Abstract
Codelivery is a promising strategy to overcome the limitations of single chemotherapeutic agents in cancer treatment. Despite progress, codelivery of two or more different functional drugs to increase anticancer efficiency still remains a challenge. Here, reduction-sensitive lipid–polymer hybrid nanoparticles (LPNPs) drug delivery system composed of monomethoxy-poly(ethylene glycol)-S-S-hexadecyl (mPEG-S-S-C16), soybean lecithin, and poly(D,L-lactide-co-glycolide) (PLGA) was used for codelivery of doxorubicin (DOX) and a Chinese herb extract triptolide (TPL). Hydrophobic DOX and TPL could be successfully loaded in LPNPs by self-assembly. More importantly, drug release and cellular uptake experiments demonstrated that the two drugs were reduction sensitive, released simultaneously from LPNPs, and taken up effectively by the tumor cells. DOX/TPL-coloaded LPNPs (DOX/TPL-LPNPs) exhibited a high level of synergistic activation with low combination index (CI) in vitro and in vivo. Moreover, the highest synergistic therapeutic effect was achieved at the ratio of 1:0.2 DOX/TPL. Further experiments showed that TPL enhanced the uptake of DOX by human oral cavity squamous cell carcinoma cells (KB cells). Overall, DOX/TPL-coencapsulated reduction-sensitive nanoparticles will be a promising strategy for cancer treatment.
Keywords: triptolide, codelivery, reduction sensitive, synergistic effect
Introduction
The existing therapy of single chemotherapeutic agents is far from perfection with undesirable severe side effects at high drug dose, low bioavailability, and the development of drug resistance. Over the past decade, due to the higher therapeutic effect at lower dose of synergistic combination, the interest in synergy therapy of two or more chemotherapeutic agents has been growing rapidly.1–3 However, due to the different biochemical activities and pharmacokinetics among the combination drugs, it is difficult to combine free drugs to obtain satisfactory therapeutic effect.4 In addition, the combination of free drugs always has more serious toxic side effects to human bodies.5
An effective codelivery system is the key to the success of combination therapy. Nanocarriers, which have been proven to reduce the side effects and increase the delivery efficiency of anticancer drugs, would be a good approach for collaborative treatment. These systems, including micelles,6 liposomes,7 and inorganic nanoparticles,8 provide the possibility to simultaneously deliver two or more chemotherapeutic drugs. However, there has not been a good solution for the codelivery of different drugs in intracellular drug release.9
Lipid–polymer hybrid nanoparticles (LPNPs), which combine the advantages of both liposomes and polymeric nanoparticles into a single delivery system, can encapsulate drugs for cancer chemotherapy, photothermal therapy, and theranostics.10–18 We previously reported the preparation of a new type of hybrid nanoparticle LPNPs using an amphiphilic reduction-sensitive polymer of monomethoxy-poly(ethylene glycol)-S-S-hexadecyl (mPEG-S-S-C16) to achieve intracellular release and then improve the therapeutic effect.19–21 The amphiphilic polymer mPEG-S-S-C16 containing a disulfide bond could maintain the stability of this formulation and serve as a switch to trigger drug release. The system of LPNPs was evaluated in vitro and in vivo and was proven as a potential drug carrier. In this study, we used reduction-sensitive LPNPs as the codelivery system of doxorubicin (DOX) and triptolide (TPL) to achieve intracellular release and synergistic therapeutic effect.
TPL, a diterpene triepoxide, is purified from the medicinal plant Tripterygium wilfordii.22 Since its antitumor effect was reported in 1972,23 the anti-inflammatory properties and antitumor activity of TPL have raised much research interest. TPL can directly induce cell apoptosis of various tumors,24 such as multiple myeloma cells,25 pancreatic carcinoma,26 ovarian cancer,27 and oral cancer.28 In addition, TPL was proved efficient in reducing tumor growth and prolonging the living time.29–31 TPL can also enhance the activities of chemotherapeutic agents in colon carcinoma cells, myeloma cell lines, and human oral cavity squamous cell carcinoma cells.32–34
As reported, the combination of TPL with other anticancer drugs resulted in synergistic effects and promoted apoptosis but did not intensify the side effects of chemotherapy.35 Hence, we attempted to determine the therapeutic potential of TPL in combination with a chemotherapeutic agent DOX, which is commonly used in clinical cancer therapy. The pairing of TPL and DOX with different mechanisms on cancer cells may produce synergistic therapeutic effect. It would be ideal if the combination of TPL and DOX at low dose can generate higher therapeutic effect and eliminate the cytotoxicity to normal tissues compared to the use of single drugs.
In this study, LPNPs through mixing mPEG-S-S-C16, soybean lecithin, and poly(d,l-lactide-co-glycolide) (PLGA) via self-assembly were developed to combine TPL and DOX (Figure 1). The TPL/DOX combination effect on human oral cavity squamous cell carcinoma cells (KB cells) was first investigated. The therapeutic potential and mechanisms of TPL in combination with DOX were determined using KB cells. The results suggest that TPL and DOX act in synergy to kill tumor cells. Dual drug-encapsulated LPNPs were also evaluated. As expected, such a codelivery system would combine the desirable properties of LPNPs and the synergistic effect of DOX and TPL and thus provide a fascinating opportunity to treat cancers.
Figure 1.
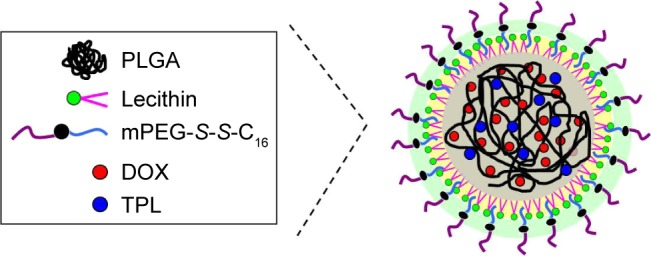
The schematic drawing of dual drug-loaded LPNPs.
Abbreviations: DOX, doxorubicin; LPNPs, lipid–polymer hybrid nanoparticles; mPEG-S-S-C16, monomethoxy-poly(ethylene glycol)-S-S-hexadecyl; PLGA, poly(d,l-lactide-co-glycolide); TPL, triptolide.
Materials and methods
Materials
PLGA (75:25, molecular weight (MW): 90,000–126,000) and soybean lecithin were purchased from Sigma-Aldrich (St Louis, MO, USA). N,N-Dimethyl formamide (DMF), triethylamine (TEA), and dimethylsulfoxide (DMSO) were purchased from Shanghai Chemical Co (Shanghai, People’s Republic of China). mPEG-S-S-C16 was synthesized using a method reported in our previous studies.36 Briefly, C16-S-S-COOH (0.52 g, 1.2 mmol), dicyclohexylcarbodiimide (0.272 g, 1.32 mmol), and 4-dimethylaminopyridine (0.03 g, 0.26 mmol) were added to an mPEG solution (MW: 2000, 0.4 g, 0.2 mmol) in 20 mL of anhydrous dichloromethane. After stirring at 25°C overnight, the mixture was filtered and the solvent was evaporated under reduced pressure. The crude product was washed three times with ethyl ether to obtain a white solid (yield: 83%).
KB cells were purchased from the China Center for Type Culture Collection (Wuhan University, Wuhan, People’s Republic of China) and cultured in Roswell Park Memorial Institute (Buffalo, NY, USA, [RPMI])-1640 medium (Beijing Dingguo Changsheng Biotechnology Co, Ltd, Beijing, People’s Republic of China), supplemented with 4×10−3 M l-glutamine, 10% fetal bovine serum (FBS), and 1% antibiotics (100 U/mL penicillin and 100 mg/mL streptomycin) at 37°C in a humidified atmosphere containing 5% CO2.
Female athymic BALB/c-nu mice (4–5 weeks old, 16±2 g) were purchased from Beijing HFK Bioscience Co, Ltd (Beijing, People’s Republic of China). All animals received care in compliance with the guidelines outlined in the Guide for the Care and Use of Laboratory Animals, and the experiment procedures were approved by the Animal Care and Use Committee of Wuhan University.
Preparation and characterization of DOX-loaded (DOX-LPNPs) and TPL-loaded LPNPs (TPL-LPNPs)
TPL-LPNPs or DOX-LPNPs were prepared using dialysis method as previously described with slight modification.36 In brief, prior to the preparation of LPNPs, the DOX hydrochloride was stirred twice with the molar amount of TEA in DMF for 10 h to obtain the lipophilic DOX base. A total of 1 mg of TPL or 5 mg of DOX was dissolved in 10 mL of DMF. PLGA (40 mg) was then added to the solution and stirred at room temperature for 2 h. mPEG-S-S-C16 and lipid soybean lecithin (weight ratio 3:1, total lipids 12 mg) were dissolved in 30 mL of 4 wt% ethanol aqueous solution at 65°C, and the solution of DOX/PLGA or TPL/PLGA was added dropwise under gentle stirring. Then, the mixed solution was vortexed vigorously for 3 min followed by dialyzing against ultrapure water at 25°C for 48 h. Afterward, the solution was filtered through a syringe filter (pore size 0.45 µM) to remove the unloaded TPL or DOX. Finally, the nanoparticles were concentrated using the Amicon® Ultra-4 Centrifugal Filter (molecular weight cutoff [MWCO]: 8,000; Beijing Dingguo Changsheng Biotechnology Co, Ltd). TPL/DOX-loaded nanoparticles were produced using the method described earlier. The only difference was that 1 mg of TPL and 5 mg of DOX were added to the PLGA solution at the same time.
Physicochemical characterizations of LPNPs
The size and size distribution of the drug-loaded nanoparticles were measured using dynamic light scattering (Zetasizer Nano Series ZEN3600; Malvern Instruments Ltd, Malvern, UK). Transmission electron microscopy (TEM; JEM-100CX II; JEOL Ltd, Tokyo, Japan) was used to observe the micelle morphology.
To evaluate the drug encapsulation efficiency (EE) and loading efficiency (LE), a predetermined aliquot of drug-loaded nanoparticles was collected by freeze-drying and, then, the dry nanoparticles were dissolved in DMSO. After that, the DOX concentration in DMSO was measured by fluorescence measurement using a calibration curve constructed from DOX solutions with different DOX concentrations.
The amount of TPL in the nanoparticles was analyzed using high-performance liquid chromatography (HPLC) (LC-15C; Shimadzu Corp., Kyoto, Japan). The EE is calculated as (actual amount of drug encapsulated in nanoparticles)/(initial amount of drug used in the fabrication of nanoparticles) ×100%. The LE is calculated as (amount of the drug in particles/amount of the feeding material and drug) ×100%.
In vitro release study
A total of 0.5 mL of the drug-loaded LPNPs was transferred into a dialysis tube (MWCO: 8,000). Then, it was immersed into a tube containing 10 mL of phosphate-buffered saline (PBS; 10 mM, pH 7.4) in a shaker shaken at 120 rpm and 37°C. At designated intervals, 5 mL of the external buffer was replaced with the corresponding fresh buffer solution. DOX quantity was determined by fluorescence measurement (excitation at 485 nm). The amount of TPL in the buffer solution was analyzed using HPLC. The error bars were obtained from triplicate samples.
In vitro cytotoxicity assay
The in vitro cytotoxicity of free drugs or drug-loaded nanoparticles against KB cells was evaluated by the MTT assay. KB cells were seeded into 96-well plate (Costar, Corning, NY, USA) at a density of 5.0×103 cells/well in 100 µL of RPMI-1640 medium containing 10% FBS. The cells were cultured for 1 day at 37°C in 5% CO2 atmosphere. Afterward, the cells were incubated with free drug, drug-free nanoparticles, or drug-loaded nanoparticles. After incubation, MTT stock solution (5 mg/mL in PBS, 20 µL) was added to each well and incubated for 4 h. The media were completely removed, and 150 µL of DMSO was added to each well to dissolve the formazan blue crystal. The absorbance of the solution was measured using a microplate reader at 570 nm. Cell viability was expressed as follows:
| (1) |
where Asample and Acontrol are the absorbance values for the treated cells and the untreated control cells, respectively. The Asample and Acontrol values were obtained after subtracting the absorbance of DMSO. Data are presented as average ± SD (n=4).
Median-effect analysis
Effects of the DOX and TPL combination were analyzed by the CalcuSyn software (Biosoft, Cambridge, UK). The combination index (CI) of the combined two drugs was determined based on the additive effect of the two drugs effect equation:
| (2) |
where da and db were the doses of drug a and drug b in the combination that kill χ% cells, Da and Db were the doses of drug a and drug b in single drug treatment to kill χ% cells. The interaction of two drugs can be categorized as synergy (CI <1), additive (CI =1), and antagonism (CI >1).
Therapeutic studies in vivo
Female athymic BALB/c-nu nude mice (4–6 weeks old, 18±2 g) were housed under specific pathogen-free conditions. Before treatment, all of the animals were kept in quarantine for a week. To establish a tumor model, H22 cells (1×107 per animal) were subcutaneously injected into the flank region of mice. When tumor grew to a volume of 50 mm3 (~6 days after inoculation), 48 mice were randomized into eight groups and numbered. After that, 200 µL of the formulated drug was injected through the tail vein, which was designated day 0. Tumor volume size (V) was monitored every 3 days for up to 18 days. Tumor volume was estimated by the following equation: V = a × b2/2, where a and b are the longest and shortest diameters, respectively.
Results and discussion
Synergistic cytotoxicity of DOX and TPL against KB cells
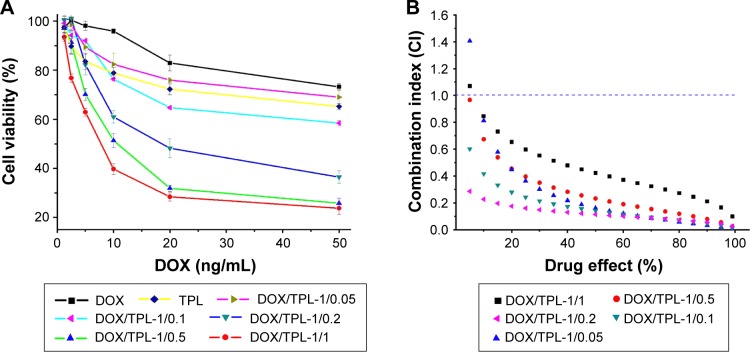
Prior to the development of codelivery systems for DOX and TPL, we first tested the cytotoxicity of DOX and TPL combination using KB cells. The cytotoxicity of DOX/TPL combination at different weight ratios was investigated to find out the most efficient ratio. Figure 2A shows the survival rate of KB cells after incubation with DOX/TPL combinations at varying ratios. Stronger inhibitory effect on KB cells viability was observed when a higher TPL/DOX ratio was treated for all DOX-based combinations (DOX/TPL-1/0.05, DOX/TPL-1/0.1, DOX/TPL-1/0.2, DOX/TPL-1/0.5, and DOX/TPL-1/1) (Figure 2A). In addition, the 50% inhibitory concentration (IC50) of DOX in DOX-based combinations was much lower than that treated with free DOX. Due to the lower IC50 of TPL, a minor change of TPL proportion in the combinations obviously impacts the viability of KB cells. The IC50 of DOX in combinations was reduced from 360.5 to 13.9 ng/mL when the combinations contained a small amount of TPL (2.78 ng/mL). The DOX/TPL-1/1 combination has the best in vitro antitumor effect.
Figure 2.
(A) Cytotoxicity of DOX-based combinations (DOX/TPL-1/0.05, 1/0.1, 1/0.2, 1/0.5, and 1/1) against KB cells after incubation for 48 h (n=4). (B) Plot of the combination index as the function of cell viability for KB cells treated with free DOX and free TPL combinations.
Abbreviations: DOX, doxorubicin; TPL, triptolide.
To further study the synergistic effect, the combined cytotoxicity data were quantitatively analyzed by the CalcuSyn software. The CI values of all DOX/TPL combinations (1/0.05, 1/0.1, 1/0.2, 1/0.5, and 1/1) were simulated with a reported method.37,38 Most of the DOX/TPL combinations showed synergistic effect (CI <1) for all treatment concentrations (Figure 2B). Moreover, when the weight ratio of TPL/DOX decreased from 1/1 to 0.2/1, the CI decreased from 0.433 to 0.114 (Figure 3). However, the CI increased with further decreasing the weight ratio of TPL/DOX from 0.2/1 to 0.05/1, which indicated that the best synergistic antitumor effect of the combination was achieved at the weight ratio of TPL/DOX of 0.2/1.
Figure 3.
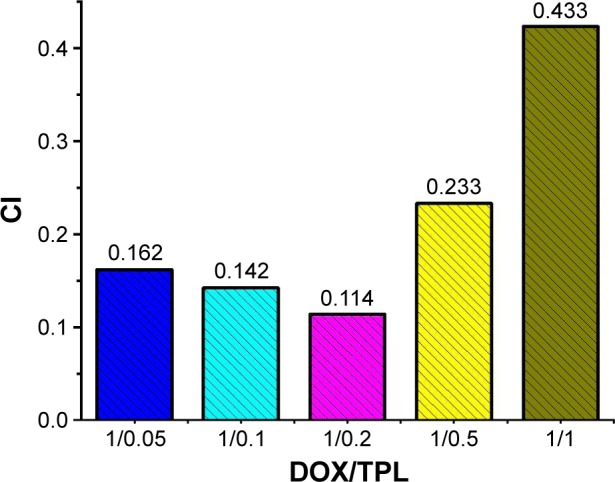
CI values at ED50 of DOX-based combinations (DOX dosage was 200 ng/mL).
Abbreviations: CI, combination index; ED50, 50% effective dose; DOX, doxorubicin; TPL, triptolide.
The simulated results that the synergistic effect of DOX/TPL combination is higher compared to that of other types of combination, such as DOX and paclitaxel, indicate a huge potential and feasibility of this combination.39,40 DOX/TPL-1/0.2 shows the lowest CI for all treatment concentrations, which showed that DOX/TPL-1/0.2 had the highest synergistic effect among the five combinations, and may be the optimal synergistic combination.
Preparation of DOX/TPL-loaded LPNPs (DOX/TPL-LPNPs)
A codelivery system that can load the two drugs in the same carrier and transport them to the same cancer cell simultaneously is significant to anticancer strategies. Therefore, we developed a reduction-sensitive drug delivery system DOX/TPL-LPNPs for the codelivery of DOX and TPL. The LPNPs comprised a biodegradable hydrophobic PLGA core, a soybean lecithin monolayer, and an outer corona layer made of mPEG-S-S-C16. The reduction-sensitive polymer mPEG-S-S-C16 was introduced into PLGA–lecithin to achieve intracellular release. After the incubation of mPEG-S-S-C16 containing LPNPs under reductive conditions, PEG segments were removed and the residual nanoparticles became unstable again, and aggregation occurred resulting in the fast release of the encapsulated drug in the hydrophobic parts of LPNPs. The DOX/TPL-LPNPs may be a promising strategy to overcome the undesirable toxicity and other side effects that limit the utilization of many potential drugs.
The foregoing experiments about the different weight ratios of DOX/TPL combination proved that favorable synergistic effect could be obtained at a weight ratio of 1/0.2 (DOX/TPL). Hence, a weight ratio of DOX/TPL-1/0.2-loaded LPNPs (DOX/TPL-1/0.2-LPNPs) was prepared (Figure 1). The characteristic absorption bands of DOX and TPL on the HPLC analysis proved that both DOX and TPL were successfully loaded into LPNPs and the drug carrier was effective for the codelivery of the two drugs. The average EE of DOX and TPL in the DOX/TPL-1/0.2-LPNPs was 75.5 and 58.3%, respectively. DOX-LPNPs and TPL-LPNPs were also prepared as controls, and their LE was 6.2 and 2.9%, respectively.
Size, morphology, and drug loading
The size and size distribution of nanoparticles were characterized by dynamic light scattering, and the results are listed in Table 1. The average sizes were generally in the same range of 100–120 nm, which is conducive to a satisfactory drug accumulation in the tumor through the enhanced permeability and retention (EPR) effect.41 The polydispersity of DOX/TPL-1/0.2-LPNPs was 0.12, indicating an unimodal size distribution.
Table 1.
Characterization of drug-loaded LPNPs
| Samples | Size (nm) | PDI | EE (%) | LE (%) | Zeta potential (mV) |
|---|---|---|---|---|---|
| DOX-LPNPs | 111±7 | 0.10±0.03 | 80.2±0.3 | 6.2±0.5 | −5.4±0.5 |
| TPL-LPNPs | 108±6 | 0.09±0.03 | 71.3±0.4 | 2.9±0.3 | −15.7±1.5 |
| DOX/TPL-1/0.2 | 120±5 | 0.12±0.04 | 75.5±0.5 (DOX) | 5.3±0.3 (DOX) | −9.7±0.8 |
| 58.3±0.4 (TPL) | 1.1±0.1 (TPL) |
Abbreviations: DOX, doxorubicin; EE, encapsulation efficiency; LE, loading efficiency; LPNPs, lipid–polymer hybrid nanoparticles; PDI, polydispersity index; TPL, triptolide.
The core–shell nanoparticles with smooth surface and spherical shape can be clearly observed from TEM (Figure 4). The amphiphilic lipid shell was fused ~8–20 nm on the hydrophobic PLGA core. The merit of this structure is that poorly soluble drugs can be encapsulated into the hydrophobic PLGA cavity with a high LE. The nanoparticles can be well dispersed in PBS with high stability. The TEM images show that the nanoparticles were dispersed individually with a well-defined spherical shape.
Figure 4.
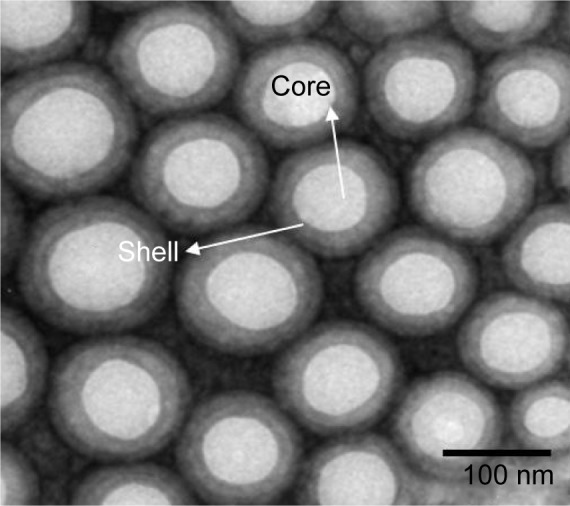
TEM image of DOX/TPL-loaded LPNPs.
Abbreviations: DOX, doxorubicin; LPNPs, lipid–polymer hybrid nanoparticles; TEM, transmission electron microscopy; TPL, triptolide.
Drug release in vitro
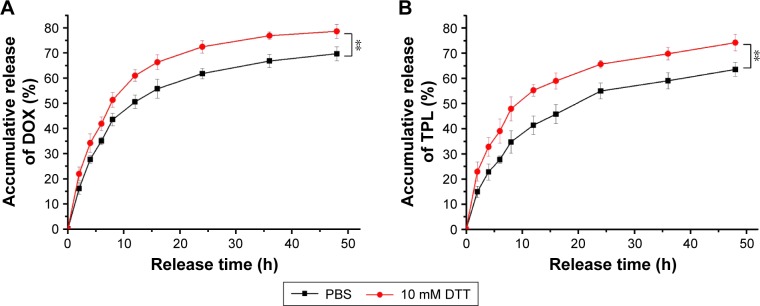
One advantage of using drug delivery systems with various nanoparticles is the controlled drug release, which improves the drug bioavailability and reduces the drug toxicity to normal tissues by the EPR effect and sensitive release.42,43 The reduction-responsive release of DOX and TPL from DOX/TPL-1/0.2-LPNPs was studied using a dialysis tube (MWCO: 8000) and HPLC analysis. The accumulative release behavior is shown in Figure 5. Due to the cleavage of the disulfide linkages, the DOX/TPL-1/0.2-LPNPs incubated with dithiothreitol (DTT) showed a more efficient release of drugs. Approximately 78% of the DOX was released over a period of 48 h in the presence of 10 mM DTT, while 64% of the DOX was released in the absence of DTT. The TPL showed similar release behavior to DOX, implying that DOX and TPL were effectively released from the LPNPs in response to the reductive environment of intracellular fluids in cancer cells. These results suggested that the LPNPs were a qualified intracellular codelivery system for DOX/TPL combination.
Figure 5.
Redox-triggered release of (A) DOX and (B) TPL from DOX/TPL-1/0.2-loaded LPNPs in PBS (0.01 M, pH 7.4) with or without 10 mM DTT.
Note: P-values were calculated by the Student’s t-test: **P<0.005.
Abbreviations: DOX, doxorubicin; DTT, dithiothreitol; LPNPs, lipid–polymer hybrid nanoparticles; PBS, phosphate-buffered saline; TPL, triptolide.
Cytotoxicity of DOX/TPL-LPNPs in vitro
To study whether the use of the carrier can fully exert the anticancer potential of the combination, the cytotoxicity of the DOX/TPL-1/0.2-LPNPs against KB cells was investigated. The cytotoxicity of DOX-encapsulated LPNPs was also characterized as parameter to determine the CI in the subsequent sections. DOX and TPL were combined to demonstrate the synergistic effect of their codelivery in the same vehicle. KB cells were first incubated in 96-well plates for 24 h and, then, were separately treated with the DOX-LPNPs, TPL-LPNPs, and DOX/TPL-1/0.2-LPNPs.
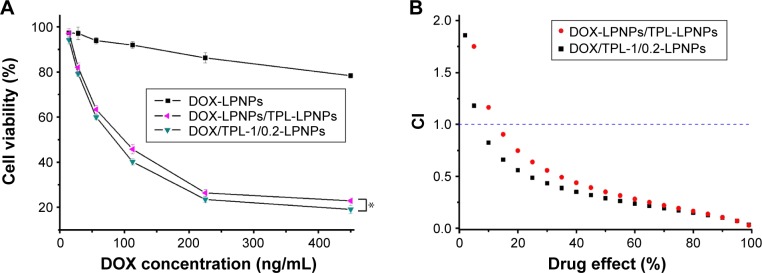
The codelivery of DOX and TPL significantly reduced cell viability (Figure 6A). Cytotoxicity experiments demonstrated that the codelivery of DOX/TPL-1/0.2-LPNPs resulted in significantly higher cytotoxicity than DOX-LPNPs and DOX-LPNPs + TPL-LPNPs at the same dose.
Figure 6.
(A) Cytotoxicity of drug-loaded LPNPs against KB cells after incubation for 48 h (n=4). (B) Plot of the CI as the function of cell viability for KB cells treated with drug-loaded LPNPs. P-values were calculated by the Student’s t-test: *P<0.05.
Abbreviations: CI, combination index; DOX, doxorubicin; LPNPs, lipid–polymer hybrid nanoparticles; TPL, triptolide.
The cytotoxicity of coencapsulation of DOX and TPL in the same vehicle was higher than the mixture of DOX-LPNPs and TPL-LPNPs due to the simultaneous drug-release profiles. Then, the synergistic effect was quantitatively analyzed by the CalcuSyn software. Figure 6B displays the analysis results of combined cytotoxicity data. DOX/TPL-1/0.2-LPNPs showed evident synergistic effect (CI <1) at all treatment concentrations, indicating that the codelivery system can preferably exert the synergistic effect of the combination. As a qualified codelivery platform, the LPNPs can transport different drugs to the same cancer cell simultaneously and may be a very potential synergistic treatment vehicle.
In vivo antitumor efficiency
On the basis of the abovementioned results, the in vivo anti-tumor efficacy and systemic toxicity of the codelivery system were further investigated on murine H22 hepatocarcinoma tumor-bearing female athymic BALB/c-nu nude mice. When tumors grew to a volume of 90 mm3 (~6 days after inoculation), 48 mice were randomized into eight groups and numbered. Mice were treated with PBS or different drug formulations every 6 days via the intravenous injection, and the tumor volume was observed and measured for 18 days. As shown in Figure 7, all the drug formulations have obvious inhibitory effect on tumor growth to different degrees compared to the rapid tumor growth of PBS treatment group. No matter the free drugs group or the drug-loaded nanoparticles-treated groups, we may find that the combination of DOX and TPL was more effective than the use of single drug through the contrastive analysis. Moreover, because of the EPR effect of nanoparticles, the DOX-LPNPs and TPL-LPNPs showed better antitumor effect compared to free drug for the same drugs, and a similar result was observed in the drug combination.
Figure 7.
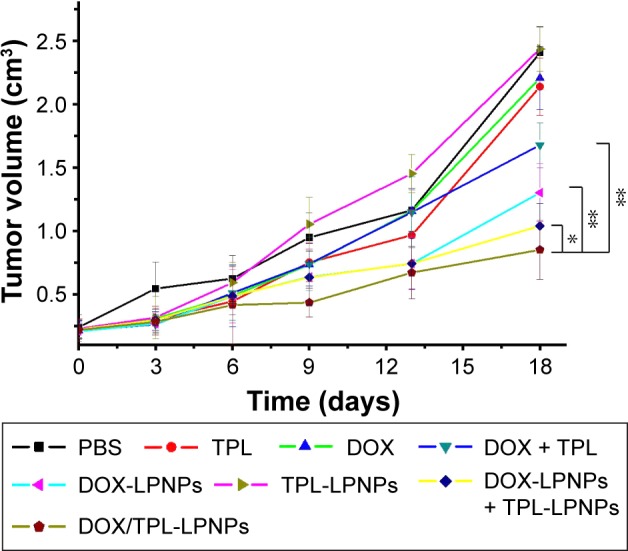
Antitumor effect of free drugs and drug-loaded LPNPs on H22 cell xenografts tumor.
Note: P-values were calculated by the Student’s t-test: *P<0.05 and **P<0.005.
Abbreviations: DOX, doxorubicin; LPNPs, lipid–polymer hybrid nanoparticles; PBS, phosphate-buffered saline; TPL, triptolide.
As seen in Figure 7, the most effective therapeutics was the DOX/TPL-1/0.2-LPNPs-treated group, with an obvious inhibition of tumor growth during the whole treatment. The tumor volume of DOX/TPL-1/0.2-LPNPs-treated group was only 34.0% of control group at the end of experiment, which was 5.5-, 4.5-, and 2.0-fold smaller than that treated with free DOX, free TPL, and free DOX/TPL, respectively. A combination of synergistic effects, passive targeting, and responsive release would mainly explain the highest antitumor effect of DOX/TPL-1/0.2-LPNPs. It is noteworthy that DOX/TPL-1/0.2-LPNPs possessed higher antitumor capacity than the combination of DOX-LPNPs and TPL-LPNPs. The results might be attributed to the higher synergistic efficiency of codelivery system. With the assistance of the codelivery system, DOX and TPL were transported to the tumor site and released in the same cancer cell. Compared to the mixture of single drug-loaded system, the codelivery system can preferably play the synergistic effect of the drug combination, which was consistent with expected.
Possible mechanism for synergistic therapeutic effect
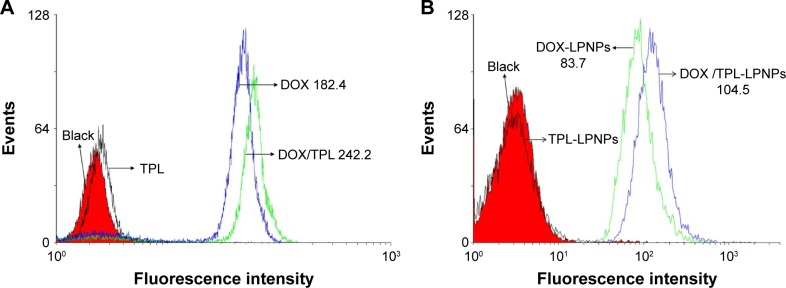
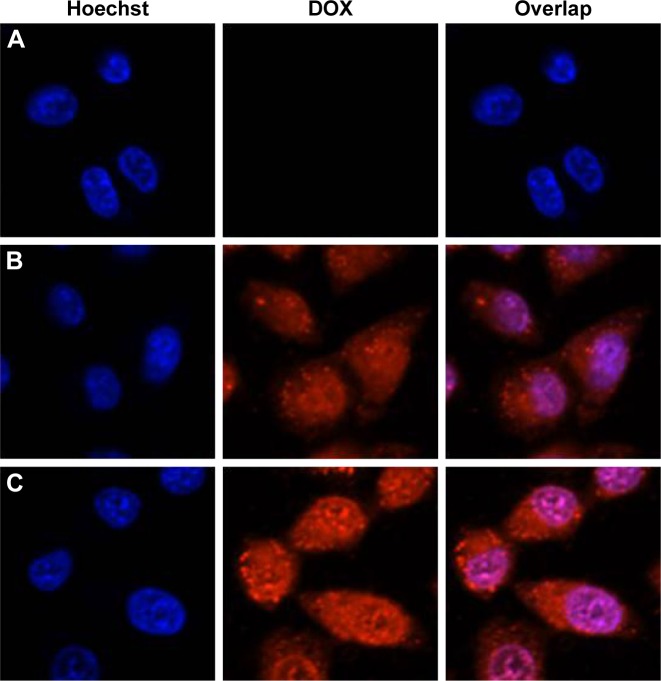
To clarify the mechanism about the synergistic effect of the DOX/TPL combination, the cellular uptake profile of DOX was examined with KB cells using flow cytometry. The mean DOX fluorescence of KB cells was 182.4 after 6 h incubation with free DOX, while there was no signifi-cant difference between the TPL group and the blank group (Figure 8). However, with the presence of TPL in the culture medium, KB cells incubated with free DOX showed stronger DOX fluorescence (242.2). Then, similar results were found when KB cells were incubated with DOX/TPL-1/0.2-LPNPs (Figure 9). Cellular uptakes of DOX and DOX-LPNPs by KB cells were further studied by confocal microscopy. The presence of TPL produced stronger DOX fluorescence than the use of DOX alone after 6 h of incubation (Figure 9). Moreover, when incubated with DOX/TPL-1/0.2-LPNPs, the KB cells showed stronger DOX fluorescence than incubation with DOX-LPNPs. These results showed that TPL enhanced the uptake of DOX by KB cells. Therefore, the synergistic effect might result from the TPL-enhanced cell uptake of DOX.
Figure 8.
Flow cytometry analyses of KB cells incubated with (A) free drugs and (B) drug-loaded LPNPs for 6 h.
Note: DOX dosage was 200 ng/mL, and TPL dosage was 40 ng/mL.
Abbreviations: DOX, doxorubicin; LPNPs, lipid–polymer hybrid nanoparticles; TPL, triptolide.
Figure 9.
Confocal laser scanning microscopy images of KB cells after treatment with (A) TPL-loaded LPNPs, (B) DOX-loaded LPNPs, and (C) DOX/TPL-1/0.2-loaded LPNPs for 6 h.
Notes: DOX dosage was 200 ng/mL, and TPL dosage was 40 ng/mL. Magnification 60×.
Abbreviations: DOX, doxorubicin; LPNPs, lipid–polymer hybrid nanoparticles; TPL, triptolide.
Conclusion
Synergistic effects of DOX/TPL combination on KB cells were investigated. The experiment in vitro and simulated CI showed that the DOX/TPL combination was highly synergistic over a wide range of treatment concentrations. The combination of DOX/TPL at the weight ratio of 1/0.2 shows the highest synergistic therapeutic effect. On this basis, a drug delivery system of LPNPs based on mPEG-S-S-C16, soybean lecithin and PLGA was developed for codelivery of DOX and TPL. The release of DOX and TPL was triggered by reductive agent in buffer or inside cancer cells after uptake by cancer cells. Furthermore, DOX and TPL coencapsulated LPNPs suppressed the tumor growth in vitro and in vivo more efficiently than DOX-LPNPs, TPL-LPNPs, or the mixture of DOX-LPNPs and TPL-LPNPs. The DOX/TPL-LPNPs codelivery system with low CI showed a higher synergistic effect than the mixture of DOX-LPNPs and TPL-LPNPs. We also suggest a possible mechanism that TPL enhances the uptake of DOX by KB cells. Overall, this DOX/TPL dual drug-encapsulated nanocarrier will be a promising strategy for cancer treatment.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (51473127, 51673152, 81372369, and 81571734) and the Opening Project of Key Laboratory of Biomedical Polymers of Ministry of Education at Wuhan University (20150103).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cai L, Xu G, Shi C, Guo D, Wang X, Luo J. Telodendrimer nanocarrier for co-delivery of paclitaxel and cisplatin: a synergistic combination nanotherapy for ovarian cancer treatment. Biomaterials. 2015;37:456–468. doi: 10.1016/j.biomaterials.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gadde S. Multi-drug delivery nanocarriers for combination therapy. Med Chem Commun. 2015;6(11):1916–1929. [Google Scholar]
- 3.Lehar J, Krueger AS, Avery W, et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat Biotechnol. 2009;27(7):659–666. doi: 10.1038/nbt.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shastri L, Abdelhamid HN, Nawaz M, et al. Synthesis, characterization and bifunctional applications of bidentate silver nanoparticle assisted single drop microextraction as a highly sensitive preconcentrating probe for protein analysis. RSC Adv. 2015;5(52):41595–41603. [Google Scholar]
- 5.Chen D, Wang G, Song W, et al. Novel CD44 receptor targeting multifunctional “nano-eggs” based on double pH-sensitive nanoparticles for co-delivery of curcumin and paclitaxel to cancer cells and cancer stem cells. J Nanopart Res. 2015;17(10):1–10. [Google Scholar]
- 6.Yin TH, Wang P, Li JG, et al. Tumor-penetrating codelivery of siRNA and paclitaxel with ultrasound-responsive nanobubbles hetero-assembled from polymeric micelles and liposomes. Biomaterials. 2014;35(22):5932–5943. doi: 10.1016/j.biomaterials.2014.03.072. [DOI] [PubMed] [Google Scholar]
- 7.Yang ZZ, Li JQ, Wang ZZ, Dong DW, Qi XR. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials. 2014;35(19):5226–5239. doi: 10.1016/j.biomaterials.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Chen AM, Zhang M, Wei DG, et al. Co-delivery of doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small. 2009;5(23):2673–2677. doi: 10.1002/smll.200900621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang F, You M, Chen T, Zhu G, Liang H, Tan W. Self-assembled hybrid nanoparticles for targeted co-delivery of two drugs into cancer cells. Chem Commun (Camb) 2014;50(23):3103–3105. doi: 10.1039/c3cc49003c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Chan JM, Gu FX, et al. Self-assembled lipid−polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano. 2008;2(8):1696–1702. doi: 10.1021/nn800275r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan JM, Zhang L, Yuet KP, et al. PLGA–lecithin–PEG core–shell nanoparticles for controlled drug delivery. Biomaterials. 2009;30(8):1627–1634. doi: 10.1016/j.biomaterials.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Krishnamurthy S, Vaiyapuri R, Zhang L, Chan JM. Lipid-coated polymeric nanoparticles for cancer drug delivery. Biomater Sci. 2015;3(7):923–936. doi: 10.1039/c4bm00427b. [DOI] [PubMed] [Google Scholar]
- 13.Zhao P, Zheng M, Yue C, et al. Improving drug accumulation and photothermal efficacy in tumor depending on size of ICG loaded lipid-polymer nanoparticles. Biomaterials. 2014;35(23):6037–6046. doi: 10.1016/j.biomaterials.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Hu J, Chan HF, Skibba M, Liang G, Chen M. iRGD decorated lipid-polymer hybrid nanoparticles for targeted co-delivery of doxorubicin and sorafenib to enhance anti-hepatocellular carcinoma efficacy. Nanomedicine. 2016;12(5):1303–1311. doi: 10.1016/j.nano.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Zheng M, Yue C, Ma Y, et al. Single-step assembly of DOX/ICG loaded lipid-polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS Nano. 2013;7(3):2056–2067. doi: 10.1021/nn400334y. [DOI] [PubMed] [Google Scholar]
- 16.Chan JM, Rhee JW, Drum CL, et al. In vivo prevention of arterial restenosis with paclitaxel-encapsulated targeted lipid-polymeric nanoparticles. Proc Natl Acad Sci U S A. 2011;108(48):19347–19352. doi: 10.1073/pnas.1115945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng M, Gong P, Zheng C, et al. Lipid-polymer nanoparticles for folate-receptor targeting delivery of doxorubicin. J Nanosci Nanotechnol. 2015;15(7):4792–4798. doi: 10.1166/jnn.2015.9604. [DOI] [PubMed] [Google Scholar]
- 18.Zheng M, Zhao P, Zheng C, et al. Indocyanine green loaded lipid-polymer nanoparticles for cancer imaging and therapy. Nanomedicine. 2016;12(2):515. [Google Scholar]
- 19.Cui C, Xue YN, Wu M, et al. Polymer-lipid hybrid nanoparticles with reduction-triggered release for improved antitumor efficiency. J Control Release. 2013;172(1):E17. [Google Scholar]
- 20.Wu B, Yu P, Cui C, et al. Folate-containing reduction-sensitive lipid-polymer hybrid nanoparticles for targeted delivery of doxorubicin. Biomater Sci. 2015;3(4):655–664. doi: 10.1039/c4bm00462k. [DOI] [PubMed] [Google Scholar]
- 21.Zhang LJ, Wu B, Zhou W, et al. Two-component reduction-sensitive lipid-polymer hybrid nanoparticles for triggered drug release and enhanced in vitro and in vivo anti-tumor efficacy. Biomater Sci. 2017;5:12. doi: 10.1039/c6bm00662k. [DOI] [PubMed] [Google Scholar]
- 22.Matsui Y, Watanabe J, Ikegawa M, Kamoto T, Ogawa O, Nishiyama H. Cancer-specific enhancement of cisplatin-induced cytotoxicity with triptolide through an interaction of inactivated glycogen synthase kinase-3beta with p53. Oncogene. 2008;27(33):4603–4614. doi: 10.1038/onc.2008.89. [DOI] [PubMed] [Google Scholar]
- 23.Kupchan SM, Court WA, Dailey RG, et al. Tumor inhibitors. LXXIV. Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J Am Chem Soc. 1972;94(20):7194–7195. doi: 10.1021/ja00775a078. [DOI] [PubMed] [Google Scholar]
- 24.Wan CK, Wang C, Cheung HY, Yang M, Fong WF. Triptolide induces Bcl-2 cleavage and mitochondria dependent apoptosis in p53-deficient HL-60 cells. Cancer Lett. 2006;241(1):31–41. doi: 10.1016/j.canlet.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Nakazato T, Sagawa M, Kizaki M. Triptolide induces apoptotic cell death of multiple myeloma cells via transcriptional repression of Mcl-1. Int J Oncol. 2014;44(4):1131–1138. doi: 10.3892/ijo.2014.2280. [DOI] [PubMed] [Google Scholar]
- 26.Sangwan V, Jensen KM, Dudeja V, et al. Abstract B119: triptolide abrogates expression of the met and epidermal growth factor receptors in pancreatic cancer. Cancer Res. 2015;75(13 suppl):B119. [Google Scholar]
- 27.Zhao H, Yang Z, Wang X, et al. Triptolide inhibits ovarian cancer cell invasion by repression of matrix metalloproteinase 7 and 19 and upregulation of E-cadherin. Exp Mol Med. 2012;44(11):633–641. doi: 10.3858/emm.2012.44.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen YW, Lin GJ, Chia WT, Lin CK, Chuang YP, Sytwu HK. Triptolide exerts anti-tumor effect on oral cancer and KB cells in vitro and in vivo. Oral Oncol. 2009;45(7):562–568. doi: 10.1016/j.oraloncology.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Subramanian A, Jaganathan S, Manikandan A, et al. Recent trends in nano-based drug delivery systems for efficient delivery of phytochemicals in chemotherapy. RSC Adv. 2016;6(54):48294–48314. [Google Scholar]
- 30.Zhou ZL, Yang YX, Ding J, et al. Triptolide: structural modifications, structure–activity relationships, bioactivities, clinical development and mechanisms. Nat Prod Rep. 2012;29(4):457–475. doi: 10.1039/c2np00088a. [DOI] [PubMed] [Google Scholar]
- 31.Qu J, Yu SS, Du D, et al. Bioactive constituents from toxic seed plants in China. RSC Adv. 2013;3(26):10078–10102. [Google Scholar]
- 32.Sai K, Li WY, Chen YS, et al. Triptolide synergistically enhances temozolomide-induced apoptosis and potentiates inhibition of NF-κB signaling in glioma initiating cells. Am J Chin Med. 2014;42(02):485–503. doi: 10.1142/S0192415X14500323. [DOI] [PubMed] [Google Scholar]
- 33.Meng G, Wang W, Chai K, Yang S, Li F, Jiang K. Combination treatment with triptolide and hydroxycamptothecin synergistically enhances apoptosis in A549 lung adenocarcinoma cells through PP2A-regulated ERK, p38 MAPKs and Akt signaling pathways. Int J Oncol. 2015;46(3):1007–1017. doi: 10.3892/ijo.2015.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li CJ, Chu CY, Huang LH, et al. Synergistic anticancer activity of triptolide combined with cisplatin enhances apoptosis in gastric cancer in vitro and in vivo. Cancer Lett. 2012;319(2):203–213. doi: 10.1016/j.canlet.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Tang XY, Zhu YQ, Tao WH, Wei B, Lin XL. Synergistic effect of triptolide combined with 5-fluorouracil on colon carcinoma. Postgrad Med J. 2007;83(979):338–343. doi: 10.1136/pgmj.2006.055426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui C, Xue YN, Wu M, et al. Cellular uptake, intracellular trafficking, and antitumor efficacy of doxorubicin-loaded reduction-sensitive micelles. Biomaterials. 2013;34(15):3858–3869. doi: 10.1016/j.biomaterials.2013.01.101. [DOI] [PubMed] [Google Scholar]
- 37.Pandita A, Kumar B, Manvati S, Vaishnavi S, Singh SK, Bamezai RN. Synergistic combination of gemcitabine and dietary molecule induces apoptosis in pancreatic cancer cells and down regulates PKM2 expression. PLoS One. 2014;9(9):e107154. doi: 10.1371/journal.pone.0107154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiskus W, Sharma S, Shah B, et al. Highly effective combination of LSD1 (KDM1A) antagonist and pan-histone deacetylase inhibitor against human AML cells. Leukemia. 2014;28(11):2155–2164. doi: 10.1038/leu.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lv S, Tang Z, Li M, et al. Co-delivery of doxorubicin and paclitaxel by PEG-polypeptide nanovehicle for the treatment of non-small cell lung cancer. Biomaterials. 2014;35(23):6118–6129. doi: 10.1016/j.biomaterials.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 40.Duong HHP, Yung LYL. Synergistic co-delivery of doxorubicin and paclitaxel using multi-functional micelles for cancer treatment. Int J Pharm. 2013;454(1):486–495. doi: 10.1016/j.ijpharm.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Ahmad Z, Shah A, Siddiq M, et al. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014;4(33):17028–17038. [Google Scholar]
- 42.Wang H, Zhao P, Su W, et al. PLGA/polymeric liposome for targeted drug and gene co-delivery. Biomaterials. 2010;31(33):8741–8748. doi: 10.1016/j.biomaterials.2010.07.082. [DOI] [PubMed] [Google Scholar]
- 43.Santander-Ortega MJ, Csaba N, González L, et al. Protein-loaded PLGA–PEO blend nanoparticles: encapsulation, release and degradation characteristics. Colloid Polym Sci. 2010;288(2):141–150. [Google Scholar]