Abstract
Exposure to drugs of abuse can produce many neurobiological changes which may lead to increased valuation of rewards and decreased sensitivity to their costs. Many of these behavioral alterations are associated with activity of D2-expressing medium spiny neurons in the striatum. Additionally, Bdnf in the striatum has been shown to play a role in flexible reward-seeking behavior. Given that voluntary aerobic exercise can affect the expression of these proteins in healthy subjects, and that exercise has shown promise as an anti-addictive therapy, we set out to quantify changes in D2 and Bdnf expression in methamphetamine-exposed rats given access to running wheels. Sixty-four rats were treated for two weeks with an escalating dose of methamphetamine or saline, then either sacrificed, housed in standard cages, or given free access to a running wheel for 6 weeks prior to sacrifice. Rats treated with methamphetamine ran significantly greater distances than saline-treated rats, suggesting an augmentation in the reinforcement value of voluntary wheel running. Transcription of Drd2 and Bdnf was assessed via RT-qPCR. Protein expression levels of D2 and phosphorylation of the TrkB receptor were measured via western blot. Drd2 and Bdnf mRNA levels were impacted independently by exercise and methamphetamine, but exposure to methamphetamine prior to the initiation of exercise blocked the exercise-induced changes seen in rats treated with saline. Expression levels of both proteins were elevated immediately after methamphetamine, but returned to baseline after six weeks, regardless of exercise status.
Keywords: Psychostimulant, Withdrawal, Inflammation, Frontocortical, Striatal, D2 receptors, TrkB
1. Introduction
In order to engage in adaptive decision making, an animal must compare previously experienced reward with currently available reward sources to determine optimal effort expenditure. Flexible responses to reward require maintenance of reward history that incorporates both frequency of reward and overall reward availability in an environment. Substance abuse can be conceptualized, in part, as a consequence of this adaptive response to a distorted percept of reward history and availability brought about by exposure to a drug (Nesse, 1994). Many drugs of abuse, including methamphetamine, exert their reinforcing effects by dramatically increasing dopamine (DA) signaling in the striatum, mimicking the effects of many natural rewards. Neuroplastic changes, especially adaptations in DA receptors and transporters, have been found to contribute to many of the cognitive and behavioral changes that are associated with compulsive drug seeking and addiction (Groman et al., 2013; Kosheleff et al., 2012; Izquierdo et al., 2010). Experience with methamphetamine, therefore, may recalibrate striatal reward circuitry to change reward valuation of and responses to future rewards. In support of this idea, we recently reported that repeated, escalating methamphetamine pretreatment increased the ability to learn from positive feedback in reversal learning during protracted methamphetamine withdrawal, when reward history and current reward experiences are most opposed (Stolyarova et al., 2014a). Responses to positive feedback have been associated with DA transporter binding (Stolyarova et al., 2014a) and variation in D2 receptor availability (Groman et al., 2011). Additionally, we found increased willingness to work for large-over-small food rewards after methamphetamine in an effortful decision-making task in rats (Stolyarova et al., 2014b), indicative of increased reward sensitivity to natural reinforcement following drug exposure. Indeed, genetic knockout of D2 receptors in the ventral striatum impairs motivation (Tran et al., 2002), while viral overexpression of postsynaptic D2 increases motivation to expend effort for rewards (Trifilieff et al., 2012), strongly implicating dysregulated D2 signaling in altered reward learning following methamphetamine.
Voluntary wheel running has also been shown to induce plastic changes in the mesolimbic reward pathways, including D2 transcription (Greenwood et al., 2011). Aerobic exercise has been proposed as a reinforcing behavior that can potentially normalize DA and glutamatergic signaling in addiction (Lynch et al., 2013; O'Dell et al., 2012; Sobieraj et al., 2014) and lead to improvements in various facets of cognition (Gomez-Pinilla and Hillman, 2013; Creer et al., 2010), including those abilities of particular relevance to cognitive flexibility, that depend on striatal DA (Eddy et al., 2014). Additionally, exercise has been shown to be protective against addiction at all stages of its progression. Six weeks of voluntary running attenuates acquisition and maintenance of methamphetamine self-administration behavior (Engelmann et al., 2014), and blocks drug- and cue-primed reinstatement to cocaine self-administration (Smith and Witte, 2012).
Altered reward learning following methamphetamine also suggests a role for proteins involved in cognition such as brain-derived neurotrophic factor (BDNF), which is critical for cell survival and synaptic signaling. Infusion of exogenous BDNF into the striatum enhances cognitive flexibility in a strategy set-shifting task (D'Amore et al., 2013), whereas methamphetamine exposure results in impairments (Groman et al., 2013; Parsegian et al., 2011). Voluntary aerobic exercise (wheel running) increases Bdnf exon IV transcription by affecting the activity of epigenetic regulatory proteins (Gomez-Pinilla et al., 2011). Given the foregoing, we hypothesized that treatment with methamphetamine would reduce the expression of Bdnf mRNA in the striatum, and that this would be normalized by voluntary aerobic exercise. We further hypothesized that exercise would also normalize methamphetamine induced alterations in Drd2 transcription. To test this we treated rats with methamphetamine or saline, and either allowed or did not allow them access to an exercise wheel. Following this treatment, we assessed transcription of Drd2 and Bdnf in the striatum, as well as protein expression levels of D2 and activation of the TrkB receptor in the striatum. Additionally, we measured Bdnf mRNA in the frontal cortex due to the region's role as a major source of striatal Bdnf.
2. Methods and materials
2.1. Subjects
Sixty-four male Long-Evans rats (Charles River Laboratories, Raleigh, NC) weighing between 250 and 300 g at the beginning of the study were maintained under a 12-hr light/12-hr dark cycle (lights on 6:00–18:00) under temperature- and humidity-controlled conditions. Food and water were available ad libitum. After arrival in the facility, animals were left undisturbed for 3 days to acclimate to the vivarium, then individually handled over the next 5 days for a minimum of 10 min per day. During acclimation, handling, and methamphetamine pre-exposure, rats were pair-housed; each methamphetamine-treated rat was housed with a saline-treated rat to minimize aggression. During exercise periods, all rats were singly-housed. All experiments were performed in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of California at Los Angeles, Chancellor's Animal Research Committee.
2.2. Drug treatment
Rats were treated with methamphetamine using a subchronic escalating regimen adapted from Segal et al. (2003), which was shown to be protective against DA neurotoxicity following subsequent binge exposure. In short, rats were given three daily injections of d-methamphetamine (“mAMPH”, n = 32; Sigma, St. Louis, MO; 0.1–6.0 mg free base/kg, s.c., escalating in 0.1 mg/kg increments up to 2.1 mg/kg, then in 0.2 mg/kg increments from 2.1 mg/kg to 6.0 mg/kg) or physiological saline solution (“Sal”, n = 32; 1 ml/kg, s.c.) for two weeks. Injections took place during the light cycle, at 10:00, 13:15, and 16:30. One methamphetamine-treated animal succumbed to cerebral ischemia following the second-highest dose, and was excluded from the dataset.
2.3. Voluntary wheel running
Following methamphetamine treatment, a subset of rats were individually housed either in standard shoebox cages (“Sed”, n = 23) or in cages equipped with running wheels with radius = 0.175 m (“Ex”, n = 24) for 6 weeks. The remaining 16 rats were euthanized the day after cessation of drug treatment (Fig. 1). Hourly wheel revolutions and running distance were recorded for exercising rats. The running intensity data from 8 rats were unanalyzable due to a malfunction in the magnetic switches used to record these data. These rats were excluded from any analysis involving running intensity, but included for analyses in which presence or absence of a running wheel was an independent variable. Subsequently, data were recorded digitally using an optical counter and Lafayette Activity Wheel Monitor (AWM) Software.
Fig. 1.
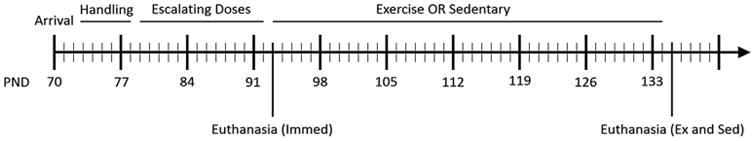
Experimental timeline. Subjects arrived at our facilities on PND70, and were allowed to acclimate to environmental conditions and experimenter handling. Escalating doses of methamphetamine or saline were given for two weeks from PND 78–92, then animals were divided into three groups. One group was sacrificed immediately (n = 16), the second was placed in standard shoebox cages for six weeks (n = 23), and the third was placed in cages equipped with running wheels for six weeks (n = 24). At the conclusion of the exercise or sedentary period, all animals were sacrificed.
2.4. Real-time quantitative PCR
As outlined above, rats were euthanized either 6 weeks (n = 47) or one day (“Immed”, n = 16) after the final methamphetamine or Sal treatment with an overdose of sodium pentobarbital (250 mg/kg, i.p.) and decapitated. Bilateral frontal cortex and striatum were rapidly dissected over a cold plate at 4 °C and flash frozen by immersion in isopentane over dry ice before being stored at −80 °C. Frontocortical dissections included ventral (orbital) and medial sectors of the frontal cortex, but excluded most lateral, posterior regions (agranular insular). Striatal dissections included both dorsal and ventral subregions. Total RNA was isolated using the Direct-zol RNA MiniPrep kit (Zymo Research) as per the manufacturer's protocol. The total RNA was converted to cDNA using qScript cDNA SuperMix kit (Quanta Bioscience) and the Bdnf and Drd2 mRNA content was measured using PerfeCTa SYBR Green FastMix kit (Quanta Bioscience) by the CFX96 Real-Time PCR Detection System (Bio-Rad). The Gapdh gene was used as an endogenous control to standardize sample loading volumes.
2.5. Western blotting
Dissected tissue was homogenized in lysis buffer (137 mM NaCl, 20 mM Tris-HCl pH 8.0, 1% NP40, 10% glycerol, 1 mM PMSF, 10 ug/ml aprotinin, 0.1 mM benzothonium, 0.5 mM sodium vanadate). After centrifuging at 12,500 g for 20 min, supernatants were collected and immediately processed for total protein concentration determination according to the Micro BCA procedure (Pierce, Rockford, IL, USA), using bovine serum albumin as standard. All chemicals were obtained from Sigma (St. Louis, MO, USA) unless otherwise noted. Total TrkB, phospho-TrkB, and Dopamine D2 Receptor protein samples were analyzed. The ratio of phospho-TrkB to total TrkB was obtained because the ratio provides information about signaling through the receptor, or the proportion of the total receptor being activated. Total TrkB and pTrkB were in the same loading. The membrane was stripped to run total TrkB after pTrkB. For D2, actin was utilized as an internal control, and each blot was standardized to its corresponding actin value. Protein samples were separated by electrophoresis on a 10% polyacrylamide gel and electrotransferred to a PVDF membrane. Non-specific binding sites were blocked in TBS with 2% BSA and 0.1% Tween-20 for 1 h at room temperature. Membranes were rinsed in buffer (0.1% Tween-20 in TBS) and incubated at 4 °C overnight, with anti-TrkB and phospho-TrkB (1:1,000, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and anti-D2 receptor (1:1000, EMD millipore, Billerica, MA, USA) followed by anti-Rabbit IgG horseradish peroxidase-conjugate (1:100,000 Santa Cruz Biotechnology Inc., CA, USA). Anti-Actin (1:1000, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) was followed by anti-goat IgG horseradish peroxidase-conjugate (1:100,000 Santa Cruz Biotechnology Inc., CA, USA). After rinsing in buffer four times for 10 min, immunocomplexes were analyzed by chemiluminescence using the ECL Plus kit (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA), according to manufacturer's instructions.
2.6. Data analyses
Software package SPSS (SAS Institute, Inc., Version 16.0) was used for statistical analysis. Statistical significance was noted when p-values were equal to or less than 0.05. Voluntary running data, daily distance and hourly running distance were analyzed using repeated-measures ANOVAs (rmANOVA) with treatment group as a between subject factor. Where significant day*treatment group interaction was observed, post hoc independent samples t-tests were conducted for each day. Where significant hour*treatment group interaction was observed, post hoc independent sample t-tests were conducted for each hour. Cumulative running data were analyzed using independent samples t-tests. Two-way ANOVA followed by Bonferroni post hoc comparisons with Sal/Sed as control was conducted for between-group mRNA comparisons, and two-way ANOVA followed by Bonferroni post hoc comparisons with Sal/Immed as control was conducted for between-group protein comparisons. There were no significant differences between Sal/Immed and Sal/Sed. The results were expressed as mean percent of control values and represent the mean ± standard error of the mean (S.E.M). Pearson correlation coefficients were calculated to examine the relationship between mRNA expression in different brain regions and running data.
3. Results
3.1. Running data
Daily running distance data were subjected to rmANOVA with day as a within- and treatment group as between-subject factors. A rmANOVA detected significant main effect of day [F(41,533) = 15.51, p < 0.0001], significant main effect of treatment group [F(1,13) = 18.79, p < 0.001], and a significant day × treatment group interaction [F(41,533) = 4.35, p < 0.0001]. Post hoc comparisons further revealed a significant simple main effect of treatment group at most time points analyzed (p < 0.05), after day 8, with methamphetamine-treated animals running more than controls. Between-group differences in cumulative running by the end of the 6-week period were analyzed with independent samples t-test. Methamphetamine treated animals ran significantly greater distance compared to saline treated animals [t(13) = 4.334, p< 0.001] (Fig. 2a).
Fig. 2.
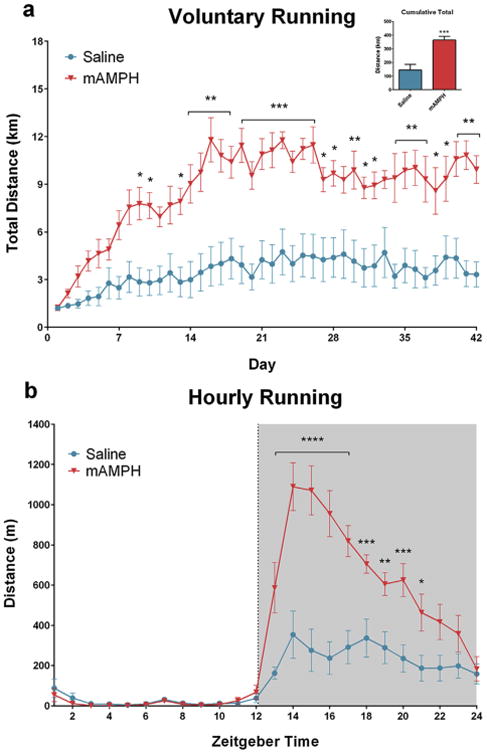
Voluntary running intensity. (a) Rats treated with methamphetamine (n = 15) ran significantly greater distances each day than their saline-treated counterparts (n = 16). This difference lasted at least six weeks after cessation of drug treatment. By the end of the six week running period, methamphetamine-treated rats had accumulated an average of 219 km more than saline-treated animals (inset). (b) Most of the running by methamphetamine-treated rats occurred during the first 4 h of the dark phase, while saline-treated rats distributed their running evenly.
Hourly running data were analyzed using rmANOVA with time of the day (ZT) as within- and treatment group as between-subject factors. A significant main effect of time [F(23,299) = 45.82, p < 0.0001], treatment group [F(1,13) = 17.55, p < 0.01] and a time*treatment group interaction [F(23,299) = 13.59, p < 0.0001] were observed. Post hoc analyses revealed a significant simple main effect of time with all animals increasing their running after ZT12 (p < 0.05) and maintained throughout the dark phase (p < 0.01) (Fig. 2b). Significant simple main effects of treatment group have also been observed with the methamphetamine treatment group increasing their running significantly more than controls at ZT13–ZT23 (p < 0.05).
3.2. Drd2 and D2
Two-way ANOVA with drug (Sal vs. mAMPH) and condition (Immed vs. Sed vs. Ex) as between-subject factors was conducted. Where appropriate, Bonferroni post hoc tests were used to correct for multiple comparisons. The results for Drd2 mRNA and D2 protein were expressed as mean percent of control values (Sal/Immed) and represent the mean ± standard error of the mean (S.E.M).
Significant main effects of drug [F(1,56) = 72.75, p < 0.0001] and condition [F(2,56) = 18.55, p < 0.0001] on striatal Drd2 mRNA expression were observed, in addition to a significant interaction between the two factors [F(2,56) = 7.74, p = 0.0011]. Post hoc tests revealed that Sal/Ex and mAMPH/Sed differed significantly from Sal/Immed controls (p < 0.0001 and p = 0.0025, respectively), and that there were significant differences between Sal/Sed and mAMPH/Sed (p = 0.0001), and between Sal/Ex and mAMPH/Ex (p < 0.0001) (Fig. 3A).
Fig. 3.
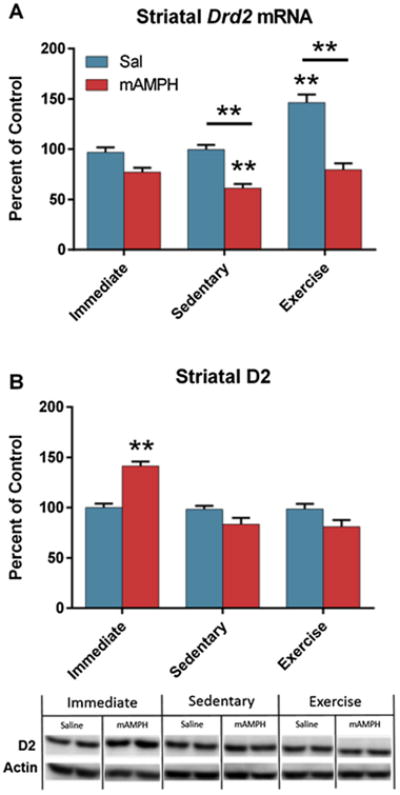
Drd2 and D2 results. (A) Exercise significantly increased striatal Drd2 mRNA (p < 0.0001). Six weeks of withdrawal from methamphetamine significantly reduced striatal Drd2 transcription relative to saline-treated controls. If exercise was preceded by methamphetamine, however, there was no effect of exercise. Stars indicate significance relative to Sal/Immed, stars with bars indicate significant differences between the groups under the bars. (B) Acute withdrawal from methamphetamine upregulated striatal D2 expression (p < 0.001). Representative Western blot bands are shown below quantification.
A significant between-group difference in striatal D2 receptor expression was observed [F(5,56) = 13.37, p < 0.0001]. Post hoc tests revealed a significant difference between Sal/Immed and mAMPH/Immed (p < 0.0001), but no differences between Sal/Immed and any other group (Fig. 3B).
3.3. Bdnf and phospho-TrkB
Two-way ANOVA with drug (Sal vs. mAMPH) and condition (Immed vs. Sed vs. Ex) as between-subject factors were conducted. Where appropriate, Bonferroni post hoc tests were used to correct for multiple comparisons. The results for Bdnf mRNA and phospho-TrkB vs. TrkB were expressed as mean percent of Sal/Immed values.
There were significant main effects of drug [F(1,56) = 7.039, p = 0.01] and condition [F(2,56) = 5.94, p < 0.01] on cortical Bdnf mRNA expression, as well as a significant interaction [F(2,56) = 9.5, p < 0.001]. Post hoc analyses revealed that levels of cortical Bdnf mRNA were elevated relative to Sal/Immed controls in the mAMPH/Sed (p < 0.01) and Sal/Ex (p = 0.001) groups, and that the mAMPH/Sed group was significantly higher compared to Sal/Sed controls (Fig. 4A).
Fig. 4.
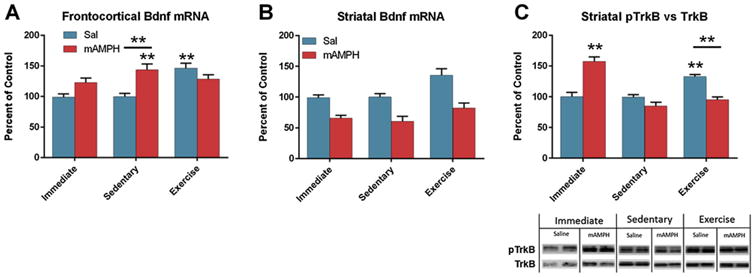
Bdnf and phospho-TrkB results. (A) In the frontal cortex, transcription of Bdnf was significantly increased by both exercise and methamphetamine withdrawal. The combination of methamphetamine and exercise produced a nonsignificant increase in frontocortical Bdnf transcription (p = 0.109). (B) Striatal Bdnf was depleted by methamphetamine exposure (p < 0.0001), and increased by exercise (p < 0.001), but no interaction was observed. (C) TrkB receptors in the striatum were activated immediately after exposure to methamphetamine (p < 0.0001) and following six weeks of exercise (p < 0.001). Exercise after methamphetamine had no effect on striatal TrkB receptor activation. Stars represent significant differences from Sal/Immed, stars with bars indicate a significant difference between the indicated groups. Representative Western blot bands are shown below quantification.
A two-way ANOVA detected significant main effects of drug [F(1,36) = 41.98, p < 0.0001] and condition [F(2,56) = 8.84, p < 0.001] on striatal Bdnf transcription. No interaction was observed (Fig. 4B).
ANOVA detected significant between group differences in TrkB activation [F(5,56) = 6.18, p < 0.0001]. Post hoc analysis revealed a significant difference between Sal/Immed and Sal/Ex (p < 0.001) and between Sal/Immed and mAMPH/Immed, but no difference between Sal/Immed and Sal/Sed, mAMPH/Sed, or mAMPH/Ex (Fig. 4C). An analysis of total striatal TrkB and phospho-TrkB compared to actin revealed that the differences in pTrkB/TrkB ratio after methamphetamine were driven by changes in phosphorylation, or activation of the receptor (Table 1).
Table 1.
Total striatal TrkB and phosphorylated TrkB. A significant main effect of methamphetamine was observed on overall TrkB expression [F(1,56) = 11.06, p = 0.0016]. A significant interaction was observed for pTrkB [F(2,56) = 21.76, p < 0.0001].
| Saline | Methamphetamine | |
|---|---|---|
| TrkB vs. Actin (% of Sal/Immed) | ||
| Immediate | 100 ± 6 | 120 ± 13 |
| Sedentary | 105 ± 5 | 131 ± 4 |
| Exercise | 117 ± 5 | 122 ± 6 |
| pTrkB vs. Actin (% of Sal/Immed) | ||
| Immediate | 100 ± 2 | 198 ± 29a |
| Sedentary | 102 ± 4 | 109 ± 6b |
| Exercise | 155 ± 6a | 115 ± 6b |
p < 0.01 vs. Sal/Immed.
p < 0.01 vs. mAMPH/Immed, Bonferonni post hoc comparisons.
3.4. Correlations between mRNA expression and running
Correlation matrices were generated to analyze the relationship between running and Bdnf and Drd2 expression across brain regions of interest. There were no significant correlations between intensity of running (measured by total kilometers over six weeks) and Bdnf and Drd2 mRNA levels in any brain region.
4. Discussion
Following a brief acquisition period, we found that animals treated with methamphetamine showed increased voluntary running activity relative to saline-treated controls. This increase was persistent, and was still present 6 weeks after cessation of drug treatment and introduction of the activity wheel. After rats had access to the running wheel, we assessed transcription of Drd2 mRNA and Bdnf, as well as protein expression levels of D2 and phosphorylation of TrkB. Saline treated animals which remained sedentary for six weeks after the injections were not different from saline treated animals which were sacrificed the day after the final injection. Interestingly, striatal Drd2 and frontocortical Bdnf transcription were not significantly different between animals treated with saline and animals treated with methamphetamine then immediately sacrificed. Transcriptional differences between methamphetamine-treated animals and saline-treated animals were only observed after 6 weeks, suggesting that withdrawal from methamphetamine plays a critical role in the neurochemical adaptations observed after chronic methamphetamine. Pretreatment with methamphetamine was found to blunt the impact of exercise on both Drd2 and Bdnf mRNA levels, though its effect on protein expression was less clear. We found robust decreases in striatal Drd2 and Bdnf mRNA transcription following protracted methamphetamine withdrawal, though protein levels were not different between saline and methamphetamine treated animals at this time point.
4.1. Voluntary running activity is enhanced during methamphetamine withdrawal
Exercise is a self-reinforcing operant behavior (Belke and Wagner, 2005; Kagan and Berkun, 1954), therefore the increased willingness to engage in exercise (i.e. expend effort on wheel running) seen in methamphetamine-treated animals may reflect an increased sensitivity to the reinforcing aspects of wheel running. Noteworthy, however, is that the only opportunities for reward available to the rat during withdrawal (and the running period) were the exercise wheel, lab chow, and water. Additionally, to monitor running wheel activity accurately, rats were transitioned from pair-housing (during drug treatment) to single-housing during withdrawal and exercise. It is possible that the observed increase in effort expenditure would not have been observed in the presence of competing reinforcers with a lower effort cost such as environmental enrichment or social housing.
Methamphetamine-treated rats began running shortly after the lights turned off, and maintained high levels of running during the first 4–5 h of the dark phase before diminishing activity toward the end of the dark phase. This binge-like pattern of running could be due to an overall increased motivation to run in methamphetamine-treated animals, interacting with muscle fatigue as running progresses. Alternatively, it could be due to animals foregoing eating and drinking early in the dark phase in favor of running, until hunger and thirst become salient enough to increase the incentive value of food and water above the value of running. Saline treated rats distributed their running relatively equally throughout the dark phase, presumably as they divide their time between available reinforcers. Body weight and food weight measures and a video of running would improve our understanding of how animals distribute their reward behaviors in the dark cycle (between food and running, for example). Future experiments should include these and other measures of exercise architecture, as well as physiological correlates.
Previous work has shown differences in self-administered vs. non-contingent methamphetamine exposure, and of voluntary vs. forced exercise effects. In the present research we were primarily interested in how methamphetamine exposure affected later reward behaviors, so we chose to non-contingently administer methamphetamine and allow voluntary running wheel access. One group (Sobieraj et al., 2014) instead had rats self-administer methamphetamine and these animals actually ran less than saline-treated animals. However, there are several important differences from the present work. First, the rats used in Sobieraj et al. were a different strain (Wistar) and rats in that experiment underwent catheterization surgery, including exposure to surgical drugs, which may have affected their propensity to engage in running activity. Additionally, drug-naïve rats in that study ran an average of 6 km per day after acquisition, whereas saline-treated animals in the present study ran approximately 3 km per day, suggesting that preexisting differences due to strain or surgery status may have contributed to the alternate finding.
4.2. Decreased striatal Drd2 mRNA and increased protein after methamphetamine
Animals that were treated with methamphetamine showed reduced Drd2 mRNA expression in the striatum relative to saline controls six weeks after cessation of drug treatment. Low D2 expression is predictive of addiction vulnerability, indicating that animals exposed to methamphetamine are at higher risk for subsequent drug-taking (Jentsch et al., 2014; Tournier et al., 2013; Sweitzer et al., 2012). Conversely, saline treated animals given access to the running wheels showed significant increases in Drd2 mRNA relative to controls, suggesting that they might be at lower risk for drug-taking behavior. Animals exposed to methamphetamine did not show this increase in Drd2 expression, indicating that experience with methamphetamine prevented the effect of exercise. Additionally, intensity of exercise did not correlate with Drd2 mRNA levels after methamphetamine, providing evidence that mere access to the exercise wheel was insufficient to elevate Drd2 expression in rats treated with methamphetamine.
These data suggest that the striatal D2 system is sensitive to major changes in the reward environment throughout life history (such as introduction of an exercise wheel or exposure to methamphetamine). Specifically, experience with major reinforcing events (e.g. methamphetamine) may “set the gain” on the impact of subsequent events on D2 expression; events that may be minor by comparison (e.g. exercise). Despite increasing the incentive value of wheel running, methamphetamine experience may limit the effects of subsequent reinforcer experience on transcriptional machinery. This robust change in reward environment leads to alterations in striatal function which affect future reward-seeking behaviors. Indeed, large dopaminergic “surprise signals” have been shown to induce long-term plasticity in corticostriatal projections, while moderate dopamine signals have minimal effects on synaptic efficacy (reviewed in Reynolds and Wickens (2002)).
In contrast to the pattern seen in the transcription of Drd2, western blots revealed significant increases in D2 receptors in the striatum one day after the last methamphetamine injection. This may be due to the fact that tissue was collected at the same time of day as the first drug injection. Anticipatory locomotor responses when stimulant drugs are given at regular daily intervals involve signaling at D2 receptors (Shibata et al., 1995). It is similarly possible that drug delivery became a salient zeitgeber during the two weeks of dosing, and that DA receptors were upregulated in anticipation for the expected methamphetamine administration. Six weeks later, however, D2 levels were not statistically different than saline treated controls, irrespective of exercise status. This suggests that the striatal D2 system is robust against perturbations, and that six weeks of withdrawal is sufficient to restore normal levels of D2. The possibility remains that re-exposure to methamphetamine following six weeks of withdrawal would have an augmented effect in pretreated animals due to transcriptional differences. This remains an empirical question for further study.
4.3. Bdnf mRNA and pTrkB vs. TrkB
Transcription of Bdnf exon IV does not normally occur in striatal neurons; rather, transcripts that arise in the cortex and other striatal input regions are trafficked anterogradely along axons, with mature Bdnf, to the striatum (Altar et al., 1997). We therefore expected to observe similar changes in the cortex and striatum. Interestingly, Bdnf mRNA expression in the striatum can be induced by excitotoxic lesions to the region, which also upregulate Bdnf transcription in the cortex (Rite et al., 2003). Additionally, increases in hippocampal BDNF have been reported 24 h after self-administered methamphetamine (Galinato et al., 2015; McFadden et al., 2014). Not surprisingly, we found increased Bdnf mRNA in the cortex and striatum of saline treated animals after 6 weeks of exercise. This was not observed following methamphetamine: while significant increases in Bdnf transcription were seen in the frontal cortex of animals that remained sedentary for 6 weeks after methamphetamine, striatal Bdnf mRNA was significantly reduced. It has previously been reported that self-administered methamphetamine transiently increases Bdnf expression in the dorsal striatum, an effect which is gone 24 h later (Krasnova et al., 2013). These data suggest that methamphetamine can cause an effective sequestration of Bdnf in the frontal cortex, possibly due to an effect on cytoskeletal polymerization (Altar et al., 1997). Alternatively, methamphetamine could affect the activity of microRNAs such as miR-30a-5p in the frontal cortex or miR124a in the striatum, which regulate BDNF expression and have been shown to play a role in regulating motivation for alcohol (Darcq et al., 2014; Bahi and Dreyer, 2013). Treatment with methamphetamine prior to initiation of aerobic exercise prevented the increases in both cortical and striatal Bdnf seen in saline-treated animals that exercised.
A different pattern emerged when we examined the activation and phosphorylation of the TrkB receptor. In the striatum of saline treated exercising rats, the increase in Bdnf mRNA was accompanied by an increase in phospho-TrkB, indicating that functional BDNF was increased in the region. However, one day after the last methamphetamine injection, there was a robust increase in TrkB phosphorylation that was not predicted by the decreased Bdnf mRNA at the same time point. There is precedence for this: eukaryotic cells in which transcription and translation occur in separate cellular compartments often exhibit discordance between mRNA and protein levels, and this discordance increases with cellular morphologic complexity (reviewed in de Sousa Abreu et al. (2009)). Differential Bdnf transcription and TrkB activity could be due to increased translation of Bdnf in the striatum. Additionally, it could be due to inhibition of protein tyrosine phosphotases such as PTPRO or PTP1B, which terminate TrkB signaling (Gatto et al., 2013; Ozek et al., 2014). Phospho-TrkB returned to pre-methamphetamine levels after six weeks, irrespective of exercise status.
5. Conclusions
Voluntary aerobic exercise and exposure to methamphetamine individually induce opposing changes in Drd2 and Bdnf transcription. However, if methamphetamine exposure precedes initiation of exercise, the putatively beneficial effects of exercise are blocked. Further studies are needed to assess the impact of exercise prior to drug exposure and the effect of exercise on cognitive functions associated with methamphetamine exposure.
Acknowledgments
This research was supported by the UCLA Division of Life Sciences Recruitment and Retention Fund (A. Izquierdo) and the Training program in Translational Neuroscience of Drug Abuse to A.B. Thompson (T32 DA024635). The authors acknowledge J.D. Jentsch for technical assistance and C.V. Grijalva for donation of the running wheels.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- Altar CA, Cai N, Bliven T, Juhansz M, Conner JM, Acheson AL, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Bahi A, Dreyer JL. Striatal modulation of BDNF expression using microRNA124a-expressing lentiviral vectors impairs ethanol-induced conditioned-place preference and voluntary alcohol consumption. Eur J Neurosci. 2013;38(2):2328–2337. doi: 10.1111/ejn.12228. [DOI] [PubMed] [Google Scholar]
- Belke TW, Wagner JP. The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behav Process. 2005;68(2):165–172. doi: 10.1016/j.beproc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Carola R, Saksida LM, van Praag H, Bussey T. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107(5):2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcq E, Warnault V, Phamluong K, Besserer GM, Liu F, Ron D. Micro-RNA-30a-5p in the prefrontal cortex controls the transition from moderate to excessive alcohol consumption. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.155. http://dx.doi.org/10.1038/mp.2014.120. Advance online publication. [DOI] [PubMed]
- de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol Biosyst. 2009;5:1512–1526. doi: 10.1039/b908315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amore DE, Tracy BA, Parikh V. Exogenous BDNF facilitates strategy set-shifting by modulating glutamate dynamics in the dorsal striatum. Neuropharmacology. 2013;75:312–323. doi: 10.1016/j.neuropharm.2013.07.033. [DOI] [PubMed] [Google Scholar]
- Eddy MC, Stansfield KJ, Green JT. Voluntary exercise improves performance of a discrimination task through effects on the striatal dopamine system. Learn Mem. 2014;21:334–337. doi: 10.1101/lm.034462.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann AJ, Aparicio MB, Kim A, Sobieraj JC, Yuan CJ, Grant Y, Mandyam CD. Chronic wheel running reduces maladaptive patterns of methamphetamine intake: regulation by attenuation of methamphetamine-induced neuronal nitric oxide synthase. Brain Struct Funct. 2014;219(2):657–672. doi: 10.1007/s00429-013-0525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinato MH, Orio L, Mandyam CD. Methamphetamine differentially affects BDNF and cell death factors in anatomically defined regions of the hippocampus. Neuroscience. 2015;286:97–108. doi: 10.1016/j.neuroscience.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto G, Dudanova I, Suetterlin P, Davies AM, Drescher U, Bixby JL, Klein R. Protein tyrosine phosphatase receptor type O inhibits trigeminal axon growth and branching by repressing TrkB and Ret signaling. J Neurosci. 2013;33(12):5399–5410. doi: 10.1523/JNEUROSCI.4707-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Hillman C. The influence of exercise on cognitive abilities. Compr Physiol. 2013;3:403–428. doi: 10.1002/cphy.c110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z, Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur J Neurosci. 2011;33(3):383–390. doi: 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HEW, Fleshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res. 2011;217(2):354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Lee B, London ED, Mandelkern MA, James AS, Feiler K, et al. Dorsal striatal D2-like receptor availability covaries with sensitivity to positive reinforcement during discrimination learning. J Neurosci. 2011;31(20):7291–7299. doi: 10.1523/JNEUROSCI.0363-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Morales AM, Lee B, London ED, Jentsch JD. Metham-phetamine-induced increases in putamen gray matter associate with inhibitory control. Psychopharmacology (Berl) 2013;299:527–538. doi: 10.1007/s00213-013-3159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O'Dell SJ, et al. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;35:505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT. Dissecting impulsivity and its relationships to drug addictions. Ann N Y Acad Sci. 2014;1327:1–26. doi: 10.1111/nyas.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Berkun M. The reward value of running activity. J Comp Physiol Psychol. 1954;47(2):108. doi: 10.1037/h0058877. [DOI] [PubMed] [Google Scholar]
- Kosheleff AR, Grimes M, O'Dell S, Marshall JF, Izquierdo A. Work aversion and associated changes in dopamine and serotonin transporter after methamphetamine exposure in rats. Psychopharmacology (Berl) 2012;219(2):411–420. doi: 10.1007/s00213-011-2367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Chiflikyan M, Justinova Z, McCoy MT, Ladenheim B, Jayanthi S, Quintero C, Brannock C, Barnes C, Adair JE, Lehrmann E, Kobeissy FH, Gold MS, Becker KG, Goldberg SR, Cadet JL. CREB phosphorylation regulates striatal transcripitional responses in the self-administration model of methamphetamine addiction in the rat. Neurobiol Dis. 2013;58:132–143. doi: 10.1016/j.nbd.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev. 2013;37(8):1622–1644. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Vieira-Brock PL, Hanson GR, Fleckenstein AE. Methamphetamine self-administration attenuates hippocampal serotonergic deficits: role of brain-derived neurotrophic factor. Int J Neuropsychopharmacol. 2014;17(8):1315–1320. doi: 10.1017/S1461145714000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse R. An evolutionary perspective on substance abuse. Ethol Sociobiol. 1994;15:339–348. [Google Scholar]
- Ozek C, Kanoski SE, Zhang ZY, Grill HJ, Bence KK. Protein-tyrosine phosphatase 1B (PTP1B) is a novel regulator of central brain-derived neurotrophic factor and tropomyosin receptor kinase B (TrkB) signaling. J Biol Chem. 2014;289:31682–31692. doi: 10.1074/jbc.M114.603621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell SJ, Galvez BA, Ball AJ, Marshall JF. Running wheel exercise ameliorates methamphetamine-induced damage to dopamine and serotonin terminals. Synapse. 2012;66(1):71–80. doi: 10.1002/syn.20989. [DOI] [PubMed] [Google Scholar]
- Parsegian A, Glen WB, Lavin A, See R. Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol Psychiatry. 2011;69(3):253–259. doi: 10.1016/j.biopsych.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JNJ, Wickens JR. Dopamine-dependent plasticity of corticostriatal synapses. Neural Netw. 2002;15:507–521. doi: 10.1016/s0893-6080(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Rite I, Venero J, Tomas-Camardiel M, Machado A, Cano J. Expression of BDNF mRNA in substantia nigra is dependent on target integrity and independent of neuronal activation. J Neurochem. 2003;87(3):70–721. doi: 10.1046/j.1471-4159.2003.02041.x. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R, O'Neil ML, Melega WP, Cho AK. Escalating dose methamphetamine pretreatment alters the behavioral and neurochemical profiles associated with exposure to a high-dose methamphetamine binge. Neuropsychopharmacology. 2003;28:1730–1740. doi: 10.1038/sj.npp.1300247. [DOI] [PubMed] [Google Scholar]
- Shibata S, Ono M, Fukuhara N, Watanabe S. Involvement of dopamine, N-methyl-D-aspartate and sigma receptor mechanisms in methamphetamine-induced anticipatory activity rhythm in rats. J Pharmacol Exp Ther. 1995;274(2):688–694. [PubMed] [Google Scholar]
- Smith MA, Witte MA. The effects of exercise on cocaine self-administration, food-maintained responding, and locomotor activity in female rats: importance of the temporal relationship between physical activity and initial drug exposure. Exp Clin Psychopharmacol. 2012;20(6):437–446. doi: 10.1037/a0029724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobieraj JC, Kim A, Fannon MJ, Mandyam CD. Chronic wheel running-induced reduction of extinction and reinstatement of methamphetamine seeking in methamphetamine dependent rats is associated with reduced number of periaqueductal gray dopamine neurons. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0905-7. http://dx.doi.org/10.1007/s00429-014-0905-7 (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- Stolyarova A, O'Dell SJ, Marshall JF, Izquierdo A. Positive and negative feedback learning and associated dopamine and serotonin transporter binding after methamphetamine. Behav Brain Res. 2014;271:195–202. doi: 10.1016/j.bbr.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolyarova A, Thompson AB, Barrientos RM, Izquierdo A. Reductions in frontocortical cytokine levels are associated with long-lasting alterations in reward valuation after methamphetamine. Neuropsychopharmacology. 2014;40(5):1234–1242. doi: 10.1038/npp.2014.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer MM, Donny EC, Hariri AR. Imaging genetics and the neurobiological basis of individual differences in vulnerability to addiction. Drug Alcohol Depend. 2012;123(S1):S59–S71. doi: 10.1016/j.drugalcdep.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier BB, Steimer T, Millet P, Moulin-Sallanon M, Vallet P, Ibanez V, Ginovart N. Innately low D2 receptor availability is associated with high novelty-seeking and enhanced behavioural sensitization to amphetamine. Int J Neuropsychopharmacol. 2013;16(8):1819–1834. doi: 10.1017/S1461145713000205. [DOI] [PubMed] [Google Scholar]
- Tran AH, Tamura R, Teruko U, Kobayashi T, Katsuki M, Matsumoto G, Ono T. Altered accumbens neural response to prediction of reward associated with place in dopamine D2 receptor knockout mice. Proc Natl Acad Sci U S A. 2002;99(13):8986–8991. doi: 10.1073/pnas.132284599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Feng B, Urizar E, Winiger V, Ward RD, Taylor KM, et al. Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Mol Psychiatry. 2012;18:1025–1033. doi: 10.1038/mp.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]


