Abstract
Lymphedema is one of the most common complications after breast cancer surgery. There are many diagnostic tools for lymphedema, but no standard method yet exists. Progressive Resistance Exercise (PRE) is expected to improve lymphedema without additional swelling. This study showed the therapeutic effects of PRE on lymphedema by using ultrasonography to measure the change in thickness of the muscle and subcutaneous tissue. The thickness of subcutaneous tissue decreased more in the PRE group than in the non-PRE group. Ultrasonography is widely used in many clinics because of its easy accessibility, safety, and inexpensiveness.Ultrasound is one of the best tools for diagnosing and determining treatment efficacy on breast cancer-related lymphedema (BCRL).
Keywords: Cancer Research, Issue 119, Ultrasonography, Evaluation, Breast cancer, Lymphedema, Exercise, Diagnosis
Introduction
Lymphedema is defined as a condition of localized protein-rich fluid retention and tissue swelling caused by a compromised lymphatic system1,2. As lymphedema progresses, fibrocytes and/or adipocytes proliferate in the affected areas, leading to changes in the texture of the skin and subcutaneous tissue and an increased vulnerability to bacterial and fungal infections3,4. The observation of these changes in various parts of the extremities may further elucidate the severity and extent of lymphedema. The subfascial lymphatic system contributes to the severity of lymphedema more than the epifascial lymphatic system.
Recent research has mainly focused on the use of ultrasonography in the diagnosis of lymphedema5,6. Ultrasonography is a relatively inexpensive method to observe soft-tissue characteristics. A previous study reported that lymphedema can be reliably diagnosed with ultrasonography by comparing the thickness of the skin and subcutaneous tissue and also by evaluating the compliance of the subcutaneous tissue in a clinical setting7,8. In addition, ultrasonography is widely used in the clinic because of its easy accessibility, safety from radiation exposure, and inexpensiveness5,6. In spite of these benefits, studies on ultrasonography for lymphedema have only been focused on diagnosis, not on evaluation of therapeutic intervention.
Therefore, the current study aimed to determine not only the usefulness of ultrasonography in elucidating the effects of PRE on BCRL, but also whether ultrasonography has a clinical significance as a follow-up test in confirming treatment efficacy. This study was conducted to establish the protocol for ultrasounds performed to examine lymphedema.
Protocol
All of the procedures were reviewed and approved by the Institutional Review Board of Chungnam National University Hospital.
1. Measurement with Ultrasonography
- Set the posture of the patient.
- Lay the patient's arm in a supine position, with both arms on the bed.
- Determine the measurement sites on the patient's upper limb.
- Mark in the mid-point of the wrist crease, the mid-point between the medial and lateral epicondyles at the level of the elbow, and the bicipital groove by a pen.
- Using a pen directly connect these three points by a ruler. Mark the two target areas on the upper arm and forearm (Figure 1).
- Mark the proximal part, 10 cm proximal to the elbow point along the line between the elbow and bicipital groove.
- Mark the distal part, 10 cm distal to the elbow point along the line between the elbow and wrist.
- Measure the thickness of the subcutaneous tissue and muscle of the upper limb. The procedure should be performed by a skilled sonographer.
- Switch on the ultrasound system. Enter 2D mode of soft tissue via the keyboard. Set the depth to 5 cm.
- Choose the 14L5 linear array transducer.
- Apply sufficient gel to the ultrasound transducer. Focus on the subcutaneous tissue.
- Place the ultrasound transducer perpendicularly to the upper limb ventral axis. Use the short-axis view. Capture the image where the thickness of the gel is at least 1 cm and the soft tissue contour is not distorted.
- Measure the thicknesses of the muscle and subcutaneous tissue.
- Draws a line by selecting the calibrator in the software via the keyboard of the ultrasound system
- Measure the thickness of the muscle, defined as the distance from the highest point on the boundary of the bone to the highest point on the boundary portion of the fascia (Figure 2).
- Measure the thickness of the subcutaneous tissue, defined as the distance from the skin to the fascia.
2. Clinical Application of Ultrasonography to BCRL
- Measure the thickness of the subcutaneous tissue and muscle of the upper limb at baseline.
- Perform the baseline measurements in the order shown in Step 1.
- Instruct the patient to perform a series of PREs using a 0.5 kg dumbbell14.
- Give the patient a compression stocking or a multilayer bandage to wear. Choose a 0.5 kg dumbbell.
- Instruct the patient to perform the PREs in the following order: (1) dumbbell fly, (2) triceps extension, (3) one arm bent-over row, (4) biceps curl, (5) dumbbell side raise, and (6) lifting the arms forward (Figure 3).
- Instruct the patient to complete the 6 exercises twice a day, according to the following schedule: 5 times each during the 1st week, 10 times each during the 2nd week, 15 times each during the 3rd week, 20 times each during the 4th week, and 25 times each during 5th-8th weeks (Figure 4).
- Use ultrasonography to identify the therapeutic effects of PRE compared to conventional therapy.
- Measure the thickness of the subcutaneous tissue and muscle of the upper limb at 4 and 8 weeks. Perform the measurements in the order shown in Step 1.
Calculate the thickness of the subcutaneous tissue and muscle at baseline, 4 weeks, and 8 weeks.
- Analyze the data.
- Compare the difference between both arms using a paired t-test. Compare the changes in subcutaneous tissue and muscle thickness using a repeated-measures ANOVA.
Representative Results
This study included 32 patients diagnosed with BCRL. The thickness of muscle and subcutaneous tissue and the circumferences of proximal and distal upper limbs were measured at baseline, 4 weeks, and 8 weeks after PRE. Examiners measured the circumference of upper limbs with a tape measure, and then the thickness of the muscle and subcutaneous tissue were measured by ultrasonography. Patients were randomly divided into a PRE group and a non-PRE group. Participants were reminded to not reveal their group assignment before measurement and evaluation sessions.
At baseline, the initial muscle thickness (cm) of all participants measured by ultrasonography was significantly lower in the lymphedematous arm compared to the unaffected arm (Table 1). The subcutaneous tissue in the arm affected with lymphedema was significantly thicker compared to the unaffected arm (Table 1).
According to the tape measurement, arm circumference did not significantly change in the PRE group after 4 weeks of exercises; however, both distal and proximal arm circumferences showed a significant reduction after 8 weeks. These circumferences did not significantly change in the control group (Figure 5).
As measured by ultrasonography in the PRE group, the thickness of muscle in the distal part showed significant increases at 4 weeks and 8 weeks, and the proximal part demonstrated a significant increase in thickness at 8 weeks (Figure 6). Statistical analyses were performed using SPSS 12.0. Statistical significance for the differences between both arms was tested using a paired t-test. Changes in the subcutaneous tissue and muscle thickness, and upper limb circumference were analyzed using repeated-measures ANOVA. Statistical significance level was set at the p-value of 0.05 or less.
In the PRE group, the thickness of subcutaneous tissue in the affected upper limb significantly decreased after 8 weeks. No significant difference in these parameters was observed in the non-PRE group (Figure 7).
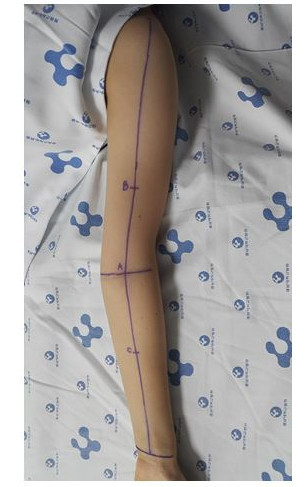 Figure 1.A Photograph Illustrating the Measurement Sites on a Patient's Upper Limb. The mid-point of the wrist crease, the mid-point between the medial and lateral epicondyles at the level of the elbow, and the bicipital groove were marked. These three points were connected linearly, and the 3 measuring sites were marked: (A) the midpoint at the elbow (elbow), (B) 10 cm above the mid-point at the elbow (proximal), and (C) 10 cm below the mid-point at the elbow (distal).
Figure 1.A Photograph Illustrating the Measurement Sites on a Patient's Upper Limb. The mid-point of the wrist crease, the mid-point between the medial and lateral epicondyles at the level of the elbow, and the bicipital groove were marked. These three points were connected linearly, and the 3 measuring sites were marked: (A) the midpoint at the elbow (elbow), (B) 10 cm above the mid-point at the elbow (proximal), and (C) 10 cm below the mid-point at the elbow (distal).
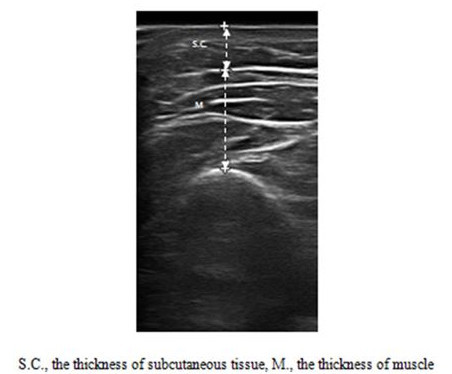 Figure 2.Measurements of the Thickness of Muscle and Subcutaneous Tissue on the Upper Limbs. Ultrasound was used to measure the thickness of the muscle and subcutaneous tissue at the determined points. S.C., the thickness of subcutaneous tissue; M., the thickness of muscle. Reprinted with permission from12.
Figure 2.Measurements of the Thickness of Muscle and Subcutaneous Tissue on the Upper Limbs. Ultrasound was used to measure the thickness of the muscle and subcutaneous tissue at the determined points. S.C., the thickness of subcutaneous tissue; M., the thickness of muscle. Reprinted with permission from12.
 Figure 3. The PRE Protocol. The prescribed exercises include: 1) dumbbell fly, 2) triceps extension, 3) one arm bent-over row, 4) biceps curl, 5) dumbbell side raise, and 6) lifting the arms forward. Reprinted with permission from12.
Figure 3. The PRE Protocol. The prescribed exercises include: 1) dumbbell fly, 2) triceps extension, 3) one arm bent-over row, 4) biceps curl, 5) dumbbell side raise, and 6) lifting the arms forward. Reprinted with permission from12.
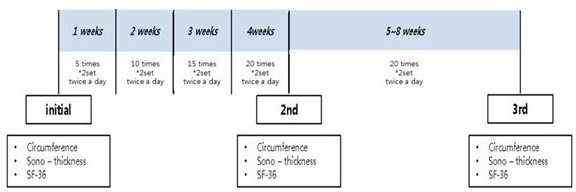 Figure 4. PRE Evaluation. Patients were evaluated at baseline and after 4 and 8 weeks. Reprinted with permission from 12.
Figure 4. PRE Evaluation. Patients were evaluated at baseline and after 4 and 8 weeks. Reprinted with permission from 12.
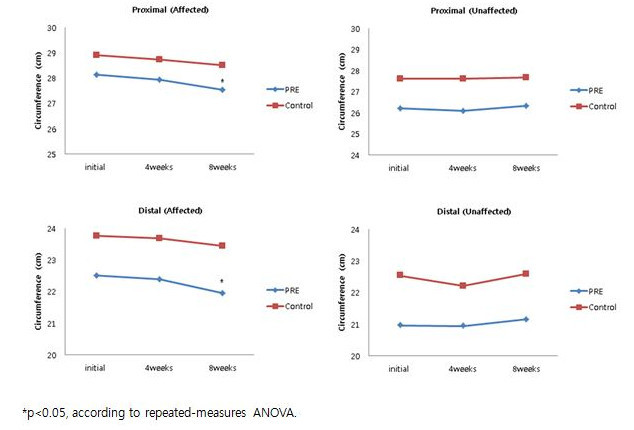 Figure 5.Tape Measurements of the Circumferences of the Affected and Unaffected Limb. The change in upper limb circumferences. *p <0.05, according to repeated measurements by ANOVA. Reprinted with permission from 12.
Figure 5.Tape Measurements of the Circumferences of the Affected and Unaffected Limb. The change in upper limb circumferences. *p <0.05, according to repeated measurements by ANOVA. Reprinted with permission from 12.
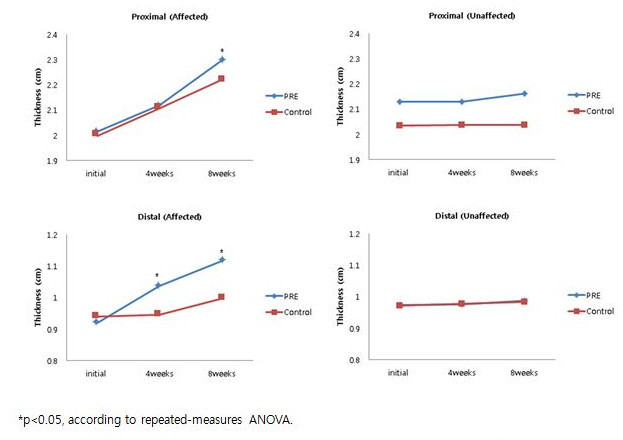 Figure 6.Ultrasonography Measurements of the Thickness of Muscle on the Affected and Unaffected Limb. The change in muscle thickness. *p <0.05, according to repeated-measurements by ANOVA. Reprinted with permission from 12.
Figure 6.Ultrasonography Measurements of the Thickness of Muscle on the Affected and Unaffected Limb. The change in muscle thickness. *p <0.05, according to repeated-measurements by ANOVA. Reprinted with permission from 12.
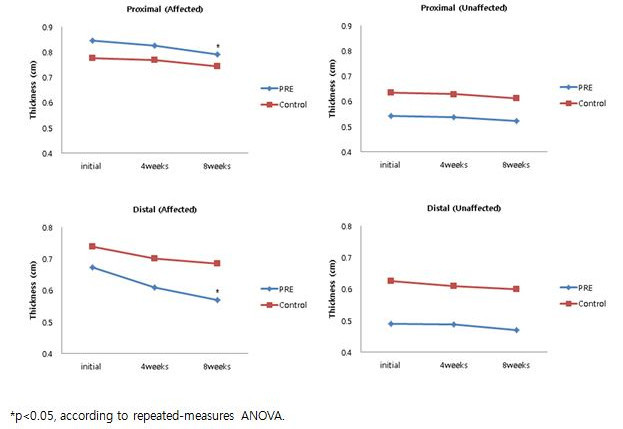 Figure 7. Ultrasonography Measurements of the Thickness of Subcutaneous Tissue on the Affected and Unaffected Limb. The change in the thickness of subcutaneous tissue. *p <0.05, according to repeated-measurements by ANOVA. Reprinted with permission from 12.
Figure 7. Ultrasonography Measurements of the Thickness of Subcutaneous Tissue on the Affected and Unaffected Limb. The change in the thickness of subcutaneous tissue. *p <0.05, according to repeated-measurements by ANOVA. Reprinted with permission from 12.
| Thickness | Affected arm | Unaffected arm | P-value | |
| Muscle | Proximal | 2.01 (0.63) | 2.12 (0.55) | 0.025* |
| Distal | 0.92 (0.37) | 1.04 (0.35) | 0.037* | |
| Subcutaneous tissue | Proximal | 0.87 (0.37) | 0.54 (0.49) | 0.016* |
| Distal | 0.67 (0.22) | 0.49 (0.42) | 0.02* |
Values are presented as mean (standard deviation)
*p<0.05, according to paired t-test.
Table 1. Ultrasonography Measurements of the Initial Difference in Thickness between Both Upper Limbs of All Participants. The initial muscle thickness (cm) of all participants measured by ultrasonography significantly decreased in the affected arm compared to the unaffected arm at baseline measurement. Values are presented as mean (standard deviation). *p <0.05, according to paired t-test. Reprinted with permission from 12.
Discussion
Evaluations of BCRL can be conducted through various techniques, including arm circumferences tape measurement, water displacement measurement, bioimpedance spectroscopy, perometry (optoelectronic volumeter), lymphoscintigraphy, clinician diagnosis, or swelling self-reports by the patient. However, there are no standards for the measurement of BCRL; the diagnosis and duration of treatment can be determined empirically in a number of ways, depending upon the clinic.
Clinically, measuring arm circumference by tape measure is the most popular and convenient method. However, this method cannot evaluate structural changes in the subcutaneous tissue, and errors can be caused by excessive tape measure pressure on the skin, inaccurately marked points, and an improper measurement angle in relation to the long axis of the limb. Furthermore, tape measurement may be less accurate than water displacement or perometry in estimating the volume of lymphedema.
Volumetry using water or an infrared light beam are excellent procedures that can give the volume of the upper limb automatically. However, volumetry cannot check the structural changes in the subcutaneous tissue and is not suitable for the proximal part of the limb and trunk. Other weaknesses are that the water displacement method is time-consuming and inconvenient, while the perometry method is very expensive.
Bioimpedance spectroscopy has been shown to be sensitive to early extracellular fluid changes, which are reliable, reproducible, and easy to manage. However, there is no standardized reference, and it has not been used in bilateral lymphedema. It also has limitations in detecting non-pitting edema at later stages, where the interstitial fluids have been replaced by deposition of adipose tissue and/or fibrotic tissue.
Ultrasonography is a clinically convenient tool; the examiner can evaluate the status of soft tissues in the office setting. The thickness of the cutaneous, epifascial, and subfascial tissue can be measured by ultrasonography, which can elucidate the fluid collection and fibrosis. In contrast to CT or MRI, ultrasonography is readily available in many clinics and is also noninvasive and inexpensive.
Ultrasonography is a relatively subjective technique whose findings can be affected by the examiner's skill. Ultrasonic measurements are operator-dependent, and technical differences in pressure application may affect the results of subcutaneous volume and compliance. However, if practitioners perform the procedure at the correct site, and if the technique can be mastered precisely, it is possible to overcome this weakness of ultrasonography.
The critical steps during ultrasonic evaluation are keeping the contour of soft tissue constant and defining the muscle thickness. The soft tissue is likely to be distorted by pressure from the examiner, so the practitioner should put sufficient gel on the transducer and maintain constant pressure to keep the subcutaneous tissue even. The thickness of the muscle is defined as the distance from the highest point on the boundary of the bone to the highest point on the boundary portion of the fascia; it is important to determine the highest point on the boundary of the bone.
This study showed that the muscle thickness in the affected arm significantly decreased more than in the unaffected side, as measured by ultrasonography. The primary causes may be the direct damage of muscles by surgery or the influence of radiation therapy or chemotherapy on the tissue. Another cause is the tendency to avoid using the affected arm. Many patients have a considerable fear that the swelling of the arm occurs due to resistive exercise. They only perform stretching and low-level aerobic exercises such as walking, so a reduction of muscle mass appears to occur. However, in the previous studies, PRE does not induce deterioration in cases of lymphedema11,13,14.
The present study found that the thickness of subcutaneous tissue was more decreased in the PRE group than in the non-PRE. Also, the PRE group had a greater increase in muscular thickness than the non-PRE group. These changes are easily measured by ultrasonography, which can more accurately distinguish between muscular and edematous areas than other tools. Ultrasonography is widely used in many clinics because of its easy accessibility, safety, and inexpensiveness3,4. Therefore, if the techniques of ultrasonography are skillfully acquired, ultrasonography may become the first-choice method of diagnosing and determining treatment efficacy on BCRL.
The main limitation of the current study relates to the subjectivity of ultrasound. However, ultrasound has been widely used in many medical fields despite this weakness. If the examiners learn the precise techniques, ultrasonography will become a valid method for measuring lymphedema. Diagnosis of lymphedema using ultrasound will be further generalized, because of its ease to measure the change of lymphedema in the clinics. Efforts will have to be made to reduce the variability of each test, such as increasing the sensitivity of the machine or finding the standard landmark.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors have nothing to acknowledge.
References
- Lasinski BB. Complete decongestive therapy for treatment of lymphedema. Seminars in Oncology Nursing. 2013;29(1):20–27. doi: 10.1016/j.soncn.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Clark B, Sitzia J, Harlow W. Incidence and risk of arm oedema following treatment for breast cancer: a three-year follow-up study. Q J Med. 2005;98(5):343–348. doi: 10.1093/qjmed/hci053. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Speck RM, Reimet E, Stark A, Schmitz KH. Does the effect of weight lifting on lymphedema following breast cancer differ by diagnostic method: results from a randomized controlled trial. Breast Cancer Res Treat. 2011;130(1):227–234. doi: 10.1007/s10549-011-1547-6. [DOI] [PubMed] [Google Scholar]
- Fu MR, Ridner SH, Armer J. Post-breast cancer lymphedema: part 1. Am J Nurs. 2009;109(7):48–54. doi: 10.1097/01.NAJ.0000357172.94131.58. [DOI] [PubMed] [Google Scholar]
- Rockson SG. Ultrasonography in the Evaluation of Breast Cancer-Related Lymphedema. Lymphatic Research and Biology. 2016;14(1):1. doi: 10.1089/lrb.2016.28999.sgr. [DOI] [PubMed] [Google Scholar]
- Suehiro K, et al. Significance of Ultrasound Examination of Skin and Subcutaneous Tissue in Secondary Lower Extremity Lymphedema. Ann Vasc Dis. 2013;6(2):180–188. doi: 10.3400/avd.oa.12.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, et al. Ultrasonographic Evaluation of Therapeutic Effects of Complex Decongestive Therapy in Breast Cancer-Related Lymphedema. Ann Rehabil Med. 2013;37(5):683–689. doi: 10.5535/arm.2013.37.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast. Lancet Oncol. 2013;14(6):500–515. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- Stanton AW, et al. Lymphatic drainage in the muscle and subcutis of the arm after breast cancer treatment. Breast Cancer Res Treat. 2008;117(3):549–557. doi: 10.1007/s10549-008-0259-z. [DOI] [PubMed] [Google Scholar]
- Godoy MFG, Pereira MR, Oliani AH, Godoy JMP. Synergic Effect of Compression Therapy and Controlled Active Exercises Using a Facilitating Device in the Treatment of Arm Lymphedema. Int J Med Sci. 2012;9(4):280–284. doi: 10.7150/ijms.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormie P, Pumpa K, Galvão DA, Turner E, Spry N, Saunders C, Zissiadis Y, Newton RU. Is it safe and efficacious for women with lymphedema secondary to breast cancer to lift heavy weights during exercise: a randomised controlled trial. J Cancer Surviv. 2013;7(3):413–424. doi: 10.1007/s11764-013-0284-8. [DOI] [PubMed] [Google Scholar]
- Bok SK, Jeon Y, Hwang PS. Ultrasonographic evaluation of the effects of progressive resistive exercise in breast cancer-related lymphedema. Lymphatic Research and Biology. 2016;14(1):18–24. doi: 10.1089/lrb.2015.0021. [DOI] [PubMed] [Google Scholar]
- Milne HM, Wallman KE, Gordon S, Courneya KS. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2008;108(2):279–288. doi: 10.1007/s10549-007-9602-z. [DOI] [PubMed] [Google Scholar]
- Schmitz KH, et al. Weight lifting for women at risk for breast-cancer-related lymphedema. JAMA. 2010;304(24):2699–2705. doi: 10.1001/jama.2010.1837. [DOI] [PubMed] [Google Scholar]
- Kiel KD, Rademacker AW. Early-stage breast cancer: Arm edema after wide excision and breast irradiation. Radiology. 1996. pp. 279–283. [DOI] [PubMed]


