Abstract
Diseases associated with Escherichia coli infection are the subject of renewed interest due to emerging conditions such as hemolytic uremia syndrome. A collection of 15 strains of beta-hemolytic E. coli was isolated from diarrheic feces and diseased tissues of ferrets. All 15 strains were positive in specific PCR assays for the presence of hlyA, pap1, and cnf1. Seven of the cnf1-positive isolates were tested and shown to have a cytopathic effect on HeLa cell monolayers. The pathogenesis of these strains warrants future study.
Cytotoxic necrotizing factor 1 (CNF1) is a 115-kDa protein toxin produced by uropathogenic and some other Escherichia coli strains which have been designated necrotoxigenic E. coli (NTEC) strains (2, 7, 23). In humans, CNF1-producing E. coli isolates have been implicated in extraintestinal infections, especially urinary tract disease and meningitis. NTEC strains cause at least 80% of uncomplicated urinary tract infections and most nosocomial urinary tract infections (18). A distinct set of virulence determinants distinguish these strains from commensal strains of E. coli found in the colon and feces; additional factors such as ibeA (invasion of brain endothelium) may be required to produce meningitis (16). Strains belonging to a limited number of O serogroups possess P fimbriae, iron acquisition systems, toxins such as hemolysin (Hly) and CNF1, capsule formation, and serum resistance (1, 14). These factors presumably allow NTEC to evade host urinary tract defense mechanisms. In animals, cnf1-positive E. coli strains have been isolated from dogs with diarrhea and urinary tract infections (17, 28), cats with urinary tract infections (11), piglets with diarrhea and edema disease (12), cattle with both extraintestinal infections and diarrhea (3, 6), and healthy animals of the aforementioned species. In cattle, the related toxin CNF2 is especially prevalent among E. coli isolates (6).
CNF1 is a member of a family of bacterial toxins that includes the dermonecrotizing toxin of Bordetella bronchiseptica and CNF1 of Yersinia pseudotuberculosis. It interferes with cellular signaling by causing the constitutive activation of Rho-GTPases. Activation leads to actin cytoskeleton changes which manifest as stress fiber formation, an increased number of focal adhesions, membrane ruffling with subsequent macropinocytosis, and the formation of multinucleate cells in cell culture systems (9, 21, 26). Later intoxication events include the activation of cell motility, filopodium formation, and enhanced internalization of bacteria (8, 10, 15, 29).
Many E. coli-associated extraintestinal diseases, including gangrenous mastitis, pyometra, vaginitis, pyelonephritis, omphalophlebitis, and septicemia, have been reported for the ferret (13). Most common among these is a severe mastitis characterized histopathologically by coagulative and liquefactive necrosis of the mammary tissues and adjacent fat, muscle, and skin (20). During the course of clinical investigations, our laboratory archived E. coli isolates from diarrheic feces and diseased tissues (mammary gland, uterus, and brain) from ferrets over a 10-year period. The purposes of this study were to characterize these E. coli isolates and to identify candidate virulence determinants as a necessary first step toward elucidating the pathogenesis of these infections.
Animals.
Ferrets (Mustela putorius furo) were purchased from a commercial vendor (Marshall Farms, North Rose, N.Y.) as time-pregnant jills (i.e., jills ordered at a specific time during gestation) or were born at our institution from such jills. Vendor jills were vaccinated for canine distemper and rabies and received type C botulism toxoid and bacterins of Pseudomonas aeruginosa and B. bronchiseptica. Jills born in-house were vaccinated for distemper. Animals were housed in an AAALAC International accredited animal facility in modified rabbit caging made of stainless steel (24 by 30 by 27 in.) or plastic (27 by 26 by 17.5 in.). Jills with litters were provided a nest box and nesting material; all ferrets were provided a cloth sleeping tube. Cage board was provided within the cage and was changed daily. Ferrets were fed an admixture of ferret chow (high-density chow 5L14; PMI, St. Louis, Mo.) and canned cat food (Science Diet feline kitten growth; Hill's, Topeka, Kans.); water was provided ad libitum. Pregnant and postparturient jills were maintained with 14 h of light and 10 h of dark per day; the temperature and humidity were controlled (68 to 74°F and 40 to 65%, respectively), and there were 12 nonrecirculated air changes per h.
Microbiology.
Fifteen isolates were collected from each animal from the following sites: milk (n = 5), feces (n = 5), vagina (n = 2), urine (n = 1), blood (n = 1), and brain (n = 1). These E. coli isolates from clinical cases were isolated by plating on MacConkey lactose agar (Remel, Lenexa, Kans.). Lactose-positive colonies were then plated on sheep blood agar (Remel). The pattern of hemolysis was determined by direct visual observation. Isolates were identified by the use of API strips (bioMerieux, Hazelwood, Mo.). Individual isolates of E. coli were frozen at −70°C in Brucella broth containing 15% glycerol. Subcultures were inoculated into Trypticase soy broth (Remel) and shipped to the E. coli reference laboratory of the Gastroenteric Disease Center of Pennsylvania State University (State College). Isolates were subjected to O and H antigen serotyping as well as select adhesin and toxin gene assays by PCR. O and H serotyping was performed as described by the testing laboratory web site (http://ecoli.cas.psu.edu/procedures.htm). Examinations for select virulence factors were performed by PCRs using 3 μl of template DNA, a 0.5 μM concentration of each primer (Integrated DNA Technologies Inc., Coralville, Iowa), a 0.18 mM concentration of each deoxynucleoside triphosphate, 3 mM MgCl2, 0.4 U of Taq DNA polymerase (PGC Scientific, Gaithersburg, Md.), 50 mM Tris (pH 8.3), 250 mg of bovine serum albumin/ml, 2% sucrose, and 0.1 mM Cresol Red. PCRs were performed in a Rapid-Cycler instrument (Idaho Technologies Inc., Salt Lake City, Utah). The cycling conditions consisted of initial denaturing at 94°C followed by 30 cycles of denaturation at 94°C, annealing at 55 or 48°C, and extension at 74°C. The amplified products were electrophoresed in 1% agarose gels at 200 V for 1 h, stained with ethidium bromide, and visualized under UV light. Positive samples were identified based on the presence of bands of appropriate sizes compared to positive control strains (strain B41 for sta, strain 80-2575 for heat-labile enterotoxin and stb, and strain 43895 for stx1 and stx2 [Gastroenteric Disease Center of Pennsylvania State University]). The primer sequences and thermocycling conditions used were from published methods for sta and stb (25), for heat-labile enterotoxin (27), and for stx1 and stx2 (31). Examinations of additional virulence factors were performed via PCRs with previously reported conditions and primers (4, 18).
Cytopathic effect on HeLa cell monolayers.
Bacterial sonicates or supernatants of seven ferret E. coli isolates and one nonpathogenic laboratory strain of E. coli without detectable cnf DNA homology or CNF activity, DH5α (Invitrogen, Carlsbad, Calif.), were evaluated for cytopathic effects on HeLa cell (CCL-2) monolayers. Five of the isolates were from clinical cases of mastitis (97-1214, 92-834, 93-1626, 98-1362, and 94-2593), one was from a urine culture (92-336), and one was from a rectal culture (02-0042). After overnight growth in Lennox broth (American Bioanalytical, Natick, Mass.), the bacteria were pelleted for 10 min at 2,040 × g at room temperature. The supernatants were filtered through a 0.2-μm-pore-size syringe filter (Pall Corporation, Ann Arbor, Mich.) and stored at −80°C. The pellets were resuspended in 1 ml of phosphate-buffered saline and disrupted by six 30-s pulses on ice with a Virsonic 50 sonicator (Virtis, Gardiner, N.Y.). Sonicates were cleared of debris by centrifugation for 3 min at 16,000 × g at room temperature and were frozen at −80°C until use.
For assays of cytopathic effect, 6 × 103 HeLa cells were plated on 13-mm-diameter glass coverslips in 24-well plates in Dulbecco's modified Eagle's medium (Mediatech, Inc., Herndon, Va.). The cells were incubated at 37°C in 5% CO2 for 3 h. Twelve microliters of either supernatant or sonicate was added to the HeLa cell monolayers, which were then incubated at 37°C in 5% CO2 for 72 h. The coverslips were stained with an eosin-azure B-based stain (Diff-Quik staining kit; Dade Behring, Newark, Del.) and mounted for visualization by light microscopy.
High-resolution genotyping.
The genomic DNA of each ferret E. coli strain was evaluated by high-resolution genotyping according to the method of Versalovic et al. (30). Briefly, repetitive element-PCR (Rep-PCR) takes advantage of interspersed repetitive elements that are found throughout the bacterial genome. Rep-PCR amplification patterns can provide strain-level discrimination. REP1R and REP2 (30) were used as primers with puRe Taq Ready-To-Go PCR beads (Amersham Biosciences, Piscataway, N.J.) and with 100 ng of genomic DNA as a template. Samples were amplified under cycling conditions of 95°C for 1 min, 40°C for 1 min, and 65°C for 8 min, with a final extension time of 16 min. The products were separated in 1% agarose gels, stained with ethidium bromide, and visualized with an Image Station 1000 (Eastman Kodak Co., Rochester, N.Y.). DNA profiles were quantitatively analyzed with GelCompar II software, v. 2 (Applied Maths, Kortrijk, Belgium). Similarity coefficients were calculated by use of the Pearson correlation and were clustered by the unweighted pair group method with arithmetic means.
Microbiology results.
The results of serotyping and the identification of virulence factors are summarized in Table 1. All 15 isolates were beta-hemolytic E. coli and contained α-hemolysin (hlyA), cnf1, and the P pilis (pap1). Isolates were negative for several other virulence factors, including heat-labile enterotoxin, st, cnf2, and eae. Serotypes included O2:H4 (five isolates), O6:H− (six isolates), O4:H− (three isolates), and O4:H5 (one isolate).
TABLE 1.
Serotypes of NTEC strainsa
| Sample no. | Source | O type | H type |
|---|---|---|---|
| 92-336 | Ferret urine culture | 2 | 4 |
| 02-0042 | Ferret rectal culture | 2 | 4 |
| 91-1918 | Ferret rectal culture | 2 | 4 |
| 01-3543 | Ferret vaginal culture | 2 | 4 |
| 94-28 | Ferret brain culture | 2 | 4 |
| 94-2593 | Ferret milk culture | 4 | 5 |
| 93-337 | Ferret blood culture | 4 | − |
| 93-1626 | Ferret milk culture | 4 | − |
| 93-178 | Ferret rectal culture | 4 | − |
| 95-5266 | Ferret rectal culture | 6 | − |
| 98-1330 | Kit rectal culture | 6 | − |
| 97-1214 | Ferret milk culture | 6 | − |
| 93-187 | Ferret vaginal culture | 6 | − |
| 92-834 | Ferret milk culture | 6 | − |
| 98-1362 | Ferret milk culture | 6 | − |
Serotyping was performed at Pennsylvania State University. All samples were positive for cnf1, hly, and pap1, and all samples were negative for cnf2, heat-labile enterotoxin, sta, stb, stx1, stx2, and eae. All samples exhibited beta-hemolysis.
Ferret NTEC strains have CNF1 activity.
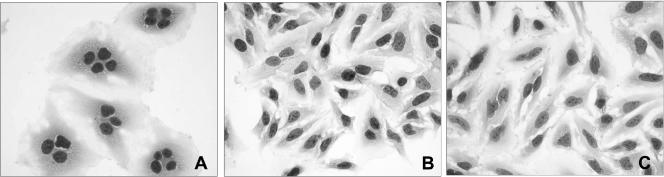
In order to determine if cnf1 sequence homology was correlated with CNF1 activity, we treated subconfluent HeLa cell monolayers with sonicates and culture supernatants from seven ferret NTEC strains. After 72 h of incubation, HeLa cells that were treated with sonicates exhibited changes that were characteristic of CNF1 activity. Treated cells were larger than untreated control cells, and >80% of the treated cells had multiple nuclei (Fig. 1). In addition, evidence of nuclear fragmentation was apparent in some of the treated cells. Sonicates of all seven ferret NTEC strains had comparable cytopathic effects on HeLa cell monolayers 72 h after treatment. Weaker activities were observed when HeLa cells were treated with culture supernatants (data not shown).
FIG. 1.
Ferret NTEC strains exhibit CNF1-mediated cytopathic effects. Subconfluent monolayers of HeLa cells were treated with sonicates of ferret NTEC strains. After 72 h, the cells exhibited distention and >80% were multinucleated. Nuclear fragmentation was also apparent in some cells. Representative monolayers of HeLa cells treated with a sonicate of ferret NTEC strain 02-0042 (A) and a sonicate of the nonpathologic strain DH5α (B) and an untreated control monolayer (C) are shown at the same magnification after being stained with Diff-Quik.
High-resolution genotyping confirms serogroup-based relationship of ferret NTEC strains.
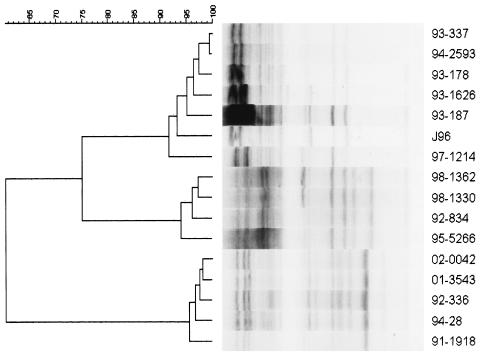
To confirm the relationship of ferret NTEC strains based on O and H serotyping, we performed PCR amplification of genomic DNAs, using primers that were complementary to interspersed repetitive elements (Rep-PCR). Three groups of ferret NTEC strains could be distinguished based on their Rep-PCR amplification patterns (Fig. 2). The human O4:K6 strain J96, three ferret O4:H− strains, one ferret O4:H5 strain, and two ferret O6:H− strains made up the first group (top seven strains). The second group (middle four strains) were all O6:H− strains. The third group (bottom five strains) were all O2:H4 strains. Despite the limited genetic diversity among the ferret NTEC strains, the strains did not stratify as a function of the date of isolation. Strains comprising the first genotype, made up of O4 and O6 strains, were isolated between 1993 and 1997. The O6:H− strains comprising the second genotype were isolated between 1992 and 1998. The O2:H4 strains comprising the third genotype were isolated between 1991 and 2002. Interestingly, the O6:H− strains 93-187 and 97-1214 appeared to be more closely related to the O4:H− strains (89 to 96% similarity) than to the other O6:H− strains (69 to 88% similarity).
FIG. 2.
Dendrogram showing similarities of Rep-PCR genotypes of ferret NTEC strains, representing five O4 strains, six O6 strains, and five O2 strains. Three groups of strains could be distinguished. From top to bottom, the strains are grouped as follows: three ferret O4:H− strains, one ferret O4:H5 strain, two ferret O6:H− strains, and the human O4:K6 strain J96 (top); four ferret O6:H− strains (middle); and five ferret O2:H4 strains (bottom).
To our knowledge, this study is the first to document cnf1-positive isolates of E. coli from diseased ferrets. Necrotizing suppurative mastitis is an important disease of the lactating jill and potentially results in neonatal death due to its effects on the dam (20). Ophthalmia neonatorum and neonatal septicemia are other E. coli-associated diseases that can reduce fecundity (13). E. coli strains have also been cultured from the urine, kidneys, vagina, brain, tail abscesses, and feces of ferrets with or without diarrhea. Hemolytic E. coli strains from ferrets can be classified as urosepsis strains, uropathogenic E. coli, or necrotoxigenic E. coli (NTEC). NTEC may be the most appropriate term for these E. coli strains. Extraintestinal diseases associated with cnf1-positive E. coli have also been demonstrated in other species.
Uropathogenic organisms contain many virulence factors, including bacterial adhesins, toxins, iron acquisition systems, and host defense avoidance mechanisms (1, 24). In the study reported here, representatives of all of these categories of virulence factors (with the exception of iron acquisition systems) were identified. All 15 ferret isolates were positive for P pili by PCRs using a pair of primers designed to amplify an internal fragment of papC (19) The human O4:K6 strain J96 contains unlinked loci encoding two distinct P pili, pyelonephritis-associated pili (pap) and Pap-related sequence (prs) (22). pap and prs cannot be distinguished by primers because the two loci are identical except for the gene encoding the adhesin (papG and prsG). Further characterization of P pili in ferret NTEC strains is needed, but a preliminary characterization of O6 strains suggested that they express Prs, while O2:H4 strains may express Pap (data not shown). The existence of pap, hly, and cnf1 in urosepsis strains is evidence of the presence of pathogenicity islands (1). Additional characterizations of pap and hly, along with challenge studies using isogenic mutants, will provide insight into the contribution of these virulence factors to disease.
The ferret may be a promising model for evaluations of the contribution of cnf1 and other genetic factors to E. coli-associated virulence. Ferrets are carnivores, are easily maintained and manipulated in a vivarium, and are of an adequate size (approximately 1 kg) to perform longitudinal studies with the ability to obtain multiple samples. Another advantage over existing models is that the ferret is the natural host for several coliform diseases. In the clinical realm, extraintestinal E. coli-associated diseases of the ferret are major causes of morbidity and mortality in postparturient jills and in neonates (5, 20). cnf1 or other virulence genes of these E. coli isolates may also be appropriate targets for vaccine development. Finally, comparisons of fecal and extraintestinal strains of pap-, hly-, and cnf1-positive E. coli from ferrets may provide insights into the critical genetic and proteomic differences required by these organisms for selecting ecological niches and their subsequent ability to elicit disease.
REFERENCES
- 1.Bingen-Bidois, M., O. Clermont, S. Bonacorsi, M. Terki, N. Brahimi, C. Loukil, D. Barraud, and E. Bingen. 2002. Escherichia coli pathogenicity island-like domains. Infect. Immun. 70:7185-7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bingen-Bidois, M., O. Clermont, S. Bonacorsi, M. Terki, N. Brahimi, C. Loukil, D. Barraud, and E. Bingen. 2002. Phylogenetic analysis and prevalence of urosepsis strains of Escherichia coli bearing pathogenicity island-like domains. Infect. Immun. 70:3216-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco, J., M. Blanco, M. P. Alonso, J. E. Blanco, J. I. Garabal, and E. A Gonzalez. 1992. Serogroups of Escherichia coli strains producing cytotoxic necrotizing factors cnf1 and cnf2. FEMS Microbiol. Lett. 96:155-160. [DOI] [PubMed] [Google Scholar]
- 4.Blanco, M., J. E. Blanco, J. Blanco, M. P. Alonso, C. Balsalobre, M. Mouriño, C. Madrid, and A. Juánez. 1996. Polymerase chain reaction for detection of Escherichia coli strains producing cytotoxic necrotizing factor type 1 and type 2 (CNF1 and CNF2). J. Microbiol. Methods 26:95-101. [Google Scholar]
- 5.Blanco, M., J. E. Blanco, A. Mora, and J. Blanco. 1998. Distribution and characterization of faecal necrotoxigenic Escherichia coli CNF1+ and CNF2+ isolated from healthy cows and calves. Vet. Microbiol. 59:183-192. [DOI] [PubMed] [Google Scholar]
- 6.Boquet, P. 1998. Cytotoxic necrotizing factor 1 from Escherichia coli: a toxin with a new intracellular activity for eukaryotic cells. Folia Microbiol. 43:285-289. [DOI] [PubMed] [Google Scholar]
- 7.Boquet, P. 2001. The cytotoxic necrotizing factor 1 (CNF1) from Escherichia coli. Toxicon 39:1673-1680. [DOI] [PubMed] [Google Scholar]
- 8.Doye, A., A. Mettouchi, G. Bossis, R. Clement, C. Buisson-Touati, G. Flatau, L. Gagnoux, M. Piechaczyk, P. Boquet, and E. Lemichez. 2002. CNF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell 111:553-564. [DOI] [PubMed] [Google Scholar]
- 9.Fabbri, A., L. Falzano, S. Travaglione, A. Stringaro, W. Malorni, S. Fais, and C. Fiorentini. 2002. Rho-activating Escherichia coli cytotoxic necrotizing factor 1: macropinocytosis of apoptotic bodies in human epithelial cells. Int. J. Med. Microbiol. 291:551-554. [DOI] [PubMed] [Google Scholar]
- 10.Falzano, L., R. Rivabene, M. T. Sanini, A. Fabgri, and C. Fiorentini. 2001. An Escherichia coli cytotoxin increases superoxide anion generation via rac in epithelial cells. Biochem. Biophys. Res. Commun. 283:1026-1030. [DOI] [PubMed] [Google Scholar]
- 11.Feria, C., J. Machado, J. D. Correia, J. Goncalves, and W. Gaastra. 2001. Virulence genes and P fimbriae PapA subunit diversity in canine and feline uropathogenic Escherichia coli. Vet. Microbiol. 82:81-89. [DOI] [PubMed] [Google Scholar]
- 12.Fournout, S., C. M. Dozois, M. Odin, C. Desautels, S. Peres, F. Herault, F. Daigle, C. Segafredo, J. Laffitte, E. Oswald, J. M. Fairbrother, and I. P. Oswald. 2000. Lack of a role of cytotoxic necrotizing factor 1 toxin from Escherichia coli in bacterial pathogenicity and host cytokine response in infected germfree piglets. Infect. Immun. 68:839-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox, J. G. (ed.). 1998. Biology and diseases of the ferret, 2nd ed., p. 321-354. Williams & Wilkins, Baltimore, Md.
- 14.Guyer, D. M., J.-S. Kao, and H. L. T. Mobley. 1998. Genomic analysis of a pathogenicity island in uropathogenic Escherichia coli CFT073: distribution of homologous sequences among isolates from patients with pyelonephritis, cystitis, and catheter-associated bacteriuria and from fecal samples. Infect. Immun. 66:4411-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guzman-Verri, C., E. Chaves-Olarte, C. von Eichel-Streiber, I. Lopez-Goni, M. Thelestam, S. Arvidson, J. P. Gorvel, and E. Moreno. 2001. GTPases of the Rho subfamily are required for Brucella abortus internalization in nonprofessional phagocytes: direct activation of Cdc42. J. Biol. Chem. 276:44435-44443. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, J. R., P. Delavari, and T. T. O'Bryan. 2001. Escherichia coli O18:K1H7 isolates from patients with acute cystitis and neonatal meningitis exhibit common phylogenetic origins and virulence factor profiles. J. Infect. Dis. 183:1546. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, J. R., N. Kaster, M. A. Kuskowski, and G. V. Ling. 2003. Identification of urovirulence traits in Escherichia coli by comparison of urinary and rectal E. coli isolates from dogs with urinary tract infection. J. Clin. Microbiol. 41:337-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landraud, L., M. Gauthier, T. Fosse, and P. Boquet. 2000. Frequency of Escherichia coli strains producing the cytotoxic necrotizing factor (CNF1) in nosocomial urinary tract infections. Lett. Appl. Microbiol. 30:213-216. [DOI] [PubMed] [Google Scholar]
- 19.Le Bouguenec, C., M. Archambaud, and A. Labigne. 1992. Rapid and specific detection of the pap, afa, and sfa adhesin-encoding operon in uropathogenic Escherichia coli strains by polymerase chain reaction. J. Clin. Microbiol. 30:1189-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberson, A. J., C. E. Newcomer, J. I. Ackerman, J. C. Murphy, and J. G. Fox. 1983. Mastitis caused by hemolytic Escherichia coli in the ferret. J. Am. Vet. Med. Assoc. 183:1179-1181. [PubMed] [Google Scholar]
- 21.Lockman, H. A., R. A. Gillespie, B. D. Baker, and E. Shakhnovich. 2002. Yersinia pseudotuberculosis produces a cytotoxic necrotizing factor. Infect. Immun. 70:2708-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lund, B., B.-I. Marklund, N. Strömberg, F. Lindberg, K.-A. Karlsson, and S. Normark. 1988. Uropathogenic Escherichia coli can express serologically identical pili of different receptor specificities. Mol. Microbiol. 2:255-263. [DOI] [PubMed] [Google Scholar]
- 23.Mainil, J. G., E. Jacquemin, F. Heraul, and E. Oswald. 1997. Presence of pap-, sfa-, and afa-related sequences in necrotoxigenic Escherichia coli isolates from cattle: evidence for new variants of the AFA family. Can. J. Vet. Res. 61:193-199. [PMC free article] [PubMed] [Google Scholar]
- 24.Mulvey, M. A., J. D. Schilling, J. J. Martinez, and S. J. Hultgren. 2000. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc. Natl. Acad. Sci. USA 16:8829-8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ojeniyi, B., P. Ahrens, and A. Meyling. 1994. Detection of fimbrial and toxin genes in Escherichia coli and their prevalence in piglets with diarrhea. The application of colony hybridization assay, polymerase chain reaction and phenotypic assays. Zentbl. Vet. Med. B 41:49-59. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt, C. K., K. C. Meysick, and A. D. O'Brien. 1999. Bacterial toxins: friends or foes? Emerg. Infect. Dis. 15:224-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schultsz, C., G. J. Pool, R. van Ketel, B. De wevek, P. Speelman, and J. Dankert. 1994. Detection of enterotoxigenic Escherichia coli in stool samples by using nonradioactively labeled oligonucleotide DNA probes and PCR. J. Clin. Microbiol. 32:2393-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Starc̆ic̆, M., J. R. Johnson, A. L. Stell, J. van der Goot, H. G. Hendriks, C. van Vorsenbosch, L. van Dijk, and W. Gaastra. 2002. Haemolytic Escherichia coli isolated from dogs with diarrhea have characteristics of both uropathogenic and necrotoxigenic strains. Vet. Microbiol. 85:361-377. [DOI] [PubMed] [Google Scholar]
- 29.Travaglione, S., L. Falzano, A. Fabbre, A. Stringaro, S. Fais, and C. Fiorentini. 2002. Epithelial cells and expression of the phagocytic marker CD68: scavenging of apoptotic bodies following Rho activation. Toxicol. In Vitro 16:405-411. [DOI] [PubMed] [Google Scholar]
- 30.Versalovic, J., M. Schnieder, F. J. de Bruijn, and J. R. Lupski. 1994. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol. Cell. Biol. 5:25-40. [Google Scholar]
- 31.Witham, P. K., C. T. Yamashiro, K. J. Livak, and C. A. Bat. 1996. A PCR-based assay for the detection of Escherichia coli Shiga-like toxin gene in ground beef. Appl. Environ. Microbiol. 62:1347-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]