Abstract
Microfluidic systems have enabled powerful new approaches to high-throughput biochemical and biological analysis. However, there remains a barrier to entry for non-specialists who would benefit greatly from the ability to develop their own microfluidic devices to address research questions. Particularly lacking has been the open dissemination of protocols related to photolithography, a key step in the development of a replica mold for the manufacture of polydimethylsiloxane (PDMS) devices. While the fabrication of single height silicon masters has been explored extensively in literature, fabrication steps for more complicated photolithography features necessary for many interesting device functionalities (such as feature rounding to make valve structures, multi-height single-mold patterning, or high aspect ratio definition) are often not explicitly outlined.
Here, we provide a complete protocol for making multilayer microfluidic devices with valves and complex multi-height geometries, tunable for any application. These fabrication procedures are presented in the context of a microfluidic hydrogel bead synthesizer and demonstrate the production of droplets containing polyethylene glycol (PEG diacrylate) and a photoinitiator that can be polymerized into solid beads. This protocol and accompanying discussion provide a foundation of design principles and fabrication methods that enables development of a wide variety of microfluidic devices. The details included here should allow non-specialists to design and fabricate novel devices, thereby bringing a host of recently developed technologies to their most exciting applications in biological laboratories.
Keywords: Bioengineering, Issue 119, Microfluidics, Pneumatic valves, Photolithography, Droplets, Hydrogels, Variable height features
Introduction
For the past 15 years, microfluidics as a field has undergone rapid growth, with an explosion of new technologies enabling the manipulation of fluids at the micrometer scale1. Microfluidic systems are attractive platforms for wet laboratory functionality because the small volumes have the potential to realize increased speed and sensitivity while at the same time dramatically increasing throughput and reducing cost by leveraging economies of scale2,3. Multilayer microfluidic systems have made particularly significant impacts in high-throughput biochemical analysis applications such as single cell analysis4,5,6, single molecule analysis (e.g., digital PCR7), protein crystallography8, transcription factor binding assays9,10, and cellular screening11.
A central goal of microfluidics has been the development of "lab on a chip" devices capable of performing complex fluidic manipulations within a single device for total biochemical analysis12. The development of multi-layer soft lithography techniques has helped realize this goal by enabling creation of on-chip valves, mixers, and pumps for actively controlling fluids within small volumes13,14,15. Despite their advantages and demonstrated applications, many of these microfluidic technologies remain largely unharnessed by non-specialist users. Widespread adoption has been challenging in part due to limited access to microfabrication facilities, but also due to inadequate communication of fabrication techniques. This is especially true for multilayer microfluidic devices featuring structures for valves or complex geometries: the paucity of detailed, practical information about important design parameters and fabrication techniques often deters new researchers from embarking on projects involving the design and creation of these devices.
This article aims to address this knowledge gap by presenting a complete protocol for making multilayer microfluidic devices with valves and variable height features, starting from design parameters and moving through all fabrication steps. By focusing on the initial photolithography steps of fabrication, this protocol complements other microfluidics protocols16 that describe downstream steps of casting devices from molds and running specific experiments.
Microfluidic devices with monolithic on-chip valves are composed of two layers: a "flow" layer, where the fluid of interest is manipulated in microchannels, and a "control" layer, where microchannels containing air or water can selectively modulate fluid flow in the flow layer14. These two layers are each fabricated on a separate silicon molding master, which is subsequently used for polydimethylsiloxane (PDMS) replica molding in a process called "soft lithography17." To form a multilayer device, each of the PDMS layers are cast on their respective molding masters and then aligned to one another, thereby forming a composite PDMS device with channels in each layer. Valves are formed at locations where flow and control channels cross one another and are separated by only a thin membrane; pressurization of the control channel deflects this membrane to occlude the flow channel and locally displace the fluid (Figure 1).
Active on-chip valves can be fabricated in multiple ways, depending on the desired final application. Valves can be configured in either a "push down" or "push up" geometry, depending on whether the control layer is above or below the flow layer (Figure 1)15. "Push up" geometries allow for lower closing pressures and higher device stability against delamination, while "push down" geometries allow for the flow channels to be in direct contact with the bonded substrate, conferring the advantage of selective functionalization or patterning of the substrate surface for later functionality18,19.
Valves can also be either intentionally leaky "sieve" valves or fully sealable, depending on the cross-sectional profile of the flow channel. Sieve valves are useful for trapping beads, cells or other macroanalytes1, and are fabricated via the use of typical negative photoresists (i.e., SU-8 series), which have rectangular profiles. When a control channel is pressurized over these valve regions, the PDMS membrane between the control and flow layer deflects isotropically into the rectangular profile of the valve without sealing the corners, permitting fluid flow but trapping macro scale particles (Figure 1). Conversely, fully-sealable microfluidic valves are fabricated by including a small patch of rounded photoresist at valve locations. With this geometry, pressurization of the control channel deflects the membrane against the rounded flow layer to completely seal the channel, halting fluid flow. Rounded profiles in the flow layer are generated via the melting and reflow of positive photoresist (e.g., AZ50 XT or SPR 220) after typical photolithography steps. We have previously demonstrated that post-reflow heights of valve regions depend on chosen feature dimensions21. This protocol demonstrates the fabrication of both valve geometries within a bead synthesis device.
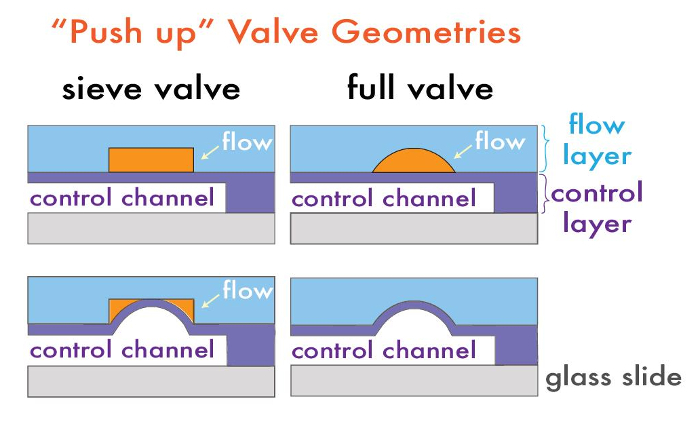 Figure 1: Multilayer Microfluidic Valve Geometries. Typical "push up" device architectures for sieve and fully sealable valves before (top) and after (bottom) pressurization. Please click here to view a larger version of this figure.
Figure 1: Multilayer Microfluidic Valve Geometries. Typical "push up" device architectures for sieve and fully sealable valves before (top) and after (bottom) pressurization. Please click here to view a larger version of this figure.
Devices can also include complex passive features such as chaotic mixers13 and on-chip resistors20 that require features of multiple different heights within a single flow layer. To achieve a variable height flow layer, different groups have employed many methods including printed circuit board etching22, multilayer PDMS relief alignment23, or multi-step photolithography24. Our group has found multi-step photolithography on a single molding master to be an effective and reproducible method. To do this, a simple photolithography technique of building thick channels of negative photoresist (e.g., SU-8 series photoresists) in layers without development in between application of each layer is employed. Each layer is spun in negative photoresist according to its thickness using manufacturer instructions25 on the silicon master. Features of this height are then patterned onto the layer using a specific transparency mask (Figure 2) affixed to a glass mask plate and aligned to the previously spun layer before exposure. In multi-step photolithography, precise alignment between layers is critical in forming a complete variable height flow channel. After alignment, each layer is subjected to a thickness-dependent post-exposure bake. Without development, the next layer is similarly patterned. In this way, tall features can be built up on a single flow wafer layer-by-layer via the use of multiple masks. By skipping development between each step, previous photoresist layers can be used to generate composite height features (i.e., two 25 µm layers can make a 50 µm feature)24. Additionally, channel floor features such as chaotic mixer herringbone grooves13 can be made using layers with previously exposed features. A final development step completes the process, creating a single flow wafer with features of variable height (Figure 3).
Here, a complete protocol for multi-step photolithography that includes examples of all procedures necessary to fabricate on-chip valves and flow channels with multiple heights is provided. This fabrication protocol is presented in the context of a multi-layer microfluidic bead synthesizer that requires valves and variable-height features for its functionality. This device includes T-junctions for generating water droplets in an oil sheath, on-chip resistors to modulate flow rates through controlling Poiseuille resistance, a chaotic mixer for homogenizing droplet components, and both fully sealing and sieve valves to enable automated workflows involving multiple reagent inputs. Using multi-step photolithography, these features are each fabricated on a different layer according to height or photoresist; the following layers are constructed in this protocol: (1) Flow Round valve layer (55 µm, AZ50 XT) (2) Flow Low layer (55 µm, SU-8 2050) (3) Flow High layer (85 µm, SU-8 2025, 30 µm additive height), and (4) Herringbone Grooves (125 µm, SU-8 2025, 40 µm additive height) (Figure 3).
Hydrogel beads can be used for a variety of applications including selective surface functionalization for downstream assays, drug encapsulation, radiotracing and imaging assays, and cell incorporation; we previously used a more complex version of these devices to produce spectrally encoded PEG hydrogel beads containing lanthanide nanophosphors20. The designs discussed here are included in Additional Resources for any lab to use in their research efforts if desired. We anticipate that this protocol will provide an open resource for specialists and non-specialists alike interested in making multi-layer microfluidic devices with valves or complex geometries to lower the barrier to entry in microfluidics and increase the chances of fabrication success.
Protocol
1. Multi-layer Device Design
NOTE: Features of different heights and/or photoresists must be added sequentially to the wafer during different fabrication steps to create final composite features. Therefore, designs for each separate height and photoresist to be included on a wafer must be printed on their own mask (Figure 4).
Download a computer-assisted design (CAD) drafting program (e.g., AutoCAD Educational Version).
Define the 4" wafer area by drawing a 4" circle. Wafer designs (Figure 4, Additional Resources) are provided as an example.
Inside the 4" wafer outline, place device borders using 300 µm polyline rectangles. Use these device borders for alignment during photolithography.
- Create different layers for each different height or photoresist needed for the final design (i.e., flow round, flow low, flow high, and control in the design) using the Layers panel.
- Design features of a particular desired height on the corresponding layer. The example design shows 4 different active layers, each with its own color (Figure 4). NOTE: Device borders, global text, and the wafer outline should be made on their own layer (i.e., 1-Negative in the designs), which, later, will appear on all layers for global alignment. Features of different photoresist (such as fully sealable valves that must be fabricated with positive resist) must appear on different layers, regardless of height.
- Using closed zero-width polylines, design device features within device borders.
- Consider design parameters in Table 1 to increase chances of successful fabrication.
- For each height, select that layer in the Layers panel and add all features of that height.
- Ready designs for transparency film printing using the Basic Mask File (Additional Resources) where each 4" wafer circle is inserted within a 5" rectangular border. Each layer will be printed on a separate transparency film for sequential addition of each photoresist layer. NOTE: This Basic Mask File represents the final designs used for printing.
- To complete design, turn all Layers off except 1-Negative and the AZ50 XT valve layer. Copy the entire wafer with the active layer (i.e., valves) and global features (i.e., device borders).
- Open the Basic Mask File and paste this design into the rectangle entitled AZ50 XT valves. Use the outer wafer border for alignment and subsequently delete it after pasting.
- Repeat for the rest of the layers (e.g., in the example design: flow square low, flow square high, and control). Example transparency files are provided (Additional Resources).
- Send files to a commercial printing company (e.g., FineLine Imaging) for printing on transparency film. Use 32,000 DPI for printing >10 µm features and up to 50,000 DPI for smaller features. If features less than 7 µm are needed, order a Chrome Mask instead of a transparency film.
Table 1: Design Parameters and Suggestions. Design considerations to avoid common pitfalls during the CAD design process of microfluidic devices. Please click here to view this table. (Right-click to download.)
2. Preparing a Wafer for Photolithography
NOTE: These steps additionally appear in table-format in Table 2.
- In cleanroom or designated clean area, clean and dehydrate a 4" test-grade silicon wafer (single-side polished).
- Rinse the wafer well with methanol. NOTE: No further cleaning steps are needed if using the SU-8 adhesion layer described below. Other adhesion layers that deviate from this protocol (e.g., HMDS) often require more thorough cleaning, such as piranha etching.
- Blow dry with N2 or compressed air.
- Bake on an aluminum hotplate at 95 °C for 10 min to fully evaporate solvent.
- Fabricate a uniform 5 µm thick layer of SU-8 2005 to improve adhesion for subsequent photoresist layers.
- Place the cleaned wafer on a spin coater, turn on the vacuum to affix it to the spin chuck, and blow away dust with N2 or compressed air.
- Apply 1-2 ml of SU-8 2005 negative photoresist in center of wafer and spin as follows: spread: 500 rpm, 10 sec, 133 rpm/sacceleration; cast: 3,000 rpm, 40 sec, 266 rpm/s acceleration.
- Remove wafer and soft bake by switching wafer between two hotplates set at 65 °C and 95 °C according to the following program: 65 °C: 2 min, 95 °C: 3 min, 65 °C: 2 min.
- Allow wafer to cool to RT.
- Place wafer in the chuck of a UV mask aligner and expose without a mask ('flood exposure') for a total energy deposition of 124 mJ (here, 20 sec at ~6.2 mW/cm2 lamp intensity). If available, select hard contact mode to achieve a 300 µm wafer: mask separation.
- Remove wafer and post-exposure bake by switching the wafer between two hotplates set at 65 °C and 95 °C as follows: 65 °C: 2 min, 95 °C: 4 min, 65 °C: 2 min.
Fabricating Rounded Valves
Use online AZ50 XT valve predictor resource26 to plan spin speeds for desired valve dimensions and heights. NOTE: The following steps will deposit a 55 µm layer of positive photoresist for valve definition and reflow rounding.
Place the wafer on a spin coater, turn on vacuum to affix it to the spin chuck, and blow away dust with N2 or compressed air.
Apply 2-3 mL of AZ50 XT positive photoresist to center of the wafer. Spin as follows: spread: 200 rpm, 10 s, 133 rpm/s acceleration; cast: 1,200 rpm, 40 sec, 266 rpm/s acceleration; Snap spin to remove edge bead: 3,400 rpm, 1 sec, 3,400 rpm/s acceleration.
In a 5" Petri dish, lay down the wafer carefully and let relax for 20 min.
Soft bake the wafer on a hotplate: 65 °C - 112 °C, 22 min, 450 °C/h ramping speed.
Remove the wafer and let rest overnight at RT in a Petri dish for ambient rehydration.
Tape Flow Round transparency mask to 5" glass plate print-side down (closest to wafer) and load into mask positioner of the UV mask aligner. Expose the wafer to 930 mJ of UV in 6 cycles (e.g., 6 cycles of 25 sec at ~6.2 mW/cm2 lamp intensity, 30 s wait time between exposures).
- Develop wafer immediately by immersing in a stirred bath of 25 mL of AZ500k 1:3 Developer in 6" glass dish for 3-5 min or until bath turns purple and features emerge.
- Remove the wafer and rinse well with DI water.
- Assess pre-reflow height using a profilometer (stylus force of 10.5 mg). NOTE: Operate the profilometer according to manufacturer instructions, carefully positioning the force stylus next to a feature channel on the desired layer before profiling. Settings used throughout this protocol were the following: stylus force 10.5 mg, length 1,000 µm, speed 200 µm/s, regime down-up.
Reflow hard bake the wafer to melt and round valve features as follows: 65 °C - 190°C, 15 hr, 10 °C/hr ramping speed.
Let the wafer cool to RT. Assess post-reflow height using a profilometer (stylus force of 10.5 mg). Heights of 55 µm ± 2 µm should be expected for this device geometry.
3. Fabricating Variable Height Features in Tandem
Proceed to variable height fabrication with the developed wafer with Flow Low, Flow High and Herringbone Mixer transparencies of the Bead Synthesizer design.
To adjust protocol for the designs, use manufacture data sheets25 to determine exposure energy, spin speeds and bake time parameters, allowing for ± 5% tolerance. NOTE: This protocol fabricates a 55 µm tall Flow Low layer using SU-8 2050 negative photoresist spun over the valve features.
- Place the cleaned wafer on spin coater, turn on the vacuum to affix it to the spin chuck, and blow away dust with N2 or compressed air.
- Apply 1-2 ml of SU-8 2050 negative photoresist to center of the wafer and spin as follows: spread: 500 rpm, 10 sec, 133 rpm/sec acceleration; cast: 3,000 rpm, 40 sec, 266 rpm/sec acceleration. Spin photoresist over developed valve features.
Carefully place the spun wafer in 5" Petri dish and let relax for 20 min on flat surface or until any streaking patterns fade.
Remove the wafer and soft bake by placing on two hotplates set at 65 °C and 95 °C as follows: 65 °C: 2 min, 95 °C: 8 min, 65 °C: 2 min.
Allow the wafer to cool to RT.
Tape the Flow Low transparency mask to a quartz 5" glass plate print-side down (closest to the wafer) and load into the mask positioner of the UV mask aligner.
Place the wafer in UV mask aligner chuck and, using microscope eyepiece or camera, carefully align new Flow Low layer features to Flow Round valve layer features. Begin by aligning horizontal, vertical and tilt axes of device borders to the device border features on mask. Next, align cross-hair features between layers. Finally, confirm that valve features intersect Flow Low features where applicable.
Expose to 170 mJ UV deposition (28 sec at ~6.2 mW/cm2).
Remove the wafer and post-exposure bake by switching between two hotplates set at 65 °C and 95 °C as follows: 65 °C: 2 min, 95 °C: 9 min, 65 °C: 2 min.
Without developing, allow the wafer to cool to RT and proceed to fabrication of Flow High layer. This Flow High layer will add 30 µm of photoresist to the undeveloped 55 µm photoresist layer to yield 85 µm features in previously unexposed locations.
- Repeat steps 3.3 to 3.10 using SU-8 2025 and the Flow High layer mask with these modifications for the spin coat settings: spread: 500 rpm, 10 sec, 133 rpm/s acceleration; cast: 3,500 rpm, 40 sec, 266 rpm/sec acceleration.
- Expose to 198 mJ UV deposition (32 s at ~6.2 mW/cm2).
Without developing, allow the wafer to cool to RT and proceed to fabrication of Chaotic Mixer Herringbone layer. Final features in this layer will have a total height of 125 µm: 55 µm from the Flow Low layer, 30 µm from the Flow Square layer, and 40 µm from this Chaotic Mixer Herringbone layer (see Figure 3) and include 35 µm herringbone grooves.
- Repeat steps 3.3 to 3.10 using SU-8 2025 and the Herringbone layer mask with the following modifications, ensuring that herringbone grooves are completely within Flow High channel outlines.
- Use the following soft bake program: 65 °C: 2 min, 95 °C: 7 min, 65 °C: 2 min.
- Expose to 148 mJ UV deposition (24 s at ~6.2 mW/cm2).
After all layers have been completed, develop by immersing the wafer in a stirred bath of 25 ml of SU-8 developer in a 6" glass dish for 3.5 min or until features clearly emerge. Check that features have clear, defined feature boundaries using a stereoscope.
Hard bake the wafer to stabilize all photoresist features on a hot plate as follows: 65 °C - 165 °C, 2 hr 30 min, 120 °C/h ramping speed.
Assess feature height in all layers using a profilometer (stylus force of 10.5 mg).
4. Control Wafer Fabrication
Clean, dehydrate, and fabricate a 5 µm adhesion layer on a new 4" silicon wafer as in Section 4.
Fabricate a 25 µm Control Layer using SU-8 2025 negative photoresist.
Place the wafer on a spin coater, turn on vacuum to affix it to the spin chuck, and blow away dust with N2 or compressed air.
Apply 1-2 ml of SU-8 2025 negative photoresist in the center of the wafer and spin as follows: spread: 500 rpm, 10 sec, 133 rpm/s acceleration; cast: 3,500 rpm, 40 sec, 266 rpm/sec acceleration.
Remove the wafer and soft bake by switching between two hotplates set at 65 °C and 95 °C as follows: 65 °C: 2 min, 95 °C: 5 min, 65 °C: 2 min.
Allow the wafer to cool to RT.
Align the control transparency mask to a 5" glass plate and load into UV mask aligner.
Place the wafer in chuck of UV mask aligner and expose to 155 mJ UV deposition (25 sec at ~6.2 mW/cm2 lamp intensity).
Remove the wafer and post-exposure bake by switching between two hotplates set at 65 °C and 95 °C as follows: 65 °C: 2 min, 95 °C: 6 min, 65 °C: 2 min.
Develop by immersing the wafer in a stirred bath of 25 ml of SU-8 Developer in 6" glass dish for 1 min or until features emerge. Check features using a stereoscope.
Hard bake the wafer to stabilize photoresist features as follows: 65 °C - 165°C, 2 hr 30 min, 120 °C/hr ramping speed.
5. Silane Wafer Treatment for Easy PDMS Lift-off
Place the completed wafers in wafer rack within a bell-jar vacuum desiccator inside a fume hood free of water or water-soluble reagents.
Under the hood, use a dropper to apply 1 drop of trichloro(1H,1H,2H,2H-perfluorooctyl) silane (PFOTS) to a glass slide and place inside the desiccator.
Close the desiccator lid and apply vacuum for 1 min.
After 1 min, turn off vacuum without re-pressurizing or evacuating bell jar.
Let the mixture sit for 10 min while aerosolized PFOTS coats wafer surface.
Open the bell jar lid and remove wafer using tweezers. Place into a Petri dish for PDMS replica molding. Dispose of silane-coated slides in proper hazardous waste. NOTE: Wafers coated with fluorinated silanes can be used hundreds to thousands of times without re-treatment. A sacrificial layer of 1:10 PDMS can be cast on wafers, cured, and discarded after the first silane treatment to remove excess silane groups from wafer surface.
6. PDMS Replica Molding
Fabricate multilayer microfluidic devices in a "push up" geometry on glass according to existing open-access protocols16. NOTE: A detailed protocol can additionally be found on the website27.
By visual inspection, ensure all valves are aligned properly to control lines and all inlets (on both the flow and control layers) are punched fully before proceeding.
7. Production of Hydrogel Beads from Droplets
Connect tubing (e.g., Tygon) loaded with water to a flow control system (e.g., syringe pumps, fluidic controllers, or an open-source solenoid valve array with reservoirs28).
Connect metal pins to tubing and connect to device ports at control line inlets. Pressurize device control lines by setting the flow control system of choice to 25 psi for each line. Ensure that valves close and re-open by inspection under the microscope. NOTE: Follow manufacturer instructions for the flow control system of choice. In this work, a custom software-controlled pneumatic system applies pressure to each line using solenoid valves that toggle between 25 psi compressed air (pressurized) and atmospheric pressure (depressurized). Details on this system can be found in Discussion.
- Prepare custom microfluidic pressure vessels for reagent and oil loading.
- Using a push-pin, punch two holes in the top of a cryogenic vial tube, insert capillary PEEK tubing into one hole, and insert a metal pin connected to the tubing into the second hole.
- Seal tubing in place with epoxy. Let dry for 1 hr.
While waiting, in a microcentrifuge tube, suspend 3.9 mg of LAP photoinitiator into 100 µl of DI water ([LAP] = 39 mg/ml) to prepare photoinitiator solution used for polymerizing droplets to hydrogel beads. Protect from light.
In a second microcentrifuge tube, add 132 µl DI water, 172 µL PEG diacrylate, 12 µl LAP solution, and 85 µL HEPES buffer to make hydrogel droplet solution.
Transfer the hydrogel droplet solution to the completed cryogenic tube vessel. NOTE: Additives for other applications such as nanocrystals, magnetic particles or biological molecules can be included within the HEPES component.
Connect the tubing of the cryogenic tube vessel to a controllable pressure source and connect the PEEK tubing to the device reagent inlet.
Prepare 10 mL of light mineral oil with 2% v/v nonionic surfactant (e.g., Span 80) and 0.05% EM90 for oil droplet emulsion. Filter using a 0.22 µm syringe filter and load 1 ml into a second cryogenic tube vessel.
Insert PEEK tubing at the device outlet for collection of droplets.
Remove air bubbles from the device by pressurizing oil, water, or PEG mixture inlets (4 psi operational pressure). Turn on all valves. Sequentially turn off each valve in a fluid pathway after 1 min or until air bubbles have permeated through the PDMS device. For instance, to de-bubble herringbone mixers, turn on valves Inlet 1, Mix 1 out, and Mix Waste. Then depressurize Inlet 1, Mix 1 out, and Mix Waste until all bubbles are gone.
When the device is repressurized after debubbling, depressurize Ro1 oil valve and set oil pressure to 10 psi.
Set PEG mixture pressure to 9 psi, depressure upstream valves (Inlet 1, Drops 1) and adjust as necessary to produce droplets of the desired size. Droplet size can be determined via microscopy using a camera with 50 fps or higher.
When the droplets have stabilized, position a 5 mm spot from a UV light source (e.g., a UV spot curing system with liquid light guide (LLG) or a focused UV LED) over the polymerization region of the device and apply 100 mW/cm2 UV (365 nm) from the UV source.
Pressurize bead sieve valve to watch polymerized beads collect and ensure that droplets have hardened into beads. Adjust LLG as necessary to achieve full polymerization.
Depressurize bead sieve valve and collect beads into tube through outlet PEEK tubing.
Representative Results
Here, we demonstrate the fabrication of valved, variable height multilayer microfluidic molds by making devices capable of generating poly ethylene glycol (PEG) hydrogel beads from droplets(Figure 2). An overview of the complete fabrication process is included in Figure 3. Using design elements from previous work, the bead synthesizer employs 4 heights in its flow layer including (1) rounded AZ50 XT valves for laminar flow modulation (55 µm) (2) flow low channels for introducing reagents at higher resistance (55 µm) (3) flow high channels for directing flow at lower resistance to the outlets and mixers (85 µm), and (4) a herringbone advection mixer (125 µm) for mixing the PEG and crosslinker into a homogenous solution (Figures 4A and 4B). A table of design parameters and suggested design constraints used in constructing this design is included in Table 1. Design files and mask transfer files are included in Materials Table.
This protocol demonstrates rounding flow valves and constructing multiple heights on the same flow wafer via tandem photolithographic steps without development between each step (Table 2). A typical post-reflow valve rounding profile from a profilometer and stereoscope images of our valves post-reflow are shown in Figures 5A and 5B. Resultant measured fabrication heights from our multi-height photolithography are listed in Table 3. Heights were measured at 10 locations per each layer across all devices in a single flow wafer, assessing both device-to-device and across wafer variation. All features observed <2% CV across the wafer.Manufacturer photoresist data sheets suggest typical height variations of ± 5% when constructing multi-layer features, so this tolerance should be taken into account if this protocol is adjusted for a different design.
As with any fabrication process, errors can result at each step if fabrication parameters such as spin speed or exposure are not optimized for the desired pattern height and geometry. Many resources are available for troubleshooting proper exposure and development times, including a website our facility maintains27. Deviations from suggested soft and hard bake times, temperatures, and ramp rates may generate cracks, bubbles or incomplete features. Additionally, rehydration of the AZ50 XT positive photoresist prior to exposure is critical. Wafers without proper rehydration may appear cracked or contain bubbles within the valve areas. If this occurs, using a 'wet box' (a closed box with a dish containing deionized water) to house wafers for the overnight rehydration may help. Shorter rehydration times (~5-6 hr) can be used for AZ50 XT features under 50 µm with similar results, but taller features require overnight rehydration to reduce the chance of feature loss during exposure and development. Newer positive resist alternatives (e.g., AZ40XT) may eliminate the need for overnight rehydration; however, we have not tested these formulations.
As a proof-of-concept, PEG-diacrylate hydrogel droplets were produced from the bead synthesizer device (Figure 6A). Figures 5C and 5D show representative images of valve operation and closure on the device when pressurizing and depressurizing valves. Common error modes for valve pressurization include: the use of insufficient pressure (valves will not fully close, which can be checked by flowing food dye), inlet tubing insertion (flow will be inhibited if tubing or metal pins are pushed down too far and device delamination may result), and incorporation of dust or fibers during the fabrication process (channels may be occluded or the device may delaminate). To troubleshoot these error modes, users should maintain clean room conditions during fabrication and systematically test each valve before proceeding to a device run. If device delamination presents as a common problem in experiments, this can be remedied by either running devices at lower pressures (<15 psi) or reducing silanization times.
Figure 6A shows the bead synthesizer device in operation producing hydrogel droplets in an oil emulsion at the T junction droplet generator and Figure 6B shows beads trapped by the on-chip sieve valve. If polymerization is not successful, beads will pass through a sieve valve. If this occurs, UV source intensity and height from the device can be modified to improve polymerization. Hydrogel beads were produced at flow rates of 10 psi (oil) and 9 psi (reagent mixture) using ~100 mW/cm2 power over a polymerization area 5 mm in diameter (Figures 6C and 6D). Resultant beads measured 52.6 ± 1.6 µm (mean ± std). Sizes were analyzed for 2,992 beads using a Hough Transform (MATLAB) in bright field images (Figures 6C and 6D) with an imposed size filter of ± 3 std (28 outliers, 0.94% of beads). Our entire hardware setup for droplet production is shown in Figure 7.
Table 2: Multi-Step Photolithography Parameters. A table format of all of the photolithography steps with applicable parameters including spin speed, soft bake times, exposure energies and hard bake times. Please click here to view this table. (Right-click to download.)
| Flow Layer Feature | Feature Height |
| Rounded Valves (Flow Round) | 54.43 µm (1.05 µm std., 1.9% CV) |
| Flow Channels (Flow Low) | 84.22 µm (0.91 µm std., 1.1% CV) |
| Flow Channels (Flow High) | 54.10 µm (1.24 µm std., 2.3% CV) |
| Herringbone Grooves (Mixer) | 124.19 µm (1.89 µm std., 1.5% CV) |
Table 3: Profilometer Heights Post-Fabrication. Total feature height post-fabrication for each of the layers fabricated via multi-step photolithography.
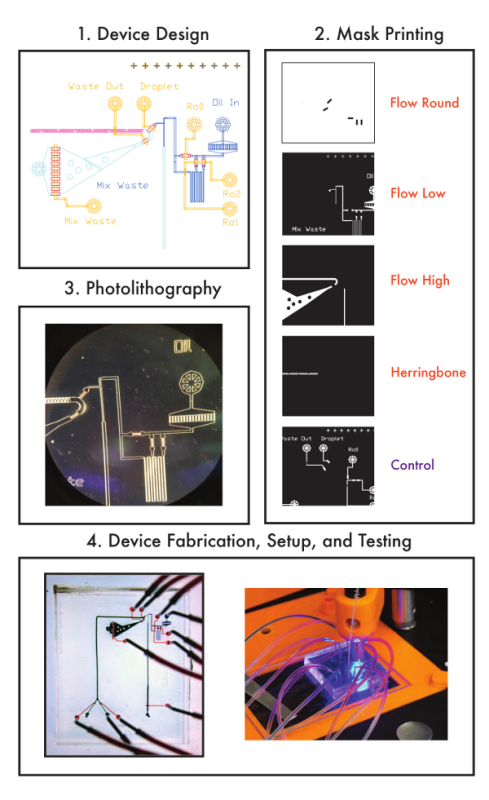 Figure 2: Overview of fabrication process. A schematic indicating steps involved in multilayer device fabrication from design to device testing. Please click here to view a larger version of this figure.
Figure 2: Overview of fabrication process. A schematic indicating steps involved in multilayer device fabrication from design to device testing. Please click here to view a larger version of this figure.
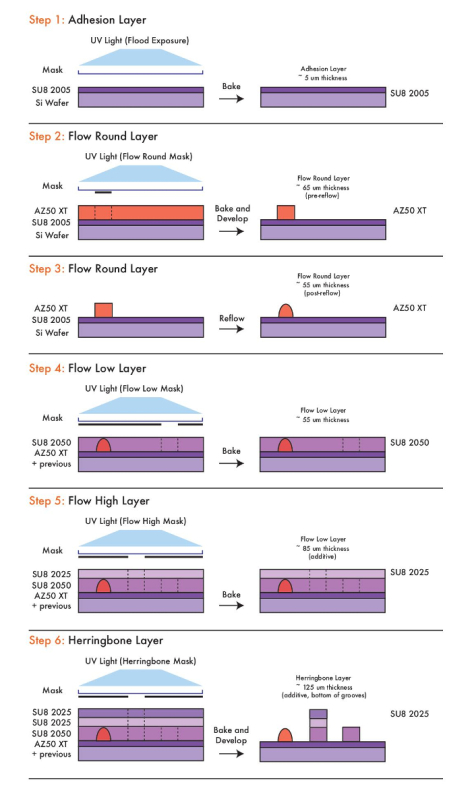 Figure 3: Schematic of multi-step photolithography. Overview of valve rounding and variable height feature fabrication in photolithography for the creation of a multilayer microfluidic device. Included here are steps for the fabrication of the bead synthesizer device. Please click here to view a larger version of this figure.
Figure 3: Schematic of multi-step photolithography. Overview of valve rounding and variable height feature fabrication in photolithography for the creation of a multilayer microfluidic device. Included here are steps for the fabrication of the bead synthesizer device. Please click here to view a larger version of this figure.
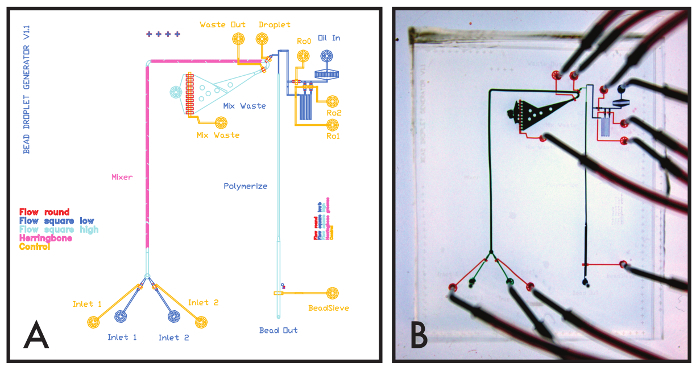 Figure 4: Bead Synthesizer Device Design and Images. (A) CAD Design of the Bead Synthesizer device with layers indicated by different colors. (B) Image of the PDMS multilayer Bead Synthesizer device. Control lines appear in orange, flow channels appear in blue and green. Please click here to view a larger version of this figure.
Figure 4: Bead Synthesizer Device Design and Images. (A) CAD Design of the Bead Synthesizer device with layers indicated by different colors. (B) Image of the PDMS multilayer Bead Synthesizer device. Control lines appear in orange, flow channels appear in blue and green. Please click here to view a larger version of this figure.
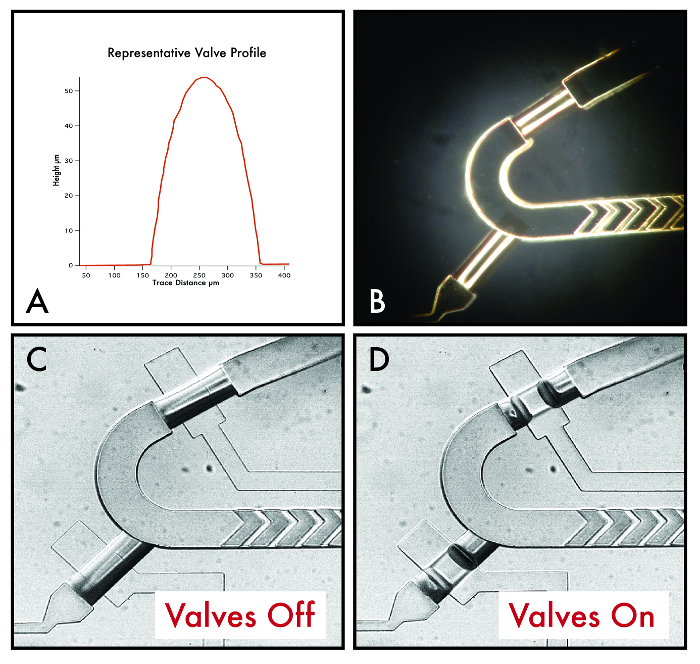 Figure 5: Valve profiles and images. (A) Representative valve height profile as assessed by a profilometer. (B) Representative images of valves and surrounding channels in the silicon molding master flow wafer. (C, D) Images of final valve operation on the multilayer PDMS device. Please click here to view a larger version of this figure.
Figure 5: Valve profiles and images. (A) Representative valve height profile as assessed by a profilometer. (B) Representative images of valves and surrounding channels in the silicon molding master flow wafer. (C, D) Images of final valve operation on the multilayer PDMS device. Please click here to view a larger version of this figure.
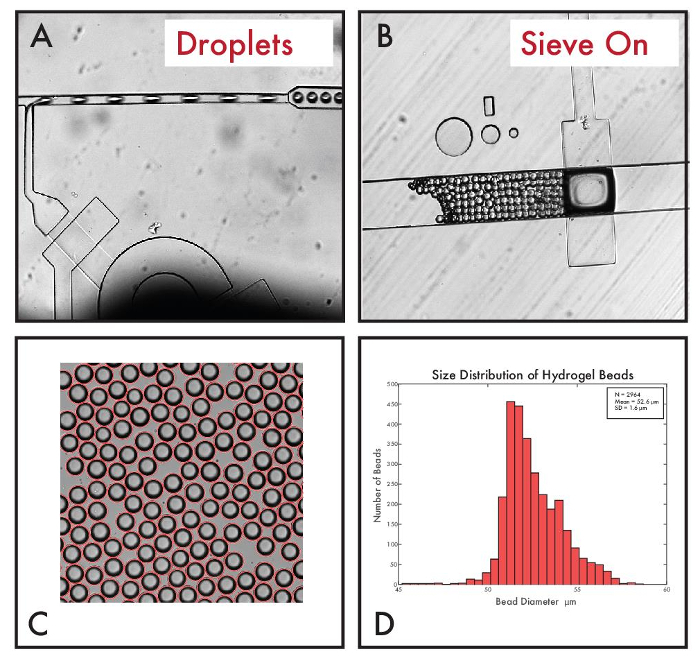 Figure 6: Production of PEG-diacrylate hydrogel droplets. (A) Image of hydrogel droplet production at the T-junction droplet generator. (B) Image of beads post-polymerization trapped at the sieve valve when the valve is pressurized. (C) Brightfield image of hydrogel beads produced in the bead synthesizer (4X). (D) Size distribution of hydrogel beads. Please click here to view a larger version of this figure.
Figure 6: Production of PEG-diacrylate hydrogel droplets. (A) Image of hydrogel droplet production at the T-junction droplet generator. (B) Image of beads post-polymerization trapped at the sieve valve when the valve is pressurized. (C) Brightfield image of hydrogel beads produced in the bead synthesizer (4X). (D) Size distribution of hydrogel beads. Please click here to view a larger version of this figure.
 Figure 7: Device operation setup. Image with annotation of all hardware required for device operation. Please click here to view a larger version of this figure.
Figure 7: Device operation setup. Image with annotation of all hardware required for device operation. Please click here to view a larger version of this figure.
Discussion
This work demonstrates a complete multi-step photolithography protocol for a multilayer microfluidic device with valves and variable height geometry that can be tuned for any application with simple modifications to fabrication parameters based on our online tool26 and manufacturer instructions25. This protocol is intended to demystify multilayer photolithography for researchers wishing to construct microfluidic devices beyond simple, passive one-layer molds.
Multilayer microfluidic devices enable the pursuit of advanced functionality within a single soft polymer device for 'lab on chip' applications ranging from single cell analysis to high-throughput biochemical assays1. The bead synthesizer device constructed here demonstrates multiple heights with a single flow layer and both fully-sealable and sieve valve geometries to produce hydrogel droplets that can be polymerized to polymer beads. The presence of valves allows for automated, programmable control over chip function that can be easily implemented with any scripting language (i.e., MATLAB, LABVIEW, or Python) that interfaces with a lab's flow control modules (i.e., syringe pumps and/or pressure regulators). In this demonstration, custom MATLAB software (code provided in reference28) communicates with Modbus-controlled solenoid valve arrays in a custom pneumatic setup28 and a MFCS flow control system to pressurize fluids on the device, but many other options for flow control are commercially available. Designs and additional suggestions are included to help maximize success for first-time microfluidic engineers.
The fabrication steps shown here should prove generally adaptable to a wide array of photolithographic patterning for microfluidic devices. In one of the steps (Flow High), 30 µm features have been added to the lower photoresist layer (Flow Low, 55 µm) to create a composite height of 85 µm. This step can be used to make thick features without the need for more viscous photoresists (which are more difficult to work with). In another step (Herringbone), features have been fabricated on top of a previously crosslinked channel, creating patterned grooves on the bottom of a microchannel when replica molded into PDMS. This step can be adapted by users to create complex geometries including well-within-well architectures, if desired.
The success of this fabrication process is demonstrated by using the bead synthesizer device to produce polymer beads from PEG-diacrylate droplets. Exploring different flow pressures can modulate the droplet regime to produce beads of different sizes. Furthermore, additives can be included easily within the polymer mixture. These hydrogel beads can be used for a variety of purposes, including spectral encoding through the incorporation of fluorescent or luminescent phosphors, drug delivery, or cellular assays via surface functionalization.
If this protocol is adopted to construct a different microfluidic device than the bead synthesizer presented here, it should be noted certain steps are critical to achieve a high chance of success on the first fabrication attempt. We and others have observed that optimizing soft bake temperature, duration, and ramp rate is critical to prevent retention of residual solvent in the resist film via crust formation (which can lead to bubbling of trapped nitrogen gas during exposure)29. In addition, an overnight rehydration step improves the reproducibility of exposure times required for thick AZ50 XT layers and reduces spatial variability in rates of development across the wafer. Finally, a long (14-15 hr) post exposure bake with a slow ramp rounds rectangular photoresist features to form valves without deforming valve geometries for a wide variety of tested photoresist thicknesses.
Several of the procedures presented here for fabricating layers from negative photoresist include small differences from manufacturer instructions. We suggest a three-step soft bake process that moves wafers between hot plates set at 65 °C, 95 °C, and 65 °C. We have found that gradual warming of wafers reduces the appearance of defects during exposure resulting from rupture of gas bubbles trapped within photoresist via the formation of a "crust" during soft baking. Conversely, gradual cooling of wafers after soft baking can reduce photoresist cracking. Finally, we have found that increasing photoresist relaxation times to ~ 20 min reduces small variations in resist height across the wafer.
Due to the flexibility of this fabrication protocol, we expect it will have broad utility for different devices across disciplines. While alternatives such as 3D printing, glass etching and embossing can also achieve microfluidic fabrication, lithographic patterning can achieve more complex functionality, such as valving, that other methods have not yet achieved at scale. The major limitation of this protocol is design-to-testing time, which takes ~3 days (mainly due to steps required to fabricate rounded valves).
We hope that open dissemination of protocols in microfluidics, especially relating to complicated photolithography steps, will encourage continued innovation in the field and that this proof-of-concept demonstration will aid in helping users troubleshoot fabrication issues.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
The authors thank Scott Longwell for helpful comments and edits to the manuscript and Robert Puccinelli for device photography. The authors acknowledge generous support from a Beckman Institute Technology Development Grant. K.B. is supported by a NSF GFRP fellowship and the TLI component of the Stanford Clinical and Translational Science Award to Spectrum (NIH TL1 TR 001084); P.F. acknowledges a McCormick and Gabilan Faculty Fellowship.
References
- Duncombe TA, Tentori AM, Herr AE. Microfluidics: reframing biological enquiry. Nat. Rev. Mol. Cell Bio. 2015;16(9) doi: 10.1038/nrm4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires TM, Quake SR. Microfluidics: Fluid physics at the nanoliter scale. Rev.Mod. Phys. 2005;77(3) [Google Scholar]
- Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442(7101) doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- Kalisky T, Blainey P, Quake SR. Genomic Analysis at the Single-Cell Level. Ann. Rev. of Genetics. 2011;45(1) doi: 10.1146/annurev-genet-102209-163607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel NH, Lou X, Wang C, He L. Peer Reviewed: Barcoding the Microworld. Anal. Chem. 2004;76(19) doi: 10.1021/ac0416463. [DOI] [PubMed] [Google Scholar]
- Lecault V, White AK, Singhal A, Hansen CL. Microfluidic single cell analysis: from promise to practice. Curr. Opin. in Chem. Bio. 2012;16(3-4) doi: 10.1016/j.cbpa.2012.03.022. [DOI] [PubMed] [Google Scholar]
- White AK, Heyries KA, Doolin C, VanInsberghe M, Hansen CL. High-Throughput Microfluidic Single-Cell Digital Polymerase Chain Reaction. Anal. Chem. 2013;85(15) doi: 10.1021/ac400896j. [DOI] [PubMed] [Google Scholar]
- Hansen CL, Classen S, Berger JM, Quake SR. A Microfluidic Device for Kinetic Optimization of Protein Crystallization and In Situ Structure Determination. J. Am. Chem. Soc. 2006;128(10) doi: 10.1021/ja0576637. [DOI] [PubMed] [Google Scholar]
- Maerkl SJ, Quake SR. A Systems Approach to Measuring the Binding Energy Landscapes of Transcription Factors. Science. 2007;315(5809) doi: 10.1126/science.1131007. [DOI] [PubMed] [Google Scholar]
- Fordyce PM, Gerber D, et al. De novo identification and biophysical characterization of transcription-factor binding sites with microfluidic affinity analysis. Nat. Biotech. 2010;28(9) doi: 10.1038/nbt.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan R, et al. Integrated barcode chips for rapid, multiplexed analysis of proteins in microliter quantities of blood. Nat. Biotech. 2008;26(12) doi: 10.1038/nbt.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovarik ML, Gach PC, Ornoff DM, Wang Y. Micro total analysis systems for cell biology and biochemical assays. Anal. Chem. 2011. [DOI] [PMC free article] [PubMed]
- Stroock AD, Dertinger SKW, Ajdari A, Mezić I, Stone HA, Whitesides GM. Chaotic Mixer for Microchannels. Science. 2002;295(5555):647–651. doi: 10.1126/science.1066238. [DOI] [PubMed] [Google Scholar]
- Unger MA, Chou H-P, Thorsen T, Scherer A, Quake SR. Monolithic Microfabricated Valves and Pumps by Multilayer Soft Lithography. Science. 2000;288(5463):113–116. doi: 10.1126/science.288.5463.113. [DOI] [PubMed] [Google Scholar]
- Thorsen T, Maerkl SJ, Quake SR. Microfluidic Large-Scale Integration. Science. 2002;298(5593) doi: 10.1126/science.1076996. [DOI] [PubMed] [Google Scholar]
- Li N, Sip C, Folch A. Microfluidic Chips Controlled with Elastomeric Microvalve Arrays. JoVE. 2007. p. e296. [DOI] [PMC free article] [PubMed]
- Kim P, et al. Soft lithography for microfluidics: a review. Biochip. J. 2008;2(1):1–11. [Google Scholar]
- Studer V, Hang G, Pandolfi A, Ortiz M, Anderson WF, Quake SR. Scaling properties of a low-actuation pressure microfluidic valve. J. Appl. Phys. 2004;95(1):393–398. [Google Scholar]
- Kartalov EP, Scherer A, Quake SR, Taylor CR, Anderson WF. Experimentally validated quantitative linear model for the device physics of elastomeric microfluidic valves. J. Appl. Phys. 2007;101(6):064505. doi: 10.1063/1.2511688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerver RE, Gómez-Sjöberg R, et al. Programmable microfluidic synthesis of spectrally encoded microspheres. Lab. Chip. 2012;12(22):4716–4723. doi: 10.1039/c2lc40699c. [DOI] [PubMed] [Google Scholar]
- Fordyce PM, Diaz-Botia CA, DeRisi JL, Gómez-Sjöberg R. Systematic characterization of feature dimensions and closing pressures for microfluidic valves produced via photoresist reflow. Lab. Chip. 2012;12(21):4287–4295. doi: 10.1039/c2lc40414a. [DOI] [PubMed] [Google Scholar]
- Li C-W, Cheung CN, Yang J, Tzang CH, Yang M. PDMS-based microfluidic device with multi-height structures fabricated by single-step photolithography using printed circuit board as masters. The Analyst. 2003;128(9):1137–1142. doi: 10.1039/b304354a. [DOI] [PubMed] [Google Scholar]
- Romanowsky MB, Abate AR, Rotem A, Holtze C, Weitz DA. High throughput production of single core double emulsions in a parallelized microfluidic device. Lab. Chip. 2012;12(4):802–807. doi: 10.1039/c2lc21033a. [DOI] [PubMed] [Google Scholar]
- Mata A, Fleischman AJ, Roy S. Fabrication of multi-layer SU-8 microstructures. JMM. 2006;16(2):276. [Google Scholar]
- Microchem. SU-8 2000 Series Data Sheet. Microchem; 2016. Available from: http://www.microchem.com/pdf/SU-82000DataSheet2025thru2075Ver4.pdf. [Google Scholar]
- Fordyce PM, DeRisi JL, Gómez-Sjöberg R. AZ50 XT Feature Height Calculator. 2016. Available from: http://derisilab.ucsf.edu/software/AZ50XT/AZ50XTToolInput.html.
- Foundry SM. Stanford Microfluidics Foundry Website. 2016. Available from: http://web.stanford.edu/group/foundry/Microfluidic%20valve%20technology.html>.
- Sjöberg R. Rafael's Microfluidics Site. 2016. Available from: https://sites.google.com/site/rafaelsmicrofluidicspage/valve-controllers.
- Wanat S, Plass R, Sison E, Zhuang H, Lu P-H. Optimized Thick Film Processing for Bumping Layers. Proc. SPIE. 2003. pp. 1281–1288.


