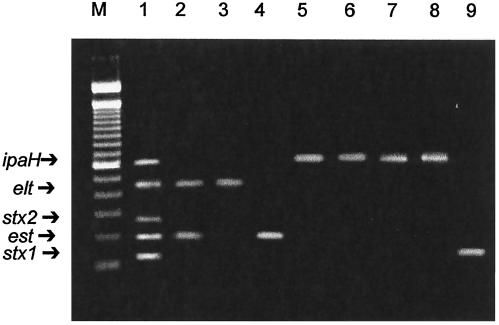
Multiplex PCR assay 2 of reference strains and clinical samples. Lane 1, ETEC H10407 (LT gene and ST gene), EIEC EDL1285 (ipaH), and STEC EDL931 (stx1 and stx2); lane 2, ETEC 1111-1 (LT gene and ST gene); lane 3, ETEC 2811-1 (LT gene); lane 4, ETEC 2021-1 (ST gene); lane 5, EIEC 733/6 (ipaH); lane 6, S. flexneri 245-5 (ipaH); lane 7, patient sample 48 (ipaH); lane 8, patient sample 75 (ipaH); lane 9, patient sample 127 (stx1); lane M, DNA molecular size markers (100-bp ladder).
To demonstrate the diagnostic usefulness of both multiplex PCR assays, we examined bacterial colonies isolated from stool specimens obtained from 150 children less than 5 years old who had been assisted in the emergency room of Hospital São Paulo, which provides public medical assistance to children of low socioeconomic status in the city of São Paulo, Brazil. Every fecal specimen was examined by standard methods for the presence of Shigella spp., Salmonella spp., Giardia lamblia, Yersinia enterocolitica, Campylobacter spp., Cryptosporidium spp., and rotavirus. Four separate lactose-fermenting colonies and two non-lactose-fermenting colonies from each patient were cultivated in commercial test systems (PROBAC do Brasil, São Paulo) for biochemical confirmation of species or genus. All isolates of E. coli and Shigella spp. were screened by colony hybridization with EPEC adherence factor (8), the E. coli attaching and effacing gene encoding intimin (eae) (6), diffuse adherence factors (daaC and AIDA-I) (2, 3), EAEC adherence factor (1), enterotoxins LT and ST (7), the enteroinvasiveness factor (Inv) (17), and Shiga toxin probes (10). These probes were labeled with [α-32P]dCTP with a random primer extension kit (Rediprime DNA labeling system; Amersham). Colony hybridization assays were performed as previously described elsewhere (13). The identified strains of E. coli and Shigella spp. were subjected to the multiplex PCR assays, and the results were compared with those obtained by DNA probe hybridization.
A total of 267 E. coli and 17 Shigella species strains isolated from 150 patients were subjected to both multiplex PCRs and DNA hybridization assays (Table 2). Thirty-six (24%) of the 150 patients had a potentially diarrheagenic strain of E. coli or Shigella spp. in their stool samples. Atypical and typical EPEC strains were isolated from nine children each. Seven children were infected with an EAEC strain, three children were infected with an EIEC strain, and one child was infected with an O26 STEC strain. Shigella spp. were isolated from three children. One child (patient 66) was infected with atypical and typical EPEC strains, one child (patient 118) was infected with atypical EPEC and EAEC strains, and two children were infected with EAEC and Shigella species (patients 55 and 131) strains. For patient 127, an STEC strain of serogroup O26 was detected, which was positive for the eae and stx1 genes. None of the other 114 patients with diarrhea had E. coli strains containing the target genes in their stools. There was agreement between results of the PCR multiplex and DNA hybridization assays for almost all strains. For patients 4 and 99, one more E. coli strain positive for the eae gene was detected by PCR assays, and, for patient 150, two more E. coli strains positive for the eae gene were detected by PCR assays. For patients 103 and 117, one more gene-positive strain was detected with the DNA probe.
TABLE 2.
Diarrheagenic isolates of E. coli and Shigella spp. from patients
| Patient | Age | E. coli group and/or Shigella sp. | Gene(s) and/or probe | No. of tested strains/no. of positive strains
|
|
|---|---|---|---|---|---|
| PCR | DNA probe | ||||
| 4 | 9 mo | Atypical EPEC | eae | 3/3 | 3/2 |
| 5 | 1 yr 4 mo | Typical EPEC | eae, bfpA | 5/2 | 5/2 |
| 6 | 1 yr 8 mo | Atypical EPEC | eae | 3/2 | 3/2 |
| 7 | 2 mo | Typical EPEC | eae, bfpA | 2/2 | 2/2 |
| 8 | 3 mo | Typical EPEC | eae, bfpA | 3/2 | 3/2 |
| 10 | 1 yr | Typical EPEC | eae, bfpA | 1/1 | 1/1 |
| 14 | 6 mo | Atypical EPEC | eae | 3/2 | 3/2 |
| 17 | 7 mo | Typical EPEC | eae, bfpA | 3/3 | 3/3 |
| 25 | 2 yr | EIEC | ipaH | 2/1 | 2/1 |
| 27 | 2 yr | EIEC | ipaH | 3/1 | 3/1 |
| 29 | 2 yr | EAEC | CVD432 | 4/1 | 4/1 |
| 30 | 2 yr | EAEC | CVD432 | 3/2 | 3/2 |
| 35 | 5 mo 11 days | Atypical EPEC | eae | 2/1 | 3/1 |
| 39 | 1 yr 6 mo | EAEC | CVD432 | 3/3 | 3/3 |
| 42 | 3 mo | Typical EPEC | eae, bfpA | 5/2 | 5/2 |
| 44 | 1 yr 8 mo | Atypical EPEC | eae | 5/1 | 5/1 |
| 48 | 10 mo | Shigella flexneri | ipaH | 5/1 | 5/1 |
| 55 | 8 mo | EAEC | CVD432 | 3/1 | 3/1 |
| Shigella flexneri | ipaH | 4/4 | 4/4 | ||
| 66 | 12 mo | Typical and atypical EPEC | eae, bfpA | 5/4 | 5/4 |
| 75 | 1 yr 6 mo | EIEC | ipaH | 5/2 | 5/2 |
| 77 | 5 mo 21 days | Atypical EPEC | eae | 3/2 | 3/2 |
| 78 | 46 days | EAEC | CVD432 | 4/2 | 4/2 |
| 80 | 2 yr | Shigella sonnei | ipaH | 3/1 | 3/1 |
| 82 | 3 mo | EAEC | CVD432 | 4/3 | 4/3 |
| 85 | 9 mo | Typical EPEC | eae, bfpA | 4/3 | 4/3 |
| 96 | 1 yr 2 mo | EAEC | CVD432 | 5/1 | 5/1 |
| 99 | 1 yr 10 mo | Atypical EPEC | eae | 3/2 | 3/1 |
| 103 | 2 yr 6 mo | Atypical EPEC | eae | 3/2 | 3/3 |
| 114 | 1 yr 8 mo | Shigella sonnei | ipaH | 2/1 | 2/1 |
| 117 | 5 mo | Typical EPEC | eae, bfpA | 3/2 | 3/3 |
| 118 | 2 mo | Atypical EPEC, EAEC | eae, CVD432 | 4/2 | 4/2 |
| 127 | 3 yr | STEC | eae, stx1 | 3/3 | 3/3 |
| 131 | 1 yr 2 mo | EAEC | CVD432 | 3/2 | 3/2 |
| Shigella flexneri | ipaH | 3/1 | 3/1 | ||
| 140 | 10 mo | Typical EPEC | eae, bfpA | 4/1 | 4/1 |
| 144 | 2 mo | EAEC | CVD432 | 6/1 | 6/1 |
| 150 | 2 mo | Atypical EPEC | eae | 3/3 | 3/1 |
To assess the sensitivity of the multiplex PCR assays, overnight cultures of prototype strains were suspended in phosphate-buffered saline at a MacFarland standard of 1, which is equivalent to 3 × 108 CFU of E. coli, and were serially diluted 10-fold. DNA was extracted from these samples and subjected to both multiplex PCRs. The sensitivity of detection was 103 CFU per assay for all target genes. The PCR assays were then used to detect diarrheagenic E. coli strains directly from fecal samples spiked with different concentrations of prototype strains. The prototype strains, suspended in phosphate-buffered saline at different concentrations, were used to spike 180 mg of stool specimen. The DNA was isolated from the spiked stool specimens by use of the QIAamp stool mini kit (QIAGEN) and subjected to both multiplex PCRs. The method accurately detected the presence of all gene products in 180 mg of feces seeded with 3 × 108 CFU of a single strain and was unable to detect prototype strains at concentrations less than 108 CFU. Also, the presence of several E. coli strains together (each of them at 3 × 108 CFU) in a fecal sample allowed visualization of bands specific for each strain (data not shown). The detection limit was found to be approximately 1.7 × 106 CFU per g of feces.
This method was then directly applied to 36 stool specimens to detect diarrheagenic E. coli and Shigella spp. These specimens were obtained from infants hospitalized in Hospital São Paulo with diarrhea or other gastrointestinal alterations. The stools were watery, and no mucus or blood was present. Most of the children were dehydrated and needed treatment with fluids and electrolytes. None of the 36 infants received antibiotics, and the duration of diarrhea was less than 1 week (median, 5 days), starting before hospitalization. Each fecal sample, obtained upon admission, was directly subjected to both multiplex PCRs and was analyzed by conventional assays, such as biochemical identification, serotyping, and DNA hybridization with specific DNA probes (Table 3). Seven (19.4%) of the 36 fecal specimens were multiplex PCR positive: 2 specimens were positive for typical EPEC isolates (O111 and O142 serogroups), 3 were positive for EAEC isolates, and 2 were positive for atypical EPEC isolates in assay 1 (Fig. 1). The same results were obtained by colony DNA hybridization with specific DNA probes. All fecal samples tested were negative by PCR assay 2.
TABLE 3.
Comparison of results with multiplex PCR, DNA probe hybridization, and conventional assays to detect diarrheagenic E. coli strains
| Stool sample | Diarrheagenic E. coli type | Target(s) detecteda:
|
Serotyping resultb | |
|---|---|---|---|---|
| By multiplex PCR assay 1 | With DNA probe | |||
| 2 | Typical EPEC | eae, bfpA | eae, EAF | E. coli O111 |
| 8 | Typical EPEC | eae, bfpA | eae, EAF | E. coli O142 |
| 3 | EAEC | CVD432 | EAEC target | E. coli OND |
| 5 | EAEC | CVD432 | EAEC target | E. coli OND |
| 12 | EAEC | CVD432 | EAEC target | E. coli OND |
| 33 | Atypical EPEC | eae | eae | E. coli OND |
| 34 | Atypical EPEC | eae | eae | E. coli OND |
EAF, EPEC adherence factor.
OND, serogroup not determined.
Both multiple PCR assays showed very high specificity when compared to conventional methods for detecting the virulence genes. This high specificity was demonstrated using several reference strains as well as clinical isolates. There was complete agreement between the results of single and multiplex PCRs for all reference strains tested. In an epidemiological study, we compared standard methods, including colony blot hybridization, with multiplex PCR assays for the identification of diarrheagenic E. coli and Shigella spp. in the diarrhea of 150 children. There was total agreement between the results of multiplex PCRs and DNA hybridization for all tested isolates.
The PCR multiplex assays were also sensitive for the detection of diarrheagenic E. coli. Stool specimens spiked with 108 CFU from cultures of prototype E. coli strains generated a specific PCR product which was visible on an ethidium bromide-stained agarose gel. This corresponds to 106 bacteria per g of feces. Of course, many more strains should be tested before concluding that the PCR assays have 100% sensitivity.
Most importantly, the two multiplex PCR assays were also found to be effective for direct detection of EPEC, EAEC, ETEC, STEC, EIEC, and Shigella spp. in stool specimens from 36 patients with diarrhea. The specificities of both multiplex PCRs were evidenced by the absence of nonspecific PCR products in feces from children without any diarrheagenic E. coli or Shigella spp.
In conclusion, the two multiplex PCR assays presented in this paper correctly determined the presence of corresponding diarrheagenic E. coli and Shigella species virulence genes in all strains tested. Multiplex PCR assay 1 correctly identified 100% of EPEC and EAEC isolates directly in stool specimens. Although assay 1 cannot detect all EAEC isolates and assay 2 cannot distinguish EIEC from Shigella species or one Shigella species from another, these multiplex PCR assays offer a practical possibility for rapid identification of diarrheagenic E. coli and Shigella spp. and could be used in the routine diagnostic laboratory.
Acknowledgments
We thank Beatriz E. C. Guth and Kinue Irino for the gift of ETEC reference strains. We also thank Elaine G. Rodrigues and Laura Quinn Leverton for critical reading of the manuscript and helpful suggestions.
This work was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
REFERENCES
- 1.Baudry, B., S. J. Savarino, P. Vial, J. B. Kaper, and M. M. Levine. 1990. A sensitive and specific DNA probe to identify enteroaggregative E. coli, a recently discovered diarrheal pathogen. J. Infect. Dis. 161:1249-1251. [DOI] [PubMed] [Google Scholar]
- 2.Benz, I., and M. A. Schmidt. 1989. Cloning and expression of an adhesin (AIDA-I) involved in diffuse adherence of enteropathogenic Escherichia coli. Infect. Immun. 57:1506-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilge, S. S., C. R. Clausen, W. Lau, and S. L. Moseley. 1989. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J. Bacteriol. 171:4281-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dulguer, M. V., S. H. Fabbricotti, S. Y. Bando, C. A. Moreira-Filho, U. Fagundes-Neto, and I. C. A. Scaletsky. 2003. Atypical enteropathogenic Escherichia coli strains: phenotypic and genetic profiling reveals a strong association between enteroaggregative E. coli heat-stable enterotoxin and diarrhea. J. Infect. Dis. 188:1685-1694. [DOI] [PubMed] [Google Scholar]
- 5.Gunzburg, S. T., N. G. Tornieporth, and L. W. Riley. 1995. Identification of enteropathogenic Escherichia coli by PCR-based detection of the bundle-forming pilus gene. J. Clin. Microbiol. 33:1375-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jerse, A. E., Y. Jun, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moseley, S. L., I. Huq, A. R. M. A. Alim, M. So, M. Samadpour-Motalebi, and S. Falkow. 1980. Detection of enterotoxigenic Escherichia coli by DNA colony hybridization. J. Infect. Dis. 142:892-898. [DOI] [PubMed] [Google Scholar]
- 8.Nataro, J. P., M. M. Baldini, J. B. Kaper, R. E. Black, N. Bravo, and M. M. Levine. 1985. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J. Infect. Dis. 152:560-565. [DOI] [PubMed] [Google Scholar]
- 9.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newland, J. W., and R. J. Neill. 1988. DNA probes for Shiga-like toxins I and II and for toxin-converting bacteriophages. J. Clin. Microbiol. 26:1292-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paton, A. W., and J. C. Paton. 1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36:598-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid, S. D., D. J. Betting, and T. S. Whittam. 1999. Molecular detection and identification of intimin alleles in pathogenic Escherichia coli by multiplex PCR. J. Clin. Microbiol. 37:2719-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 14.Scaletsky, I. C. A., S. H. Fabbricotti, K. R. Aranda, M. B. Morais, and U. Fagundes-Neto. 2002. Comparison of DNA hybridization and PCR assays for detection of putative pathogenic enteroadherent Escherichia coli. J. Clin. Microbiol. 40:1254-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt, H., C. Knop, S. Franke, S. Aleksic, J. Heesemann, and H. Karch. 1995. Development of PCR for screening of enteroaggregative Escherichia coli. J. Clin. Microbiol. 33:701-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sethabutr, O., M. Venkatesan, G. S. Murphy, B. Eampokalap, C. W. Hoge, and P. Echeverria. 1993. Detection of Shigella and enteroinvasive Escherichia coli by amplification of the invasion plasmid antigen H DNA sequence in patients with dysentery. J. Infect. Dis. 167:458-461. [DOI] [PubMed] [Google Scholar]
- 17.Small, P. L., and S. Falkow. 1986. Development of a DNA probe for the virulence plasmid of Shigella spp. and enteroinvasive Escherichia coli, p. 121-124. In L. Leive, P. F. Bonventre, J. A. Morello, S. D. Silver, and W. C. Wu (ed.), Microbiology—1986. American Society for Microbiology, Washington, D.C.
- 18.Stacy-Phipps, S., J. J. Mecca, and J. B. Weiss. 1995. Multiplex PCR assay and simple preparation method for stool specimens detect enterotoxigenic Escherichia coli DNA during course of infection. J. Clin. Microbiol. 33:1054-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]