Abstract
The evolutionarily conserved genes that encode the tripartite motif (TRIM) protein family are involved in various biological processes, including cellular immunity, inflammatory reaction, antiviral activity, and tumor progression. One member of this protein family, Trim59, has been reported as a novel biomarker for the occurrence and progression of multiple human carcinomas, such as lung cancer, gastric cancer, cervical cancer, and osteosarcoma. However, little is known about the relationship between Trim59 and bladder carcinogenesis. In this study, we examined the expression of Trim59 in bladder cancer (Bca) specimens and cell lines, and investigated its biological roles in Bca cell lines. We found that Trim59 was upregulated in Bca tissues and cell lines. In addition, using transwell chamber assays and the cell scratch test, we determined that knockdown of Trim59 significantly inhibited the epithelial-mesenchymal transition (EMT) and the processes of cell invasion and migration in Bca cell lines. Furthermore, we found that downregulated Trim59 expression could also inhibit cell proliferation and promote apoptosis. As a result, we demonstrated that the effects of Trim59-induced EMT and invasion/migration in Bca cells were achieved by the activation of the transforming growth factor beta/Smad2/3 signaling pathway. Our findings also revealed that Trim59 can present oncogenic activity, and may serve as a novel candidate target for bladder carcinoma treatment.
Keywords: Trim59, bladder carcinoma, EMT, metastasis, TGF-β, Smad2/3
Introduction
Worldwide, bladder cancer (Bca) is one of the most common and lethal tumors of the genitourinary system.1 Surgery is now considered as the most effective treatment for Bca patients. Although diagnosis and therapies for Bca have developed in recent decades, patients still exhibit poor prognoses, with high rates of recurrence and mortality.2 Therefore, exploring potential molecular mechanisms and developing new therapeutic targets are goals of great urgency for Bca clinical treatment.
The tripartite motif (TRIM) protein family consists of various members and involve into multiple biological processes, including cellular growth,3 metastasis,4 and development.5 Due to the presence of the RING finger domain, many TRIM proteins function as E3 ubiquitin ligases. TRIM proteins promote ubiquitin-related modifications of various substrates, as well as transcriptional silencing of specific target genes.6,7 Several TRIM proteins, such as Trim13, Trim19, Trim24, Trim29, and Trim37, have recently been verified to act as oncogenic or suppressive factors that are implicated in the progression of various cancers.8 Trim59 has been reported as a tumor promoter in multiple human carcinomas, including lung cancer, gastric cancer, cervical cancer, and osteosarcoma. Trim59 was first detected as a tumorigenic gene of various cancer types in 2011, such as breast, lung, parotid, gastrointestinal, female genital tract, bladder, kidney, and prostate cancer.9 In a 2015 study, Zhan discovered that Trim59 promotes metastasis and proliferation through cyclinB1/CDC25C/CDK1 proteins in non-small cell lung cancer.10 Furthermore, Trim59 was reported to interact with P53, and promote its ubiquitination and degradation, in gastric tumors and osteosarcoma.4,11
However, little is known about the expression profile of Trim59 in human Bca, and its biological role in this disease remains unclear. In the present study, we determined that Trim59 was more highly upregulated in Bca sample tissues than in normal tissues, and that knockdown of Trim59 significantly suppressed capabilities for invasion, migration, and proliferation in Bca cell lines.
Materials and methods
Patient samples
Written informed consent was obtained from all patients, and the study was approved by the Ethics Committee of Nanjing Medical University. Human bladder tissues used in this study were obtained from Bca patients who underwent radical cystectomy, without preoperative antitumor treatment, at the Urology Department of The First Affiliated Hospital of Nanjing Medical University (Table 1).
Table 1.
Patients’ characteristics
| Characteristics | Bca (n=15) |
|---|---|
| Age (years) | |
| Median (range) | 60 (56–77) |
| Sex | |
| Male | 10 |
| Female | 5 |
| Tumor stage | |
| Ta–T1 | 6 |
| T2–T4 | 9 |
| Tumor grade | |
| G1 | 7 |
| G2–G3 | 8 |
Abbreviation: Bca, bladder cancer.
Cell lines and cell culture
Three human Bca cell lines, TCCSUP, T24, and 5637, were chosen, and were obtained from the Chinese Academy of Sciences Cell Bank (Shanghai, People’s Republic of China). TCCSUP and T24 cells were grown in Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific). 5637 cells were grown in (RPMI) 1640 medium supplemented with 10% FBS (Thermo Fisher Scientific). All cells were cultured in an atmosphere of 5% CO2 at 37°C.
Cell transfection
Before transfection, 106 cells were planted in 6-well plates. When cells grew to 50%–70% degree of fusion, cell transfection was performed in accordance with the manufacturer’s protocol of Lipofectamine 2000 (Invitrogen, Grand Island, NY, USA) for cell transfection. Small interference RNAs (small interfering-Trim59 [si-Trim59] and negative control) were purchased from Genepharma (Shanghai, People’s Republic of China). When the cells grew to 50%–70% confluence in DMEM medium, Bca cells were transfected with Lipofectamine 2000. After transfection for 4–6 h, the medium surrounding the cells was replaced with DMEM.
Recombinant human TGF-β stimulation
Recombinant human transforming growth factor beta (Rh TGF-β) (R&D Systems, Minneapolis, MN, USA) was reconstituted at 20 µg/mL in sterile 4 mM HCl containing 0.1% bovine serum albumin for storing and use. Cells of the si-Trim59 group cells were seeded in 6-well plates at 40% confluence on the day before stimulation. At 48 h after 2 ng/mL Rh TGF-β stimulation, cells were harvested for transwell assay. Si-Trim59 group cells stimulated with Rh TGF-β were defined as si-Trim59 + TGF-β group.
RNA isolation and quantitative reverse transcription-polymerase chain reaction
Total RNA was isolated from tumor tissues and cells using Trizol Reagent (Thermo Fisher Scientific), according to the manufacturer’s instructions, and spectroscopy was used to detect the purity and concentration of the RNA samples. The conditions of the amplification reactions were as follows: 95°C for 30 s, 40 cycles at 95°C for 5 s, and 60°C for 1 min. The primers for Trim59 were as follows: forward (F), 5′-TGACTGACACACACTGGACA-3′, and reverse (R), 5′-CTGCTGCTCTCGTATTTCCT-3′. The primers for β-actin were as follows: F, 5′-ACTGGAACGGTGAAGGTGAC-3′, and R, 5′-AGAGAAGTGGGGTGGCTTTT-3′. The ABI 7900 system (Applied Biosystems, Carlsbad, CA, USA) was used to perform the reactions, and the data were analyzed using the 2−ΔΔCt method of quantitation. Each reaction was run in triplicate.
Cell proliferation
Cell proliferation assays were carried out using a Cell Counting Kit-8 (Beyotime, Shanghai, People’s Republic of China). After 48 h of transfection, cells were plated in 96-well plates at a density of 3,000 cells per well, and were then cultured for 24, 48, 72, and 96 h. Absorbance was detected at the wavelength of 450 nm, and cell viability was measured for 3 wells in each treatment group.
Colony formation assays
After transfection, cells were trypsinized and counted, and 800 cells were seeded in 6-well plates for 2 weeks. The wells were then washed twice with phosphate buffer solution; the cells were fixed with methanol for 30 min and were then stained with 0.5% crystal violet at room temperature for 30 min. Images were obtained using a digital camera.
Cell apoptosis
Cell apoptosis was carried out using an FITC Annexin V Apoptosis Detection Kit with PI (BioLegend, San Diego, CA, USA). Cells were washed twice with cold volumes of BioLegend’s Cell Staining Buffer, and were then resuspended in Annexin V Binding Buffer at a concentration of 1.0×106 cells/mL. More specifically, this suspension (100 µL) consisted of 10 µL of FITC Annexin V, 5 µL of propidium iodide, and 85 µL of Binding Buffer. The cells were then vortexed gently, and were incubated for 15 min, at room temperature (25°C), in the dark. To each tube, 400 µL of Binding Buffer was then added, and the cells were analyzed via flow cytometry (FACS Calibur; Becton Dickinson, Franklin Lakes, NJ, USA).
Cell cycle analysis
After 48 h of transfection, freshly prepared cells were harvested and re-suspended in 0.5 mL phosphate-buffered saline (PBS), and were then fixed with 70% alcohol at 4°C overnight. Then, the supernatant was discarded and the pellet was washed once with PBS. Subsequently, cells were incubated in 50 mg/mL of propidium iodide and 1 mg/mL of RNase, for 30 min at 37°C. Each sample consisted of at least 20,000 cells, and all cells were analyzed via flow cytometry (FACS Calibur).
Transwell migration assay and wound healing assay
The transwell migration assay was performed using 24-well chambers with polyethylene terephthalate track-etched membranes (8 µm pore size; Corning, Inc., Corning, NY, USA). A total of 5×104 transfected cells was suspended in the upper chamber with serum-free medium, while the lower chamber was filled with 20% FBS containing medium. After incubation at 37°C for 48 h, the remaining cells on the upper surface were removed. The cells on the lower surface of the membrane were fixed, then stained with crystal violet, and counted under a light microscope (Olympus IX71; 40× magnification). For cell invasion assays, the membranes were covered with Matrigel (BD Biosciences, San Diego, CA, USA) to form matrix barriers; otherwise, the remainder of this procedure was performed using the same steps previously described for the transwell migration assay. For wound healing assays, cells were cultured to form a monolayer of about 80% confluence in cell culture dishes. Using a sterile 200 mL tip, three separate wounds through the cell monolayer were created per well, and the cells were then cultured in free serum media. Wound closure was monitored at 0 and 2 d, and images were captured using phase contrast microphotography (200× magnification per field).
Protein isolation and western blot
Cells and human Bca tissues were lysed in radioimmunoprecipitation assay buffer (Beyotime, Beijing, People’s Republic of China), supplemented with protease inhibitors (Roche, Shanghai, People’s Republic of China) and the serine protease inhibitor phenylmethylsulfonyl fluoride (Roche), at 4°C for 1 h. Proteins from tissues and cells were separated using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and were then transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). Membranes were incubated first with primary antibodies, then with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody. Blots were detected using a Bio-Rad Bioimaging system (Bio-Rad, Hercules, CA, USA), and antibodies against GAPDH served as a negative control. Rabbit monoclonal antibodies (1:1,000) against TGF-β, Smad2/3, phosphorylated Smad2 (p-Smad2), p-Smad, vimentin, N-cadherin, and matrix metallopeptidase-9 (MMP-9) were purchased from Cell Signaling Technology (St Louis, MO, USA). Rabbit monoclonal antibody against Trim59 (1:200) was purchased from Abcam (Cambridge, MA, USA). All experiments were performed in triplicate.
Statistical analysis
All data values are expressed as mean ± standard deviation, and statistical analyses were performed using the SPSS17.0 software. Statistical evaluations were analyzed using the Student’s t-test. Values of P<0.05 were considered statistically significant.
Results
Trim59 is upregulated in Bca tissues and cultured cell lines
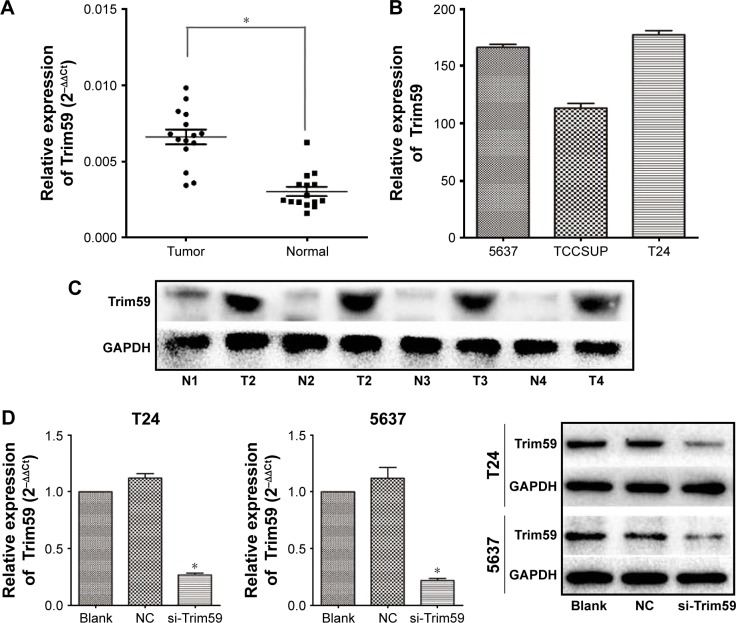
To determine the role of Trim59 in Bca, we examined the expression of Trim59 in clinical Bca tissues and their adjacent normal tissues by quantitative polymerase chain reaction. The results indicated that Trim59 was significantly overexpressed in Bca tissues, compared with adjacent normal tissues (Figure 1A). We also tested the mRNA levels of Trim59 in three Bca cultured cell lines: T24, TCCSUP, and 5637. Relatively higher levels of expression were found in the T24 and 5637 cell lines than in the TCCSUP cell line (Figure 1B). This might indicate that T24 and 5637 are the optimal cell lines for further investigations of Bca. Furthermore, western blot analyses also confirmed upregulated levels of Trim59 in Bca tissues, compared with normal tissues (Figure 1C). These results revealed that Trim59 is highly expressed in human Bca tissues and in cultured Bca cell lines.
Figure 1.
Trim59 is upregulated in Bca cell lines and tissues.
Notes: (A) The mRNA expression of Trim59 in Bca samples was highly regulated than the paired adjacent normal tissues. (B) Trim59 level in 5637 and T24 cell lines was relatively high compared with TCCSUP cells. (C) Trim59 protein level in Bca tissues was upregulated than that in the adjacent normal tissues. (D) Trim59 mRNA expression of T24 and 5637 cells with si-Trim59 transfection decreased significantly compared to NC or blank. The protein level of Trim59 in T24 and 5637 cells reduced significantly compared with NC or blank after si-Trim59 transfection. Data are presented as mean ± SD. *P<0.05 by independent-sample t-test.
Abbreviations: Bca, bladder cancer; NC, negative control; SD, standard deviation; si-Trim59, small interfering-Trim59.
Transfection with a specific small interfering RNA to reduce the expression of Trim59 in Bca cell lines
To further understand the function of Trim59 in bladder carcinoma progression, two cell lines (T24 and 5637) were transfected with a specific small interfering RNA (siRNA) against Trim59 (siTrim59). Reverse transcription-polymerase chain reaction results demonstrated that the relative mRNA levels of Trim59 in siTrim59-transfected T24 and 5637 cells decreased to 20%–30%, compared to cells without transfection (Figure 1D). We also checked levels of the Trim59 protein after siRNA transfection; Trim59 expression was notably downregulated in the siRNA-transfected T24 and 5637 cell lines, compared to negative control cells.
Knocking down the expression of Trim59 inhibits the migration and invasion of Bca cell lines
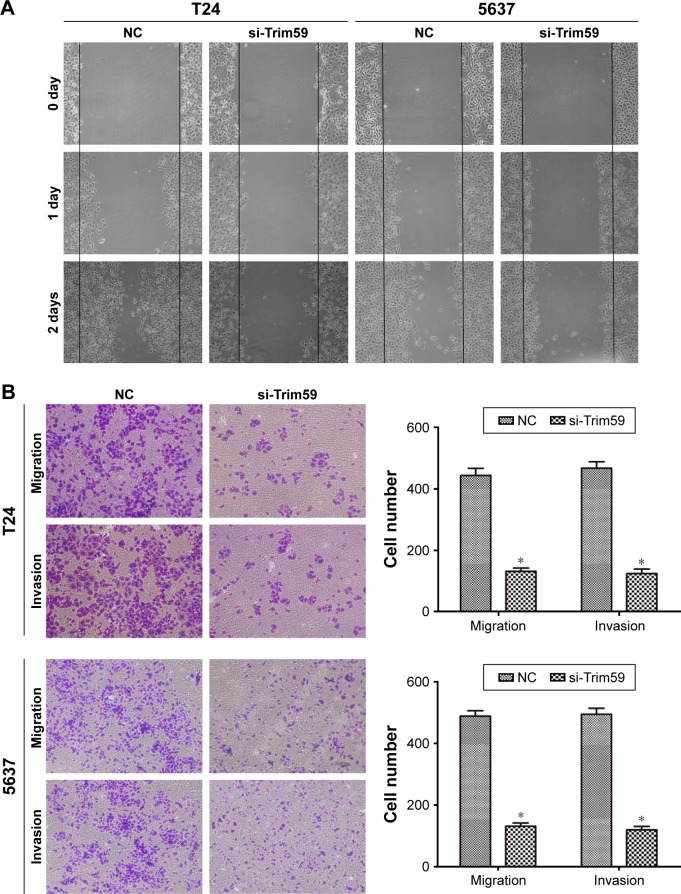
Bca has a marked tendency to metastasize and reappear; therefore, investigations of the functions of Trim59 are essential. In our cell scratch experiment, we found that downregulated Trim59 expression in T24 and 5637 cells significantly inhibited the ability of a cell monolayer to repair wounds (Figure 2A). Using crystal violet staining to count migrated cells in transwells, we found that the cell numbers for the si-Trim59-transfected group were markedly decreased compared to the negative control group (Figure 2B). Furthermore, in the invasion assay, silencing Trim59 notably suppressed the cells’ ability to invade through Matrigel and membrane pores (Figure 2B). These results confirmed that Trim59 could promote metastasis in bladder carcinoma.
Figure 2.
Knockdown of Trim59 inhibits migration and invasion of Bca cell lines.
Notes: (A) Effect of Trim59 on wound healing of T24 and 5637 cells. The invasion cells in which Trim59 was downregulated were analyzed by a wound healing assay. (B) The decreased Trim59 expression suppressed the invasion and migration of T24 and 5637. The invasion of the treated cells was evaluated in an invasion assay using a transwell insert coated with Matrigel. Data are presented as mean ± SD. *P<0.05 compared with NC.
Abbreviations: Bca, bladder cancer; NC, negative control; SD, standard deviation; si-Trim59, small interfering-Trim59.
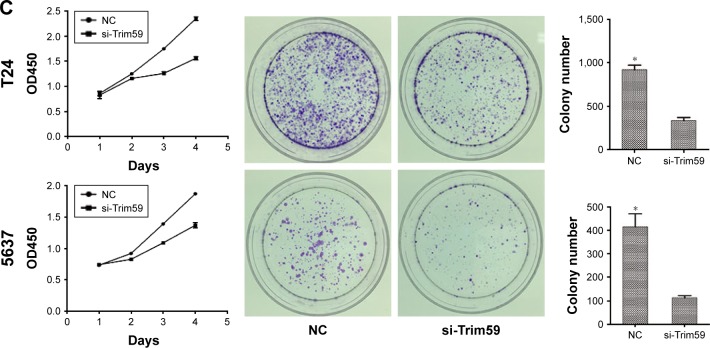
Knockdown of Trim59 suppresses cell proliferation and growth ability of Bca cell lines
To characterize the functional roles of Trim59 on cell proliferation, and to detect the effects of Trim59 on Bca growth, we performed cell-counting kit 8 assays and colony formation assays. Silencing of Trim59 using siRNA to achieve RNA interference markedly inhibited cell proliferation, compared to negative control cells (Figure 3C). In addition, knockdown of Trim59 expression attenuated the colony-forming ability of T24 and 5637 cells (Figure 3C). Given these results, Trim59 may promote cell proliferation and growth in Bca cell lines.
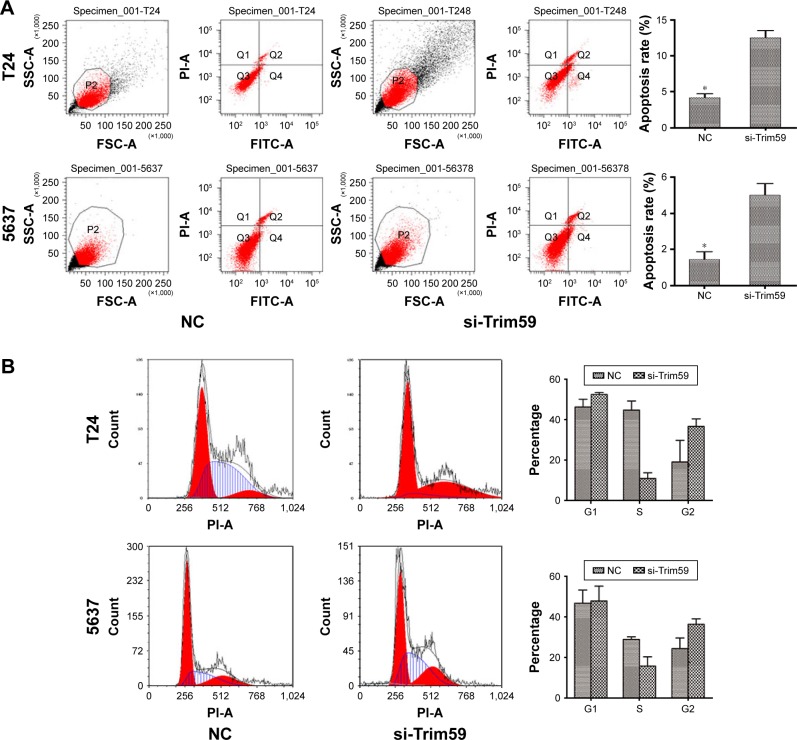
Figure 3.
Trim59 inhibits cell apoptosis and promotes cell proliferation of Bca cell lines.
Notes: (A) Flow cytometry analysis showed that cell apoptosis rates increased significantly after the transfection of si-Trim59. (B) si-Trim59 transfection of T24 and NC cells did not contribute to the cell cycle arrest compared with NC. Knockdown of Trim59 did not increased G1 phase percentage of T24 and 5637 cells. (C) Cell proliferation by CCK-8 assay and colony formation experiments. The proliferation capacity of T24 and 5637 cells was significantly inhibited by the downregulation of Trim59. The colony number of T24 and 5637 cells with si-Trim59 transfection decreased significantly compared with NC.
Abbreviations: Bca, bladder cancer; CCK-8, cell counting kit-8; NC, negative control; SD, standard deviation; si-Trim59, small interfering-Trim59.
Knockdown of Trim59 promotes apoptosis but does not affect the cell cycle transition in Bca cell lines
To investigate the role of Trim59 on apoptosis in Bca cell lines, we used the FITC Annexin V Apoptosis Detection Kit with PI (BioLegend) on siRNA-transfected T24 and 5637 cells. As shown in Figure 3A, depleting Trim59 significantly increased the apoptosis rates in these cell lines, compared with the negative control. These results demonstrated that Trim59 might serve as a factor inhibiting apoptosis and promoting abnormal proliferation in Bca cells; however, in our cell cycle analyses, we found no changes in the proportion of cells in the G1 phase in si-Trim59-transfected T24 and 5637 cells, compared to non-transfected cells. Of note, following knockdown of Trim59 expression, the percentage of cells in the S phase decreased distinctly, and G2 cells presented a growth trend (Figure 3B). Taken together, these results might lead us to propose that Trim59 regulates cell apoptosis, but does not facilitate cell cycle progression, in bladder carcinoma cells.
Trim59 promotes the epithelial-mesenchymal transition and increases protein levels of MMP-9 in Bca cells
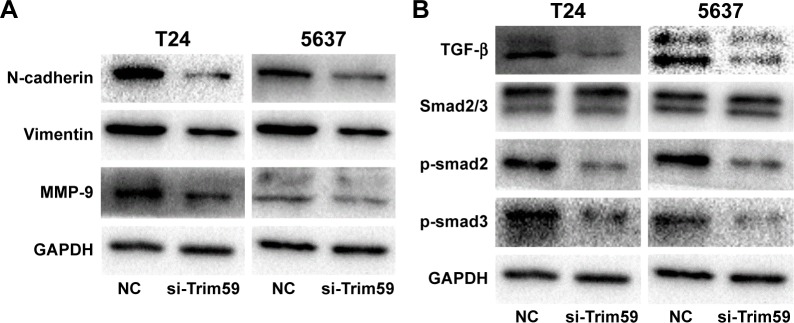
Considering the role of the epithelial-mesenchymal transition (EMT) process in promoting bladder carcinoma metastasis, we investigated whether Trim59 could affect EMT markers in Bca cell lines. Compared with the negative control group, knockdown of Trim59 notably mediated the expression levels of EMT markers, contributing to the downregulation of N-cadherin and vimentin; this indicated that Trim59 could promote the EMT process in Bca cells (Figure 4A). Additionally, this suggested that downregulation of Trim59 could inhibit the expression of MMP-9 in Bca cell lines (Figure 4A). These results further demonstrate the carcinogenic role of Trim59 in bladder cell carcinoma.
Figure 4.
Effect of si-Trim59 on EMT and TGF-β/Smad2/3 signaling pathway of Bca cell lines.
Notes: (A) Knockdown of Trim59 blocks EMT and MMP-9 protein levels in Bca cells. Western blot analysis was used to detect the changes in EMT markers in NC group and si-Trim59 group cells. The reduction of N-cadherin, vimentin, and MMP-9 was observed in si-Trim59 group cells. Data are presented as mean ± SD. (B) Knockdown of Trim59 inhibits TGF-β/Smad2/3 signaling pathways. Western blot analysis was used to detect the changes of TGF-β signaling markers in NC and si-Trim59 group. The reduction of p-Smad2 and p-Smad3 was observed in si-Trim59 group cell markers in NC group and si-Trim59 group cells. The reduction of n-cadherin, vimentin, and MMP-9 was observed in si-Trim59 group cells.
Abbreviations: Bca, bladder cancer; EMT, epithelial-mesenchymal transition; MMP-9, matrix metallopeptidase-9; NC, negative control; p-Smad2, phosphorylated Smad2; SD, standard deviation; si-Trim59, small interfering-Trim59; TGF-β, transforming growth factor beta.
Trim59 promotes Bca cells migration and invasion by activating the TGF-β/Smad2/3 signaling pathway
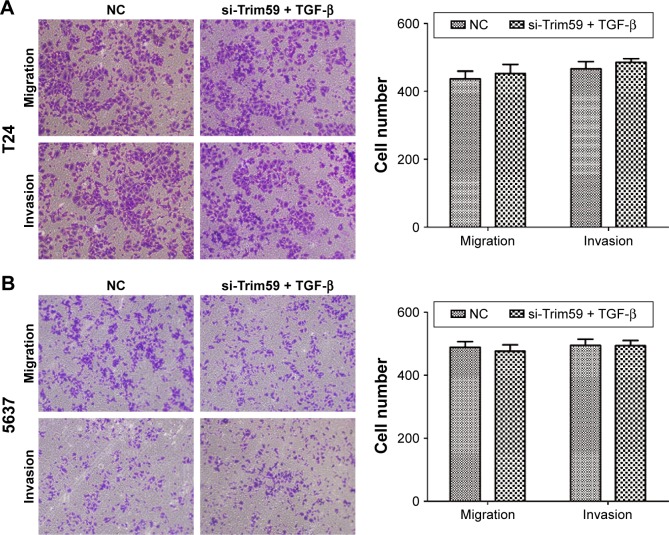
To further analyze the mechanism of Trim59 on Bca cells’ migration and invasion, we examined the protein levels of TGF-β, Smad2/3, and p-Smad2/3, which regulate the EMT process. The expression of TGF-β significantly decreased with the downregulation of Trim59 expression. In addition, in the Smad2/3 signaling pathway, p-Smad2 and p-Smad3 were both inhibited in the T24 and 5637 cell lines (Figure 4B), indicating that Trim59 might promote Bca metastasis through the TGF-β/Smad2/3 signaling pathway. Furthermore, we performed an experiment using TGF-β to rescue the migration and invasion abilities of Trim59-knockdown Bca cells. T24 and 5637 cells were transfected with si-Trim59, and then were stimulated with Rh TGF-β1 to upregulate TGF-β protein levels. Using the transwell assays described previously, we observed that the decreased migration and invasion capabilities induced by si-Trim59 transfection were recovered by Rh TGF-β stimulation. No significant differences were observed between the (si-Trim59 + Rh TGF-β) cells and the negative control cells (Figure 5A and B). These results confirmed that, within the context of downregulated Trim59 levels, the biological behaviors of bladder carcinoma cells could be rescued by restoring TGF-β expression.
Figure 5.
TGF-β promotes cell migration and invasion in Bca cell lines.
Notes: (A) TGF-β promotes migration and invasion of T24 cells. (B) TGF-β promotes migration and invasion of 5637 cells. Data are expressed as the mean ± SD.
Abbreviations: Bca, bladder cancer; NC, negative control; SD, standard deviation; si-Trim59, small interfering-Trim59; TGF-β, transforming growth factor beta.
Discussion
Worldwide, bladder carcinoma has become the most frequent neoplasm of the urinary tract.12,13 Remarkable advances in diagnosis and treatment have been made recently, but high mortality and poor prognoses are still major concerns in Bca patients. Even after early radical cystectomy and reasonable drug therapy, numerous patients with bladder carcinomas experience primary invasion and metastasis. Therefore, there is a severe need for new biomarkers for the early diagnosis and treatment of this neoplasm.
As one member of super family protein, TRIM has been reported to involve into multiple cellular processes and serve as an important regulator, including tumorigenesis, cell proliferation, cell apoptosis, and antiviral activity.3,8,14–16 Among TRIM proteins family, Trim59 is a novel molecule and has been proven to possess carcinogenic and malignant activity. It has been reported to be highly regulated in several malignant tumors, such as those associated with gastric cancer, lung cancer, cervical cancer, and osteosarcoma. Overexpression of Trim59 promotes cancer progression and metastasis, and correlates with “clinical stage” of tumor staging in various neoplasms.3,4,11 However, the expression density and biological functions of Trim59 in bladder carcinoma have never been investigated, and are still not very clear. In the present study, we have confirmed that Trim59 is upregulated in clinical Bca tissues, at both the mRNA and protein levels. In cultured Bca cell lines, we demonstrated that Trim59 mRNA is more overexpressed in T24 and 5637 cells than in TCCSUP cells. Furthermore, by transfecting these cells with a specific siRNA, we determined that downregulated Trim59 levels affect the biological activities of Bca cell lines and inhibit cancer progression. As a result, we found that knocking down Trim59 expression markedly decreased the proliferative, migratory, and invasive abilities of Bca cells of the T24 and 5637 lines. To investigate the molecular mechanisms of Trim59 on Bca migration and invasion, we detected the expression of several related molecules. Previously, the EMT process was shown to induce a series of tumor behaviors, such as enhanced migration, invasion, and metastasis.17 Likewise, MMP-9 belongs to the family of matrix metalloproteinases that can degrade the extracellular matrix and basement membranes, inducing cell metastasis as an outcome. We found that knockdown of Trim59 was accompanied by the blocking of the EMT process in Bca cell lines, with decreased expression of N-cadherin, vimentin, and MMP-9. These results suggested that Trim59 might facilitate Bca cell migration and metastasis, through regulation of the levels of MMP-9 and other EMT-related proteins.
In this study, we found that downregulation of Trim59 promoted apoptosis in Bca cells. Interestingly, we also found that knockdown of Trim59 did not affect the number of cells in the G1 phase, indicating that Trim59 might not participate in the regulation of the cell cycle. Additionally, Trim59 could enhance proliferation rates and colony formation capabilities in Bca cells. Taking all of these facts into account, although Trim59 does not seem to affect the cell cycle, it does promote proliferation and colony formation.
The results described confirmed our speculation that Trim59 might function as a tumor-promoting factor in Bca. We further investigated the possible mechanisms of Trim59-mediated effects. Recently, mounting evidence has made it clear that TGF-β plays a significant role in inducing the EMT process through various signaling pathways.18–22 We examined several molecular markers and found that Trim59 upregulated TGF-β, Smad2/3, p-Smad2, and p-Smad3 expression in Bca cell lines. It has recently been suggested that the TGF-β/Smad2/3 signaling pathway plays significant roles in cancer metastasis and progression.23,24 TGF-β is known to be capable of activating the phosphorylation of Smad2 and Smad3, and plays key roles in regulating various cellular responses including cell proliferation, extracellular matrix production, and apoptosis.25,26 Recent studies have proposed that the activation of TGF-β signaling involves the EMT process and increases the potential for tumor metastasis.27–29 In addition, upregulated MMP-9 expression has been shown to enhance the invasion and migration capabilities of cancer cells in various tumors.30–32 Therefore, the oncogenic role of Trim59 in Bca may partly depend upon its interaction with the TGF-β signaling pathway. We observed that knocking down Trim59 contributed to the inhibition of the TGF-β/Smad2/3 signaling pathway, consequently reducing levels of p-Smad2 and p-Smad3. These results revealed the molecular mechanisms by which Trim59 facilitates or induces invasion, migration, and the EMT process in Bca cells.
Consequently, we performed rescue experiments to further demonstrate that Trim59 could regulate Bca biological behaviors by activating the TGF-β/Smad2/3 signaling pathway. We found that the inhibitory effects of Trim59 knockdown on tumor invasion and migration could be recovered by treating the cells with Rh TGF-β. These results suggested that TGF-β was partially responsible for Trim59-promoted Bca metastasis and progression. However, the specific regulatory mechanisms of Trim59-mediated, aberrant activation of TGF-β/Smad2/3 signaling remain to be elucidated.
Conclusion
In conclusion, our study has identified the biological functions of Trim59 in Bca. Using siRNA to knockdown Trim59 expression significantly inhibited the invasive and migratory abilities of Bca cell lines through disruption of the TGF-β/Smad2/3 signaling pathway. In addition, we have demonstrated that Trim59 could promote cell proliferation and inhibit apoptosis in Bca cells. This may enhance our understanding of how tumor progression works and could identify Trim59 as a potential target for the treatment of bladder carcinomas.
Acknowledgments
The study was supported by National Natural Science Foundation of China (grant numbers 81270685 and 81402104) and Project of Nanjing Science and Technology Committee (201605001). We would like to appreciate all the colleagues who helped to design and perform this experiment.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin. 2012;62(5):283–298. doi: 10.3322/caac.21153. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Chen DT, Kurtyka C, et al. Tripartite motif containing 28 (Trim28) can regulate cell proliferation by bridging HDAC1/E2F interactions. J Biol Chem. 2012;287(48):40106–40118. doi: 10.1074/jbc.M112.380865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Z, Ji Z, Wang Y, et al. TRIM59 is up-regulated in gastric tumors, promoting ubiquitination and degradation of p53. Gastroenterology. 2014;147(5):1043–1054. doi: 10.1053/j.gastro.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Tocchini C, Keusch JJ, Miller SB, et al. The TRIM-NHL protein LIN-41 controls the onset of developmental plasticity in Caenorhabditis elegans. PLoS Genet. 2014;10(8):e1004533. doi: 10.1371/journal.pgen.1004533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of ‘single protein RING finger’ E3 ubiquitin ligases. Bioessays. 2005;27:1147–1157. doi: 10.1002/bies.20304. [DOI] [PubMed] [Google Scholar]
- 7.Bhatnagar S, Gazin C, Chamberlain L, et al. TRIM37 is a new histone H2A ubiquitin ligase and breast cancer oncoprotein. Nature. 2014;516(7529):116–120. doi: 10.1038/nature13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatakeyama S. TRIM proteins and cancer. Nat Rev Cancer. 2011;11(11):792–804. doi: 10.1038/nrc3139. [DOI] [PubMed] [Google Scholar]
- 9.Valiyeva F, Jiang F, Elmaadawi A, et al. Characterization of the oncogenic activity of the novel Trim59 gene in mouse cancer models. Mol Cancer Ther. 2011;10(7):1229–1240. doi: 10.1158/1535-7163.MCT-11-0077. [DOI] [PubMed] [Google Scholar]
- 10.Zhan W, Han T, Zhang C, et al. Trim59 promotes the proliferation and migration of non-small cell lung cancer cells by upregulating cell cycle related proteins. PLoS One. 2015;10(11):e142596. doi: 10.1371/journal.pone.0142596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang J, Xing D, Li Z, Shen J, Zhao H, Li S. Trim59 is upregulated and promotes cell proliferation and migration in human osteosarcoma. Mol Med Rep. 2016;13(6):5200–5206. doi: 10.3892/mmr.2016.5183. [DOI] [PubMed] [Google Scholar]
- 12.Nagata M, Muto S, Horie S. Molecular biomarkers in bladder cancer: novel potential indicators of prognosis and treatment outcomes. Dis Markers. 2016;2016:8205836. doi: 10.1155/2016/8205836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gschwend JE, Dahm P, Fair WR. Disease specific survival as endpoint of outcome for bladder cancer patients following radical cystectomy. Eur Urol. 2002;41(4):440–448. doi: 10.1016/s0302-2838(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 14.Zaman MM, Nomura T, Takagi T, et al. Ubiquitination-deubiquitination by the TRIM27-USP7 complex regulates tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol. 2013;33(24):4971–4984. doi: 10.1128/MCB.00465-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Versteeg GA, Benke S, Garcia-Sastre A, Rajsbaum R. InTRIMsic immunity: positive and negative regulation of immune signaling by tripartite motif proteins. Cytokine Growth Factor Rev. 2014;25(5):563–576. doi: 10.1016/j.cytogfr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manocha GD, Mishra R, Sharma N, Kumawat KL, Basu A, Singh SK. Regulatory role of TRIM21 in the type-I interferon pathway in Japanese encephalitis virus-infected human microglial cells. J Neuroinflammation. 2014;11:24. doi: 10.1186/1742-2094-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 18.Papageorgis P. TGFbeta signaling in tumor initiation, epithelial-to-mesenchymal transition, and metastasis. J Oncol. 2015;2015:587193. doi: 10.1155/2015/587193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zavadil J, Böttinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24(37):5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 20.Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:347–376. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- 21.Lamouille S, Derynck R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol. 2007;178:437–451. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 23.Kim TW, Michniewicz M, Bergmann DC, Wang ZY. Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature. 2012;482:419–422. doi: 10.1038/nature10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu H, Luo H, Shen Z, Hu X, Sun L, Zhu X. Transforming growth factor-beta1 in carcinogenesis, progression, and therapy in cervical cancer. Tumour Biol. 2016;37(6):7075–7083. doi: 10.1007/s13277-016-5028-8. [DOI] [PubMed] [Google Scholar]
- 25.Kamato D, Burch ML, Piva TJ, et al. Transforming growth factor-beta signalling: role and consequences of Smad linker region phosphorylation. Cell Signal. 2013;25(10):2017–2024. doi: 10.1016/j.cellsig.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Gratchev A. TGF-beta signalling in tumour associated macrophages. Immunobiology. 2017;222(1):75–81. doi: 10.1016/j.imbio.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Zhao JJ, Hao S, Wang LL, et al. Long non-coding RNA ANRIL promotes the invasion and metastasis of thyroid cancer cells through TGF-beta/Smad signaling pathway. Oncotarget. 2016;7(36):57903–57918. doi: 10.18632/oncotarget.11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan Z, Marshall JF. The role of integrins in TGFbeta activation in the tumour stroma. Cell Tissue Res. 2016;365(3):657–673. doi: 10.1007/s00441-016-2474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moustakas A, Heldin CH. Mechanisms of TGFβ-induced epithelial-mesenchymal transition. J Clin Med. 2016;5(7):63. doi: 10.3390/jcm5070063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q, Yu W, Huang T, Zhu Y, Huang C. RUNX2 promotes hepatocellular carcinoma cell migration and invasion by upregulating MMP9 expression. Oncol Rep. 2016;36(5):2777–2784. doi: 10.3892/or.2016.5101. [DOI] [PubMed] [Google Scholar]
- 31.Xiao B, Chen D, Luo S, et al. Extracellular translationally controlled tumor protein promotes colorectal cancer invasion and metastasis through Cdc42/JNK/MMP9 signaling. Oncotarget. 2016;7(31):50057–50073. doi: 10.18632/oncotarget.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Kempen LC, Coussens LM. MMP9 potentiates pulmonary metastasis formation. Cancer Cell. 2002;2(4):251–252. doi: 10.1016/s1535-6108(02)00157-5. [DOI] [PubMed] [Google Scholar]