Abstract
DNA degradation (which results in a smear pattern) occurs with almost 50% of Mycobacterium abscessus strains during pulsed-field gel electrophoresis (PFGE). We assessed the potential benefit of using thiourea-containing buffer with M. abscessus by studying 69 isolates not previously typeable by PFGE (i.e., those with a smear pattern). Random (epidemiologically unrelated) isolates that were typeable (no smear pattern) were included as controls. Genomic DNA was digested with DraI, XbaI, and AseI. PFGE gels were run in regular gel buffer with and without 100 μM thiourea. All 69 isolates that generated smear patterns had clear band profiles when the thiourea buffer was used. These isolates were divided into only 30 patterns with DraI, 20 patterns with XbaI, and 20 patterns with AseI. The molecular profiles were all closely or possibly related, and the differences between the isolates ranged from zero to six bands. By multilocus enzyme electrophoresis (MEE), 45 of 53 smear isolates (85%) belonged to two closely related electrophoretic types. These isolates contained at least one enzyme allele seen almost exclusively in this group. Isolates without smear patterns were unaffected by thiourea and produced unrelated PFGE profiles, as well as multiple MEE types. The hsp65 and 16S rRNA gene sequences of the isolates with smear patterns were identical to those of M. abscessus type strain ATCC 19977, which had a nonsmear pattern, suggesting that this clone is a subgroup within M. abscessus. This demonstrates that the inability to type M. abscessus by PFGE is associated with a single clone of organisms.
Mycobacterium abscessus is a species of rapidly growing mycobacteria. It most commonly causes wound infections, abscess formation, and chronic pulmonary disease (23). Health care setting-associated outbreaks or pseudo-outbreaks due to the species have been reported with increasing frequencies since the mid-1970s (19, 21, 22, 27). Repetitive insertional sequences have not yet been recognized in M. abscessus, and usable probes are still unknown. Plasmids are also present in only some strains (5). Pulsed-field gel electrophoresis (PFGE) analysis of genomic DNA is the standard molecular technique for epidemiologic studies of the species (22). Unfortunately, approximately 50% of M. abscessus strains lyse spontaneously during electrophoresis, resulting in a smear pattern, and these strains cannot be assessed by PFGE (22). Random amplified polymorphic DNA PCR (RAPD-PCR) has been used as an alternative method for these strains (27). However, this method often produces highly similar profiles for clinically unrelated isolates. It is much more method dependent than PFGE and generally requires the use of multiple primers to differentiate strains. The potential for the misinterpretation of the results obtained by RAPD-PCR is also greater than the potential for misinterpretation of those obtained by PFGE (27).
In the early 1990s, it was observed that the DNA of two Streptomyces species contained site-specific modifications which undergo Tris-dependent strand scission during gel electrophoresis (3, 8, 28). The use of non-Tris-containing HEPES gel running buffer or the addition of 50 μM thiourea in Tris-containing buffer may prevent DNA degradation (3, 8, 9, 28). Recently, these methods were reported to have been used with a number of bacterial species to prevent DNA degradation (1, 6, 10, 11).
We applied the technique of adding thiourea into regular Tris-borate-EDTA (TBE) gel running buffer to study 69 M. abscessus isolates which had previously given smeared PFGE profiles with regular buffer (referred to as “smear isolates”) (22). Some of the isolates (mainly isolates from clinical outbreaks) were previously studied by RAPD-PCR because of their inability to be differentiated by PFGE typing (27).
MATERIALS AND METHODS
Strains.
Sixty-nine clinical M. abscessus isolates not previously typeable by PFGE were chosen. This included 49 random (epidemiologically unrelated) isolates from 18 states within the United States (1 isolate from Arkansas, 1 from Alabama, 2 from California, 1 from Colorado, 1 from Connecticut, 1 from Georgia, 1 from Illinois, 3 from Indiana, 3 from Louisiana, 1 from Massachusetts, 3 from Michigan, 2 from Minnesota, 3 from North Carolina, 1 from Ohio, 3 from Pennsylvania, 2 from Rhode Island, 19 from Texas, and 1 from Utah) and 20 isolates from four separate outbreaks in Florida, Texas (two outbreaks), and one foreign country (Colombia) (19, 21, 27). Random smear isolates were from (i) patients treated at the University of Texas Health Center at Tyler, (ii) among those isolates submitted to the Mycobacteria/Nocardia Reference Laboratory of the University of Texas Health Center at Tyler for antimicrobial susceptibility testing, or (iii) among those isolates submitted to the Mycobacteriology Reference Laboratory, National Center for Infectious Diseases, Centers for Disease Control and Prevention (Atlanta, Ga.). The outbreak isolates were provided for epidemiological study by individual investigators. Three of the four outbreaks have been described previously (19, 21, 27), while the fourth outbreak in Texas has not been described. A total of 15 random (epidemiologically unrelated) isolates that were typeable by PFGE were included as PFGE study controls. Thirty-one random (epidemiologically unrelated) isolates that were typeable by PFGE were included as multilocus enzyme electrophoresis (MEE) controls. The type strain of M. abscessus (ATCC 19977) was kindly provided by the American Type Culture Collection, Manassas, Va.
Species identification of the isolates was performed by published standard growth and biochemical methods and antimicrobial susceptibility patterns (12, 16, 26). Of the 69 isolates, 27 seen after 1995 were also identified by PCR-restriction enzyme analysis (PRA) of the 441-bp Telenti fragment of the hsp65 gene (13, 17, 24, 26). Susceptibility testing was performed by the broth microdilution method by use of the recently published NCCLS standards (4, 25). Isolates had been stored at −70°C in tryptic soy broth with 15% glycerol following isolation.
PFGE.
Analysis of large restriction fragment profiles by PFGE was carried out as described previously (22) with all isolates included in this study. Briefly, organisms cast into low-melting-point agarose plugs were lysed with lysozyme, sodium dodecyl sulfate, and proteinase K. The genomic DNA contained in the plugs was digested with restriction endonucleases DraI, XbaI, and AseI and separated by PFGE with a CHEF Mapper system (Bio-Rad Laboratories, Richmond, Calif.). The gel photos were scanned and analyzed by using Advanced Quantifier 1-D Match software (Bio Image, Ann Arbor, Mich.). PFGE gels were run in regular 0.5× TBE buffer, as well as 0.5× TBE buffer plus 100 μM thiourea. All PFGE procedures, including DNA preparation and restriction endonuclease digestion, were unchanged except for the addition of 50 or 100 μM thiourea to the gel running buffer.
Differences between PFGE profiles were determined, and strain relatedness was determined by use of a modification of the definitions recommended by Tenover et al. (18) (with the recognition that these definitions were designed for studies of outbreaks, not population studies, and were not examined for their validity with mycobacterial species). Isolates were considered indistinguishable, closely related, or possibly related if they exhibited zero, two to three, or four to six band differences with all three enzymes, respectively. In all these circumstances the isolates were considered clonal. Isolates were considered not related if they exhibited seven or more band differences with all three enzymes.
MEE.
Five enzyme systems (6-phosphogluconate dehydrogenase, glutamate oxalacetic transaminase, adenylate kinase, phosphoglucose mutase, and esterase) which exhibited allelic differences for M. abscessus isolates were studied by MEE as described previously (26). Forty-four of the 69 isolates included in this study were evaluated.
hsp65 gene sequencing.
A 360-bp portion of the hsp65 gene was sequenced as described previously (7, 14, 15) by using an Applied Biosystems (Foster City, Calif.) model 377 sequencer. Nine of 16 isolates with smear profiles by PFGE with regular buffer and seven isolates with clear profiles by PFGE were analyzed.
16S rRNA gene sequencing.
The 16S rRNA gene sequences of two selected smear isolates were sequenced as described previously (20), and the results were compared to those obtained with type strain ATCC 19977.
RESULTS
Strains.
All isolates were identified as M. abscessus, with no differences between smear and nonsmear isolates in their growth, biochemical reactions, or drug susceptibility patterns. All smear and nonsmear isolates had the type I restriction pattern by PRA of the 441-bp Telenti fragment of the hsp65 gene sequence (2).
PFGE.
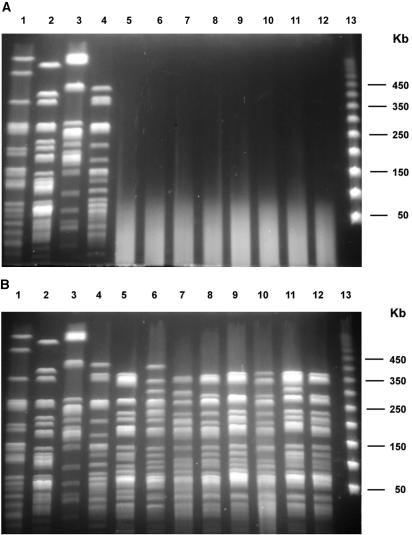
The use of 50 μM thiourea produced only a partial reduction in DNA smearing. All 69 isolates with smear patterns with regular buffer produced band profiles as clear as those for isolates that were typeable with regular buffer when the smear isolates were run with the buffer containing 100 μM thiourea (Fig. 1). Isolates from three of the four outbreaks each gave indistinguishable PFGE profiles, while isolates from the remaining outbreak had two XbaI profiles with only one band difference between the two.
FIG. 1.
PFGE patterns of M. abscessus isolates digested with AseI and run with regular TBE buffer (top) and buffer to which thiourea was added (bottom). Lanes 1 to 4, random isolates which produced clear patterns with regular buffer; lanes 5 to 12, smear patterns with regular buffer; lane 13, bacteriophage lambda DNA size standard.
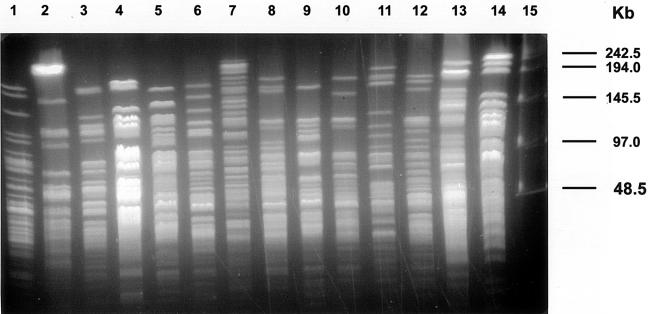
The results for 1 isolate from each of the three outbreaks which had indistinguishable PFGE profiles and the 2 isolates from the other outbreak which had two PFGE profiles, along with the 49 random isolates, were then compared in detail. These 54 isolates were divided into only 30 profiles by DraI, 20 profiles by XbaI, and 20 profiles by AseI (Table 1). DraI digestion produced more easily discernible profiles. The profiles were all indistinguishable, closely related, or possibly related (Fig. 2). The differences between the genotypes of a selected reference strain from one Texas outbreak and the other isolates mostly ranged from zero to six bands. Only one isolate had a seven-band difference with DraI, one isolate presented a seven-band difference with XbaI, and three isolates yielded seven-band differences with AseI. No single isolate gave seven-band differences with all three restriction enzymes, and hence, all smear isolates were considered related. The molecular profiles of the isolates from four outbreaks in two U.S. states (Florida and Texas) and Colombia were also closely related. By AseI digestion, isolates from outbreaks in Texas (Sherman, Texas), Florida, and Colombia were indistinguishable and differed by only three bands from an isolate from a second outbreak in Texas.
TABLE 1.
PFGE band differences between the 54 isolates with smear patterns by PFGEa
| Enzyme | Zero to three band differences
|
Four to six band differences
|
Seven band differences
|
Total
|
||||
|---|---|---|---|---|---|---|---|---|
| No. (%) of isolates | No. (%) of patterns | No. (%) of isolates | No. (%) of patterns | No. (%) of isolates | No. (%) of patterns | No. of isolates | No. of patterns | |
| DraI | 42 (78) | 19 (63) | 11 (20) | 10 (33) | 1 (2) | 1 (3) | 54 | 30 |
| XbaI | 48 (89) | 15 (75) | 5 (9) | 4 (20) | 1 (2) | 1 (5) | 54 | 20 |
| AseI | 34 (63) | 11 (55) | 17 (31) | 8 (40) | 3 (6) | 1 (5) | 54 | 20 |
All patterns were compared to the genotype of a selected reference isolate.
FIG. 2.
Schematic representation of PFGE patterns obtained by DraI digestion of all isolates that produced a smear pattern with regular buffer and that was visualized only with thiourea buffer. Lanes 1 to 30, different DraI patterns; lane 31, yeast DNA size standard (the values on the right are in kilobases).
Fifteen nonsmear isolates, including ATCC 19977, gave unrelated PFGE profiles which all differed by more than seven bands with all three enzymes. These profiles are shown in Fig. 3 and are categorized as unrelated.
FIG. 3.
PFGE patterns of 14 random M. abscessus isolates (lanes 1 to 14, respectively) that produced clear DNA patterns with regular TBE buffer. The chromosomal DNA was digested with XbaI. The patterns are highly diverse and are considered unrelated. Lane 15, bacteriophage lambda DNA size standard.
The isolates typeable with regular buffer showed the same PFGE profile with thiourea-containing buffer (Fig. 1).
MEE.
The 44 smear isolates of M. abscessus gave highly similar MEE profiles (Table 2). They were separated into only six electrophoretic types (ETs) (ETs 1 and 13 to 17). Only 2 of 31 (6%) nonsmear control isolates belonged to these ETs. A total of 37 of the 44 (84%) smear isolates belonged to ETs 13 and 14, which were two closely related ETs (Fig. 4). The smear isolates contained a 6-phosphogluconate dehydrogenase enzyme allele (allele 1 in Table 2), which was seen almost exclusively in this group, while the nonsmear isolates had multiple alleles that were seen almost exclusively with the nonsmear isolates. Twenty-nine of the 31 isolates with nonsmear PFGE profiles were separated into 19 other ETs. This also suggested that the smear group of isolates was clonal.
TABLE 2.
M. abscessus enzyme profiles for 44 isolates with PFGE smear patterns and 31 isolates with nonsmear patterns with regular buffer
| ET | No. of isolatesa
|
Allele no. at locus for corresponding enzymeb
|
|||||
|---|---|---|---|---|---|---|---|
| Clear | Smear | 6PD | GOT | ADK | PGM | EST | |
| 1 | 0 | 2 | 1 | 2 | 2 | 1 | 1 |
| 2 | 1 | 0 | 3 | 2 | 2 | 1 | 1 |
| 3 | 1 | 0 | 3 | 1 | 3 | 3 | 2 |
| 4 | 1 | 0 | 3 | 1 | 3 | 2 | 2 |
| 5 | 1 | 0 | 2 | 1 | 2 | 1 | 4 |
| 6 | 1 | 0 | 2 | 1 | 2 | 0 | 4 |
| 7 | 1 | 0 | 2 | 1 | 2 | 2 | 1 |
| 8 | 2 | 0 | 2 | 1 | 2 | 1 | 1 |
| 9 | 2 | 0 | 2 | 1 | 2 | 3 | 1 |
| 10 | 1 | 0 | 2 | 1 | 2 | 0 | 2 |
| 11 | 1 | 0 | 2 | 1 | 2 | 2 | 2 |
| 12 | 1 | 0 | 2 | 1 | 2 | 2 | 3 |
| 13 | 0 | 13 | 1 | 1 | 2 | 2 | 1 |
| 14 | 1 | 24 | 1 | 1 | 2 | 1 | 1 |
| 15 | 0 | 1 | 1 | 1 | 2 | 0 | 1 |
| 16 | 1 | 3 | 1 | 1 | 2 | 1 | 2 |
| 17 | 0 | 1 | 1 | 1 | 2 | 0 | 2 |
| 18 | 1 | 0 | 3 | 1 | 2 | 2 | 6 |
| 19 | 1 | 0 | 3 | 1 | 2 | 2 | 5 |
| 20 | 5 | 0 | 3 | 1 | 2 | 2 | 1 |
| 21 | 2 | 0 | 3 | 1 | 2 | 2 | 0 |
| 22 | 1 | 0 | 3 | 1 | 2 | 1 | 2 |
| 23 | 1 | 0 | 3 | 1 | 2 | 4 | 2 |
| 24 | 4 | 0 | 3 | 1 | 2 | 2 | 2 |
| 25 | 1 | 0 | 3 | 1 | 2 | 3 | 2 |
| Total | 31 | 44 | |||||
Clear isolates produced PFGE patterns with regular buffer; smear isolates did not.
6PD, 6-phosphogluconate dehydrogenase; GOT, glutamate oxalacetic transaminase; ADK, adenylate kinase; PGM, phosphoglucose mutase; EST, esterase.
FIG. 4.
Dendrogram of MEE results. Forty-two of the 44 smear isolates belonged to ETs 13 to 17, which clustered together at a genetic distance of 0.19.
hsp65 gene sequencing.
Nine smear isolates of M. abscessus and seven nonsmear control isolates had identical hsp65 gene sequences which matched (100%) the sequence of the nonsmear M. abscessus type strain, ATCC 19977.
16S rRNA gene sequencing.
The 16S rRNA gene sequences of the two smear isolates of M. abscessus showed 100% identity with type strain ATCC 19977 when the nearly complete 16S rRNA gene (1,463 bp) was sequenced.
DISCUSSION
We have been conducting epidemiologic and clinical studies with M. chelonae, M. abscessus, and M. fortuitum isolates for many years. Strain comparison has relied on PFGE as the standard. However, the genomic DNA of approximately 50% of M. abscessus strains and 10% of M. chelonae strains lyses spontaneously during gel electrophoresis, resulting in smeared profiles when PFGE is performed (6, 22, 27). Different cell treatment procedures and DNA digestion protocols were applied to prevent DNA degradation, but with little to no success. Recently, Römling and Tümmler (10) reported that more than 50 Pseudomonas aeruginosa strains which were previously affected by DNA degradation produced macrorestriction profiles with no background DNA smearing with the addition of 50 μM thiourea to the Tris-containing buffer. Corkill et al. (1) found that genomic DNA was degraded in all PCR ribotype 1 isolates of Clostridium difficile in the absence of thiourea, while band profiles were observed when thiourea was present. In our study the addition of thiourea to the TBE gel running buffer resolved the problem of DNA degradation in M. abscessus. Isolates previously affected by DNA degradation gave PFGE profiles as clear as those not previously affected. The addition of thiourea had no effect on strains previously typeable with regular buffer.
The use of HEPES buffer has also been recommended for the prevention of DNA degradation (3, 6, 8). We applied and tested this method. With HEPES buffer, however, PFGE gels must run for a longer time to keep the current within the normal range because of the buffer's higher ionic strength, and the bands in the gels were not as clear as those run in gels with thiourea-containing TBE buffer (Y. Zhang, unpublished observations). However, thiourea is a carcinogenic agent, on the basis of the results of animal studies, and special caution should be taken when handling this agent.
The 69 clinical M. abscessus smear isolates included in this study were from 18 states within the United States and one foreign country (Colombia). These isolates were divided into only 30 characterization patterns with DraI, 20 patterns with XbaI, and 20 patterns with AseI. Compared with the profiles for random M. abscessus isolates previously typeable, which produced highly diverse (unrelated) restriction profiles (22), the PFGE profiles of random smear isolates showed that they were all closely or possibly related (Table 1). The large restriction fragment differences between isolates generally ranged from zero to six bands. Thus, M. abscessus isolates requiring thiourea for PFGE represent a single clone by PFGE and MEE characterization, even though they were from a wide range of sources and geographic locations. This is a surprising observation. It suggests that this inability to type and characterize M. abscessus isolates by PFGE with regular gel running buffer is not a random event but is associated with a closely related clone of organisms. No differences between smear and nonsmear isolates were evident by standard growth and biochemical testing, drug susceptibility patterns, PRA of the hsp65 gene, partial sequencing of the hsp65 gene, and sequencing of the 16S rRNA gene, suggesting that this group of smear organisms is a subgroup within M. abscessus and not a different species.
However, we do not know if the isolates with the same characteristics within other species in which some isolates have smear patterns may form a subspecies. Corkill et al. (1) studied a small number of Clostridium difficile isolates and found that genomic DNA was degraded in all PCR ribotype 1 isolates. As shown by Römling and Tümmler (10), P. aeruginosa strains from different sources affected by DNA degradation had various PFGE profiles. They concluded that DNA degradation is not a clonal trait and that it could be lost by individual isolates of a clone (10). The taxonomic significance of this feature for other species may need further studies.
Isolates from four outbreaks involving thiourea-requiring strains had almost indistinguishable PFGE profiles. Three of the four outbreaks were previously studied by RAPD-PCR and are reported elsewhere (19, 21, 27). RAPD-PCR was used as an alternative method for these strains. However, PFGE analysis is still considered the “gold standard” molecular technique for epidemiologic and clinical microbiological studies of the species. While doing epidemiologic studies, one must keep in mind the fact that thiourea-requiring strains have closely or possibly related PFGE profiles, regardless of their sources. Therefore, a definition more strict than that of Tenover et al. (18) should be considered when interpreting strain relatedness by PFGE. In the previous study (27), RAPD-PCR separated outbreak from random smear isolates most of the time. It would be of interest to reassess these smear isolates now that PFGE analysis is possible.
REFERENCES
- 1.Corkill, J. E., R. Graham, C. A. Hart, and S. Stubbs. 2000. Pulse-field gel electrophoresis of degradation-sensitive DNA from Clostridium difficile DNA ribotype 1 strains. J. Clin. Microbiol. 38:2791-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devallois, A., K. S. Goh, and N. Rastogi. 1997. Rapid identification of mycobacteria to species level by PCR-restriction fragment length polymorphism analysis of the hsp65 gene and proposition of an algorithm to differentiate 34 mycobacterial species. J. Clin. Microbiol. 35:2969-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans, M., F. S. Kaczmarek, K. Stutzman-Engwall, and P. Dyson. 1994. Characterization of a Streptomyces-lividans-type site-specific DNA modification system in the avermectin-producer Streptomyces avermitilis permits investigation of two novel giant linear plasmids, pSA1 and pSA2. Microbiology 140:1367-1371. [DOI] [PubMed] [Google Scholar]
- 4.Ferraro, M. J., W. A. Craig, M. N. Dudley, G. Eliopoulos, D. W. Hecht, J. F. Hindler, L. B Reller, A. T. Sheldon, J. M. Swenson, F. C. Tenover, R. T. Testa, M. P. Weinstein, and M. A. Wikler. 2002. Performance standards for antimicrobial susceptibility testing; Twelfth informational supplement M100-S12. NCCLS, Wayne, Pa.
- 5.Hector, J. S. R., Y. Pang, G. H. Mazurek, Y. Zhang, B. A. Brown, and R. J. Wallace, Jr. 1992. Large restriction fragment patterns of genomic Mycobacterium fortuitum DNA as strain-specific markers and their use in epidemiologic investigation of four nosocomial outbreaks. J. Clin. Microbiol. 30:1250-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koort, J. M. K., S. Lukinmaa, M. Rantala, E. Unkila, and A. Siitonen. 2002. Technical improvement to prevent DNA degradation of enteric pathogens in pulsed-field gel electrophoresis. J. Clin. Microbiol. 40:3497-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pai, S., N. Esen, X. Pan, and J. M. Musser. 1997. Routine rapid Mycobacterium species assignment based on species-specific allelic variation in the 65-kilodalton heat shock protein gene (hsp65). Arch. Pathol. Lab. Med. 121:859-864. [PubMed] [Google Scholar]
- 8.Ray, T., J. Weaden, and P. Dyson. 1992. Tris-dependent site-specific cleavage of Streptomyces lividans DNA. FEMS Microbiol. Lett. 96:247-252. [DOI] [PubMed] [Google Scholar]
- 9.Ray, T., A. Mills, and P. Dyson. 1995. Tris-dependent oxidative DNA strand scission during electrophoresis. Electrophoresis 16:888-894. [DOI] [PubMed] [Google Scholar]
- 10.Römling, U., and B. Tümmler. 2000. Achieving 100% typeability of Pseudomonas aeruginosa by pulsed-field gel electrophoresis. J. Clin. Microbiol. 38:464-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silbert, S., L. Boyken. R. Hollis, and M. A. Pfaller. 2003. Improving typeability by pulsed-field gel electrophoresis. Diagn. Microbiol. Infect. Dis. 47:619-621. [DOI] [PubMed] [Google Scholar]
- 12.Silcox, V. A., R. C. Good, and M. M. Floyd. 1981. Identification of clinically significant Mycobacterium fortuitum complex isolates. J. Clin. Microbiol. 14:686-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steingrube, V. A., J. L. Gibson, B. A. Brown, Y. Zhang, R. W. Wilson, M. Rajagopalan, and R. J. Wallace, Jr. 1995. PCR amplification and restriction endonuclease analysis of a 65-kilodalton heat shock protein gene sequence for taxonomic separation of rapidly growing mycobacteria. J. Clin. Microbiol. 33:149-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swanson, D. S., X. Pan, and J. M. Musser. 1996. Identification and subspecific differentiation of Mycobacterium scrofulaceum by automated sequencing of a region of the gene (hsp65) encoding a 65-kilodalton heat shock protein. J. Clin. Microbiol. 34:3151-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swanson, D. S., V. Kapur, K. Stockbauer, X. Pan, R. Frothingham, and J. M. Musser. 1997. Subspecific differentiation of Mycobacterium avium complex strains by automated sequencing of a region of the gene (hsp65) encoding a 65-kilodalton heat shock protein. Int. J. Syst. Bacteriol. 47:414-419. [DOI] [PubMed] [Google Scholar]
- 16.Swenson, J. M., R. J. Wallace, Jr., V. A. Silcox, and C. Thornsberry. 1985. Antimicrobial susceptibility of five subgroups of Mycobacterium fortuitum and Mycobacterium chelonae. Antimicrob. Agents Chemother. 28:807-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Telenti, A., F. Marchesi, M. Balz, F. Bally, E. C. Böttger, and T. Bodmer. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31:175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 35:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiwari, T. S. P., B. Ray, K. C. Jost, Jr., M. K. Rathod, Y. Zhang, B. A. Brown-Elliott, K. Hendricks, and R. J. Wallace, Jr. 2003. Forty years of disinfectant failure: outbreak of postinjection Mycobacterium abscessus infection caused by contamination of benzalkonium chloride. Clin. Infect. Dis. 36:954-962. [DOI] [PubMed] [Google Scholar]
- 20.Turenne, C. Y., L. Tschetter, J. Wolfe, and A. Kabani. 2001. Necessity of quality-controlled 16S rRNA gene sequence databases: identifying nontuberculous Mycobacterium species. J. Clin. Microbiol. 39:3637-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villanueva, A., R. Villanueva, B. A. Vargas, F. Ruiz, S. Aguero, Y. Zhang, B. A. Brown, and R. J. Wallace, Jr. 1997. Report on an outbreak of postinjection abscesses due to Mycobacterium abscessus, including management with surgery and clarithromycin therapy and comparison of strains by random amplified polymorphic DNA polymerase chain reaction. Clin. Infect. Dis. 24:1147-1153. [DOI] [PubMed] [Google Scholar]
- 22.Wallace, R. J., Jr., Y. Zhang, B. A. Brown, V. Fraser, G. H. Mazurek, and S. Maloney. 1993. DNA large restriction fragment pattern of sporadic and epidemic nosocomial strains of Mycobacterium chelonae and Mycobacterium abscessus. J. Clin. Microbiol. 31:2697-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace, R. J., Jr., J. L. Cook, J. Glassroth, and D. E. Griffith. 1997. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. American Thoracic Society Statement. Am. J. Respir. Crit. Care Med. 156:S1-S25. [DOI] [PubMed] [Google Scholar]
- 24.Wilson, R. W., V. A. Steingrube, B. A. Brown, and R. J. Wallace, Jr. 1998. Clinical application of PCR-restriction enzyme pattern analysis for rapid identification of aerobic actinomycete isolates. J. Clin. Microbiol. 36:148-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woods, G. L., B. A. Brown-Elliott, E. P. Desmond, G. S. Hall, L. Heifets, G. E. Pfyfffer, J. C. Ridderhof, R. J. Wallace, Jr., N. G. Warren, and F. G. Witebsky. 2003. Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes: approved standard M24-A. NCCLS, Wayne, Pa. [PubMed]
- 26.Yakrus, M. A., S. M. Hernandez, M. M. Floyd, D. Sikes, W. R. Butler, and B. Metchock. 2001. Comparison of methods for identification of Mycobacterium abscessus and M. chelonae isolates. J. Clin. Microbiol. 39:4103-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang, Y., M. Rajagopalan, B. A. Brown, and R. J. Wallace, Jr. 1997. Randomly amplified polymorphic DNA PCR for comparison of Mycobacterium abscessus strains from nosocomial outbreaks. J. Clin. Microbiol. 35:3132-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou, X., Z. Deng, J. L. Firmin, D. A. Hopwood, and T. Kieser. 1988. Site-specific degradation of Streptomyces lividans DNA during electrophoresis in buffer contaminated with ferrous iron. Nucleic Acids Res. 16:4341-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]