Abstract
A newly developed reagent strip assay for the diagnosis of schistosomiasis based on parasite antigen detection in urine of infected individuals was evaluated. The test uses the principle of lateral flow through a nitrocellulose strip of the sample mixed with a colloidal carbon conjugate of a monoclonal antibody specific for Schistosoma circulating cathodic antigen (CCA). The strip assay to diagnose a group of highly infected schoolchildren in Mwanza, Tanzania, demonstrated a high sensitivity and association with the intensity of infection as measured both by egg counts, and by circulating anodic antigen and CCA levels determined by enzyme-linked immunosorbent assay. A specificity of ca. 90% was shown in a group of schistosome-negative schoolchildren from Tarime, Tanzania, an area where schistosomiasis is not endemic. The test is easy to perform and requires no technical equipment or special training. The stability of the strips and the conjugate in the dry format lasts for at least 3 months at ambient temperature in sealed packages, making it suitable for transport and use in areas where schistosomiasis is endemic. This assay can easily be developed to an end-user format.
Diagnosis of schistosomiasis, one of the major parasitic diseases in tropical areas, is usually performed by parasitological (microscopic detection of eggs), and/or immunological methods (antibody and antigen detection) (11). The demonstration of parasite eggs in urine or feces directly indicates the presence of the worms, but the disadvantages of this approach include a high fluctuation in egg counts, easily missed low infections, and a relatively time-consuming methodology. Immunological methods such as enzyme-linked immunosorbent assays (ELISAs) usually require more advanced laboratory settings but may yield a higher sensitivity (particularly for antibody detection). However, for antibody detection, specificity may be a problem, and the efficacy of treatment remains difficult to determine since specific antibodies continue to be present long after the worms have disappeared. In this respect, detection of parasite antigens (such as circulating anodic antigen [CAA] and circulating cathodic antigen [CCA]) by ELISA (1, 3, 11) shows many advantages, such as the demonstration of active infections or of the effect of treatment, and has a high specificity. However, ELISA procedures (total assay time of ca. 3 h) remain relatively slow, even in an optimized and standardized format, and they require skilled personnel and well-equipped laboratories. In most studies involving the CAA and/or CCA ELISA on serum and urine samples, the best diagnostic performance was achieved with the urine CCA assay, with sensitivities ranging from 80 to 100% (11). For this reason and because of the relative ease of obtaining urine samples, we have investigated ways to develop a rapid field-applicable test for the detection of CCA in the urine of schistosome-infected individuals. Here, we describe the development of a lateral-flow assay with carbon-labeled anti-CCA monoclonal antibodies (9). The assay can determine the presence of CCA in urine within 30 min, and strips and reagents can be transported and stored at room temperature.
MATERIALS AND METHODS
Antibodies.
A panel of 11 monoclonal antibodies (immunoglobulin M [IgM], IgG1, and IgG3 isotypes) directed against Schistosoma CCA was used (2) for initial screening for optimal performance as coating and detecting antibodies. Colloidal carbon particles (Spezial Schwartz 4 [SS4]; Degussa AG, Frankfurt, Germany) were used as the label. These particles have an irregular shape (bunches of grapes/aggregates) with a size range of 25 to 250 nm and are easily dispersible in aqueous buffers of low ionic strength (<5 mM). Conjugates were prepared by standard methods (6) by mixing a 0.2% suspension of carbon particles in 5 mM borate buffer (pH 8.8) with the antibody solution (0.5 mg/ml). After being mixed for 2 h, the conjugate was washed three times with 1% bovine serum albumin in 5 mM borate buffer (pH 8.8), in which buffer it can be stored at 4°C for at least 1 year.
Test principle.
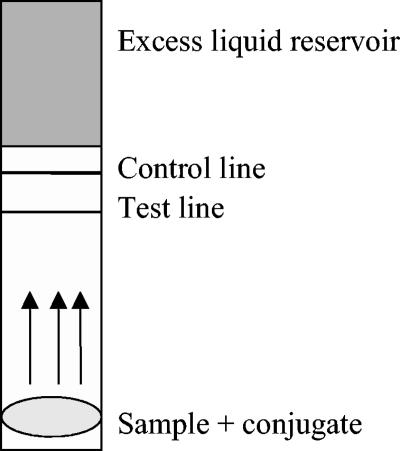
The lateral flow assay involves the use of nitrocellulose strips through the capillaries of which a mixture of the urine sample and a detection conjugate (monoclonal antibody labeled with carbon particles) flows. The presence of the analyte (CCA) is made visible by capture of the immune complex of antigen and carbon-labeled antibody by the anti-CCA monoclonal antibody that is immobilized on the strip as a test line. In addition, a line of immobilized polyclonal anti-mouse antibodies is used to capture the excess carbon-labeled antibodies to act as a positive control line (schematic diagram, Fig. 1).
FIG. 1.
Schematic diagram of the test principle of a lateral flow test.
Strips and buffers.
Unbacked nitrocellulose membrane cards (30 cm wide, type AE-99; Schleicher & Schuell, Dassel, Germany) were used and fixed to a vinyl backing. The antibodies were applied to these cards by using a Linomat IV (Camag, Muttenz, Switzerland) in 1 μl/7 mm at a concentration of 0.5 μg/μl in 5 mM borate buffer (pH 8.8) with 7.5% ethanol. As a positive control, a line of goat anti-mouse IgG (Sigma Chemicals) was applied. After application of the antibodies, the cards were cut into strips of 7 mm wide and stored at room temperature in a dry helium atmosphere, in which they were stable for at least 3 months. During optimization, the test line of the antibodies was applied in a series of concentrations in different buffers, with optimal results being obtained by 1 μl/strip of a 1-μg/μl antibody solution in 5 mM borate buffer (pH 8.8) with 7.5% ethanol. To avoid background staining and particle aggregation at the bottom of the strip, a series of blocking agents in the running buffer was tested. The best results were obtained with 0.1 M borate buffer (pH 8.8) containing 1% bovine serum albumin, 0.05% Tween 20, and 0.02% NaN3 (running buffer).
Test format.
Strips were tested in two different formats: the wet and dry formats. In the wet format, the carbon conjugate was mixed with the 1/10-diluted urine sample to a final volume of 100 μl (in a well of a microtitration plate or on a flat Parafilm-covered surface) into which the strip was placed. For the dry format the conjugate was mixed with a solution of mannitol (5% [wt/vol]) in running buffer, and 25 μl of this solution was dried in the wells of a 96-well microtitration plate overnight in a 37°C oven. The plates were then packed in sealed plastic bags under helium gas with a dry pellet included. In this way, the plates can be stored for at least 3 months in the dark at ambient temperature. As for the wet format, the urine samples were tested in a 1/10 dilution, prepared by adding 90 μl of buffer and 10 μl of urine into the wells, and were mixed well to dissolve the conjugate before the strip was put into the well.
In both formats, urine samples were tested along with a series of five standards of different antigen concentrations per group of 50 samples: a pool of negative control urine samples and four samples containing a series of 0, 0.3, 3, and 30 ng of CCA/ml of running buffer. These standards were used as negative and positive controls and also to facilitate scoring of the samples semiquantitatively on a 0-to-4 scale. A test line intensity similar to the standard concentration of 0.3 ng of CCA/ml would give a score of 1, 3 ng of CCA/ml would give a score of 2, 30 ng of CCA/ml would give a score of 3, and an even more intense line would result in a score of 4.
Samples.
For the initial laboratory evaluation of the test, we used samples from a group of 49 schoolchildren from the Mwanza region, Tanzania, where schistosomiasis mansoni is highly endemic. These children ranged in age from 7 to 18 years, and the prevalence by egg counts was determined to be 80% (median, 84 eggs/g of feces). Duplicate stool samples (42-mg samples) were examined by the Kato-Katz technique as previously described (5), and egg excretion was expressed as the mean of the two counts. Venous blood samples were taken, from which serum samples were prepared, and these were stored at −20°C until transported to the laboratory in Leiden for analysis of CAA and CCA by ELISA. Urine samples were taken as well and transported to the National Institute for Medical Research in Mwanza where, after sedimentation for at least 30 min, they were tested for CCA by using the strips in the wet format. In addition, urine aliquots were prepared and stored at −20°C until transported to the laboratory in Leiden where the CCA levels were determined by ELISA and by using the strip in the dry format. Both CAA and CCA ELISAs were performed as described previously (7) with minor modifications. The specificity of the strip test was evaluated in a group of 45 schoolchildren from a village in a mountainous area near Mwanza, where schistosomiasis is not endemic.
Permissions.
Permissions for the present study were obtained from the National Institute for Medical Research, Mwanza Medical Research Center, Mwanza, Tanzania, in accordance with the principles and practice of the Helsinki Declaration.
RESULTS
Only 2 of the 11 monoclonal antibodies used for the first screening were found to give working carbon conjugates. Of these two, one showed insufficient sensitivity in the strip test, which resulted in one monoclonal antibody (line 54-4C2-A; IgG1 isotype) that could also be used as a capture antibody.
By using strips coated with this antibody in an optimized buffer system, the required sensitivity and cutoff value (2 ng of CCA/ml [7], implying that when tested as a 1/10-diluted sample the detectable concentration on the strip is 0.2 ng/ml) were determined by adjusting the conjugate dilution and the amount of antibody deposited per strip. For the dry strip format, the width was reduced to 5 mm to allow the strips to fit into the wells of an ELISA plate. The concentration of the antibody solution for striping was not adjusted. Examples of the strips run in the wet format and an indication of the semiquantitative scoring are given in Fig. 2.
FIG. 2.
Examples of CCA strips run in wet format. (A) Set of standards to allow semiquantitative scoring of the samples: Neg. and 0 are scored as 0, 0.3 is scored as 1, 3 is scored as 2, 30 is scored as 3, and an even more intense line is scored as 4. (B) Five random samples that are scored against the standard series. The respective scores for samples 1 to 5 are 3, 0, 4, 3, and 2, respectively. It should be noted that the faint line at 0.3 ng/ml may not be well reproduced in print.
Sensitivity.
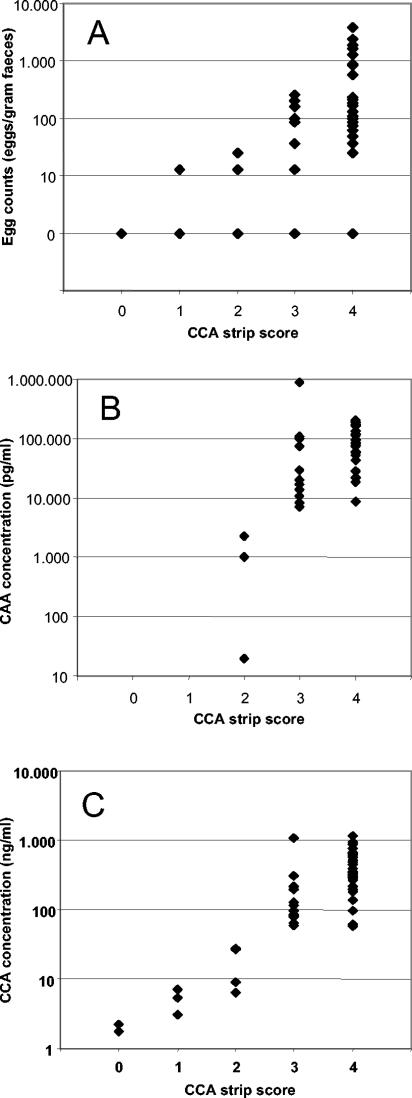
In Table 1 the numbers of positive results detected by the different tests are given. In this high-prevalence, high-intensity area, the antigen tests were found to have a higher positivity score than the egg counts. The agreement between the two strip formats was found to be nearly 100%. The correlation with the intensity of infection is shown in Fig. 3 and indicates that a semiquantitative score of the strips is feasible.
TABLE 1.
Comparison between stool egg counts, antigen ELISAs, and strip tests
| Test | No. of positivesa | % Positives |
|---|---|---|
| Egg counts | 39 | 80 |
| CAA serum ELISA | 42 | 86 |
| CCA urine ELISA | 47 | 96 |
| CCA serum ELISA | 45 | 92 |
| Strip wet format | 47 | 96 |
| Strip dry format | 49 | 100 |
The total number of cases examined in all tests was 49.
FIG. 3.
Association of CCA strip score with the intensity of schistosome infection as determined by egg counts in stool (A), CAA concentration in serum (B), and CCA concentration in urine (C). The strips were run in the wet format.
Specificity.
Urine samples from a group of 45 schoolchildren from Tarime, a mountainous area in Tanzania where schistosomiasis is not endemic, were used as negative controls. They were confirmed to be egg negative and were also negative in the serum CAA and urine CCA ELISAs. Of these 45 children, 2 were positive according to the CCA strip in the wet format and 6 were positive according to the dry format strip, resulting in 96 and 87% specificities, respectively. Other helminth parasites found in 24% of this group were Strongyloides stercoralis, Ascaris lumbricoides, hookworm, and Trichuris trichiura. Only in two of the six dry-format strip-positive cases were helminths found: in one case hookworm was found, and in the other case hookworm, Ascaris, and Trichuris were found.
Scoring.
A random series of 211 samples collected for an unrelated study running in the lab (mixed S. mansoni and S. haematobium infection, prevalence of ∼50%) were tested with the strip assay. The strips were scored independently by three observers, resulting in 78% identical scores. Of the 22% nonidentical scores, 14% would make a negative-to-positive (or vice versa) change, and 8% would differ only one point at the semiquantitative scale but remain a positive score. The prevalence of this series based on the strip results was 42%.
Stability.
After storage of the strips for 3 months at room temperature in an inert and dry atmosphere (a sealed package), the reactivity was not diminished. The carbon conjugate in solution is stable for at least 1 year at 4°C, although it tends to aggregate, which might necessitate a regular dissociation by ultrasonic vibration. In addition, in the dry format, both the strips and the dried carbon conjugate are stable for at least 3 months at ambient temperature in an inert and dry atmosphere.
DISCUSSION
Since the development of ELISAs for the detection of schistosome antigens in host serum and urine, the potential of a simple field-applicable test has been recognized. However, initiatives up to now have been hampered by the lack of commercial interest, funds, and availability of the right technology. In our laboratory, we have developed an ELISA-like reagent strip assay for the detection of CCA in urine (10), although this assay involves a number of steps and thus cannot be regarded as truly user-friendly and field applicable. In the last few years, various rapid lateral-flow assays have been developed for many different applications. By using available technology and know-how, we were able to develop an assay that is simple, user-friendly, and field applicable.
The sensitivity of the strip test is equal to that of the corresponding CCA ELISA. In this high-prevalence, high-intensity area, however, the sensitivity of the strip test has been found to be considerably higher than the parasitologic diagnosis. From the “pocket-chart” described by de Vlas et al. (4), it can be extrapolated that in this endemic setting (prevalence 80%, geometric mean of 7.3 eggs per 50 mg of stool) the true prevalence is 99%. Table 1 shows that, indeed, the results of the strip test correspond well with this predicted 96 to 100% prevalence. The sensitivity of the different antigen ELISAs was found to be as reported previously (8, 11).
The specificity was examined in a region where schistosomiasis does not occur due to geographical circumstances. In this population the specificities were 87 and 96%, respectively, for the dry and wet formats. As already described, for a noninvasive screening test this is completely acceptable (7). It should be noted that, as with all strip tests, the determination of the cutoff remains a difficulty because of the need to discriminate between a very weak line and no line at all. Retesting of problematic samples might solve this problem to some extent. On the other hand, for precise epidemiological analyses or for experimental animal studies, the highly specific CAA ELISA would remain the method of choice.
The identical test principles and, indeed, the good association of the strip test with the CAA and CCA ELISAs (used routinely in our laboratory) on urine and serum samples indicates that the well-described characteristics of these assays—such as sensitivity, determination of the effect of treatment, species specificity, and correlation with worm burden—would probably also apply to the strip. Particularly, the potential of sensitively detecting active infections would give this strip test an advantage above strip tests based on antibody detection (12). In conclusion, the developed strip test shows sufficient sensitivity and specificity for further validation studies and development into an end-user format where only a drop of urine and, if necessary, buffer needs to be added to the strip. Preliminary results already obtained with such a format are promising, indicating that the final goal of a field-applicable strip test based on parasite antigen detection for the diagnosis of schistosomiasis is within sight.
Acknowledgments
We thank L.-Å. Nilsson for advice and assistance with the field work and S. Spillane, D. Kornelis, C. W. A. Naus, and P. E. A. van Beek for advice and technical assistance in the lab.
This study received financial support from the European Communion under contract IC18CT97024.
REFERENCES
- 1.Deelder, A. M., N. De Jonge, O. C. Boerman, Y. E. Fillie, G. W. Hilberath, J. P. Rotmans, M. J. Gerritse, and D. W. Schut. 1989. Sensitive determination of circulating anodic antigen in Schistosoma mansoni infected individuals by an enzyme-linked immunosorbent assay using monoclonal antibodies. Am. J. Trop. Med. Hyg. 40:268-272. [DOI] [PubMed] [Google Scholar]
- 2.Deelder, A. M., G. J. van Dam, D. Kornelis, Y. E. Fillie, and R. J. Van Zeyl. 1996. Schistosoma: analysis of monoclonal antibodies reactive with the circulating antigens CAA and CCA. Parasitology 112(Pt. 1):21-35. [DOI] [PubMed] [Google Scholar]
- 3.De Jonge, N., P. G. Kremsner, F. W. Krijger, G. Schommer, Y. E. Fillie, D. Kornelis, R. J. Van Zeyl, G. J. van Dam, H. Feldmeier, and A. M. Deelder. 1990. Detection of the schistosome circulating cathodic antigen by enzyme immunoassay using biotinylated monoclonal antibodies. Trans. R. Soc. Trop. Med. Hyg. 84:815-818. [DOI] [PubMed] [Google Scholar]
- 4.De Vlas, S. J., B. Gryseels, G. J. Van Oortmarssen, A. M. Polderman, and J. D. F. Habbema. 1993. A pocket chart to estimate true Schistosoma mansoni prevalences. Parasitol. Today 9:305-307. [PubMed] [Google Scholar]
- 5.Håkangård, C., A. M. Deelder, R. M. Gabone, L. Å. Nilsson, and Ø. Ouchterlony. 1996. A comparative study on specific antibodies and circulating antigen (CAA) in serum and parasitological findings for diagnosis of schistosomiasis mansoni in an area of endemicity in Tanzania. Acta Trop. 61:213-222. [DOI] [PubMed] [Google Scholar]
- 6.Niessen, M. J. F., J. H. Wichers, H. A. Lee, M. Alcocer, M. R. A. Morgan, and A. van Amerongen. 1998. Rapid sol particle immunoassay for the detection of aflatoxin in food products, p. 23-29. In V. Gaukel and W. E. L. Spiess (ed.), Proceedings, part 3, of the third Karlsruhe Nutrition Symposium. European Research toward Safe and Better Foods, Karlsruhe, Germany.
- 7.Polman, K., M. M. Diakhate, D. Engels, S. Nahimana, G. J. van Dam, S. T. Falcao Ferreira, A. M. Deelder, and B. Gryseels. 2000. Specificity of circulating antigen detection for schistosomiasis mansoni in Senegal and Burundi. Trop. Med. Int. Health 5:534-537. [DOI] [PubMed] [Google Scholar]
- 8.Polman, K., F. F. Stelma, B. Gryseels, G. J. van Dam, I. Talla, M. Niang, L. Van Lieshout, and A. M. Deelder. 1995. Epidemiologic application of circulating antigen detection in a recent Schistosoma mansoni focus in northern Senegal. Am. J. Trop. Med. Hyg. 53:152-157. [DOI] [PubMed] [Google Scholar]
- 9.Van Amerongen, A., D. van Loon, L. B. Berendsen, and J. H. Wichers. 1994. Quantitative computer image analysis of a human chorionic gonadotropin colloidal carbon dipstick assay. Clin. Chim. Acta 229:67-75. [DOI] [PubMed] [Google Scholar]
- 10.Van Etten, L., C. C. Folman, T. A. Eggelte, P. G. Kremsner, and A. M. Deelder. 1994. Rapid diagnosis of schistosomiasis by antigen detection in urine with a reagent strip. J. Clin. Microbiol. 32:2404-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Lieshout, L., A. M. Polderman, and A. M. Deelder. 2000. Immunodiagnosis of schistosomiasis by determination of the circulating antigens CAA and CCA, in particular in individuals with recent or light infections. Acta Trop. 77:69-80. [DOI] [PubMed] [Google Scholar]
- 12.Zhu, Y., W. He, Y. Liang, M. Xu, C. Yu, W. Hua, and G. Chao. 2002. Development of a rapid, simple dipstick dye immunoassay for schistosomiasis diagnosis. J. Immunol. Methods 266:1-5. [DOI] [PubMed] [Google Scholar]