Abstract
We carried out an epidemiological study covering 2,365,067 patient days of hospitalization between 2000 and 2003. During this time, 413 Staphylococcus aureus bloodstream infections occurred. This corresponds to 15% of the 2,676 bloodstream infections observed during this period in the 31 hospitals in our region of France, which has 2.5 million inhabitants. The incidence of nosocomial S. aureus bloodstream infections was 0.11 per 1,000 days of hospitalization. The prevalence of methicillin-resistant S. aureus (MRSA) strains, of which 13% were nonmultiresistant MRSA (NORSA), was 33%, and this percentage was stable over the 4 years. In contrast, the prevalence of S. aureus strains susceptible to methicillin but resistant to quinolones or susceptible to methicillin but multiresistant to antibiotics (EMSSA strains) increased from 4% in 2000 to 23% in 2003. As previously reported, MRSA strains were mostly recovered from nosocomial bloodstream infections, whereas NORSA strains—generally considered to be responsible for community-acquired infections—were always isolated from nosocomial bloodstream infections. Pulsed-field gel electrophoresis (PFGE) analysis of 109 MRSA strains and 15 EMSSA strains demonstrated clonal diffusion of the three major French MRSA clones and revealed considerable genetic heterogeneity among EMSSA strains. Although no epidemiologically related NORSA strains clustered in particular PFGE groups, the distribution of MRSA strains isolated from bloodstream infections according to the portal of entry (vascular devices, pulmonary, and urinary) was not random for the major PFGE clones, suggesting that each MRSA lineage displays particular virulence features.
In France, methicillin-resistant S. aureus (MRSA) strains became endemic in many health care institutions (HCI) during the 1980s and early 1990s, accounting for up to 40% of all S. aureus isolates (1, 11). These strains frequently cause disease outbreaks and cross infection and have become endemic in many regions, adding to the morbidity, mortality, and cost of care associated with hospital-acquired infections. Enhanced surveillance and infection control measures, together with the molecular typing of MRSA isolates, facilitate the identification of genotypes that spread, decreasing the annual frequency of hospital-acquired MRSA infections (21). The surveillance of antibiotic resistance is essential if we are to identify trends in resistance, develop accurate treatment, and assess the effectiveness of interventions appropriately (9).
Data describing the incidence and epidemiology of MRSA in the Centre region of France have been available since 2000, when regional surveillance for MRSA began. An extensive, prospective, longitudinal, regionwide survey of bloodstream infection (BSI) is carried out for 3 months of each year in a large number of HCI in the region to establish a comprehensive picture of the epidemiology of severe hospital-acquired S. aureus infections. S. aureus BSIs are extensively studied within this framework. All the S. aureus strains isolated during successive study periods are sent to a central laboratory for susceptibility testing and molecular typing, with the aim of determining the spread and diversity of MRSA at the regional level.
We report here the results obtained during the first 4 years of surveillance (2000 to 2003) of S. aureus BSIs. The data obtained were used to determine the epidemiology of BSI and especially of S. aureus BSIs. We evaluated the change in the antibiotic susceptibilities of S. aureus strains responsible for BSI during the 4-year period. We sought a correlation between antibiogroups and the nature of the portal of entry of the BSI and between antibiogroups and the vital status during the 7 days following BSI diagnosis. We genetically characterized S. aureus BSI strains to identify peculiar characteristics of some antibiogroups of strains or of strains classified on the basis of the portal of entry of the BSI.
MATERIALS AND METHODS
BSI epidemiological survey method.
The Relais d'Hygiène du Centre (RHC) is the center responsible for monitoring nosocomial infections in the Centre Region of France (2.5 million inhabitants). It was set up with the support of the Agence Regionale de l'Hospitalisation. Since 2000, an experimental system has been in operation in which the surveillance of BSI is closely linked to the study of S. aureus strains isolated from BSI cases. The goal of the S. aureus survey is to identify the strains responsible for severe infections that persisted and spread between HCI in the Centre region.
Thirty-one HCI, comprising a total of 8,570 beds (including 5,224 short-stay beds), are participating in this program, which involves an annual 3-month survey of all cases of BSI. This prospective study is carried out to determine the incidence of BSI. BSI is defined as a positive (not contaminated) blood culture in a patient with clinical or laboratory evidence of infection. A blood culture was considered to be contaminated when only one blood culture was positive for a bacterium that is frequently found as a contaminant (i.e., coagulase-negative staphylococci, Corynebacterium spp., or Propionibacterium spp.) and when the clinician did not prescribe any antibiotic treatment against the bacterium. In the last 4 years, the survey covered 2,365,067 patient days. The variables studied included patient age and sex, portal of entry (skin [primitive cutaneous form or superinfection of a skin breach], surgical site, pulmonary, urine, intravascular devices, or digestive), community- or hospital-acquired BSI, death within 7 days of BSI diagnosis, and duration of hospital stay. The data were analyzed with Epi Info version 6 software. Means were compared by using the chi-square test, and the Bonferroni correction was applied in cases of multiple testing (7). The incidences of community-acquired and nosocomial BSI were determined with respect to the number of patient days.
Microbiological methods. (i) Bacteriology.
A microbiological study was conducted with the 360 BSI-associated S. aureus strains collected during the study. The strains were sent to our laboratory, which is the reference laboratory of the RHC. All specimens were cultured on sheep blood agar (trypticase soy agar with 5% sheep blood; bioMérieux, Marcy l'Etoile, France). The plates were incubated for 48 h at 35°C in the presence of 5 to 7% CO2. The bacteria were identified as S. aureus on the basis of their characteristic growth pattern, colony morphology, Gram staining, catalase test, coagulase production (rabbit plasma; Difco Laboratories, Elancourt, France), slide test for clumping factor (Pastorex Plus-Staph; Bio-Rad, Ivry-sur-Seine, France), and results obtained with ID 32 STAPH strips (API System; bioMérieux), a rapid identification system for the genera Staphylococcus, Micrococcus, Stomatococcus, and Aerococcus. These strips were used according to the manufacturer's recommendations.
(ii) Antimicrobial susceptibility testing.
We used the disk diffusion method (Bio-Rad) to test the antibiotic susceptibilities of the S. aureus strains. The antibiotics tested were penicillin G, oxacillin, erythromycin, lincomycin, pristinamycin, tetracycline, kanamycin, tobramycin, gentamicin, rifampin, fusidic acid, fosfomycin, pefloxacin, cotrimoxazole, vancomycin, and teicoplanin. The cefinase test (bioMérieux) was used to detect β-lactamase production, and the mecA gene was detected by PCR. In this paper, classical multisensitive methicillin-susceptible S. aureus (MSSA) is referred to as CMSSA, and methicillin-susceptible strains displaying resistance to quinolones or to at least two antibiotics (not including penicillin) are referred to as emerging MSSA (EMSSA). MRSA strains displaying typical resistance to multiple antibiotics are referred to as classical multiresistant MRSA (CMRSA), and MRSA strains resistant to no more than three antibiotics (excluding penicillin and methicillin) are referred to as nonmultiresistant MRSA (NORSA).
(iii) Multiplex PCR assay for typing of major SCCmecs.
Multiplex PCR was used to type the staphylococcal cassette chromosome mec element (SCCmec) in oxacillin-resistant NORSA as described by Oliveira and de Lencastre (33).
(iv) DNA macrorestriction and PFGE.
Pulsed-field gel electrophoresis (PFGE) was used as a typing technique, as multilocus sequence typing and PFGE—the most powerful techniques for high-precision epidemiological typing of MRSA strains—provide very similar results (20). Genomic DNA was extracted from the isolates and digested with SmaI. It was then subjected to PFGE, as previously described for S. aureus (30). The gels were stained with ethidium bromide and photographed with a Polaroid instant camera. The PFGE patterns were compared by eye. Patterns (pulsotypes) were considered to be indistinguishable if all bands were shared. The patterns were analyzed with the Taxotron package (Taxolab; Institut Pasteur, Paris, France). The images were transferred to a microcomputer, and the distances migrated by each band in each lane were determined in pixel units by RestrictoScan. The molecular size of each fragment was calculated from the distance migrated using cubic Schaffer and Sederoff method algorithms with RestrictoTyper. This software also generated a schematic representation of electrophoretic patterns and produced a distance matrix. The relationships between pulsotypes were determined by the unweighted pair group method using arithmetic means and the Adanson pulsogrouping program (dissimilarity). A dendrogram was drawn with Dendrograf.
(v) Virulence factors.
Given the diffusion of Panton-Valentine leukocidin (PVL)- and TSST-1-producing MRSA strains in France since 2002 (19, 39), we sought sequences corresponding to lukS-PV and lukF-PV, encoding PVL, and tst, encoding TSST-1. Genomic DNA was extracted from staphylococcal cultures and used as a template for PCR amplification, using the procedure and primers described by Jarraud et al. (24).
RESULTS
Epidemiology of BSI and situations in which S. aureus infections occurred.
During the study period, 2,676 cases of BSI were identified in 2,676 patients, 53% of which were community acquired and 47% of which were nosocomial (Table 1). The mean and median ages of the patients were 69 years. The sex ratio (male to female) was 1.7. The mean incidence of nosocomial BSI was 0.53 cases per 1,000 days of hospitalization. This ranged from 0.37 to 0.61 cases, depending on the year.
TABLE 1.
Classification of 276 BSI
| Yr | No. of BSIs (%)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Community-acquired
|
Nosocomial
|
All BSI | |||||||||
| All | S. aureus | S. pneumoniae | E. coli | KESa | All | S. aureus | CNSb | E. coli | KES | ||
| 2000 | 283 (54) | 21 (7) | 23 (8) | 134 (47) | 8 (3) | 243 (46) | 45 (19) | 47 (19) | 46 (19) | 20 (8) | 526 |
| 2001 | 301 (56) | 28 (14) | 25 (8) | 139 (46) | 20 (7) | 235 (44) | 56 (24) | 39 (17) | 65 (28) | 31 (13) | 536 |
| 2002 | 439 (51) | 49 (11) | 40 (9) | 179 (41) | 21 (2) | 426 (49) | 86 (20) | 114 (27) | 55 (13) | 18 (4) | 865 |
| 2003 | 406 (54) | 48 (12) | 44 (11) | 158 (39) | 14 (3) | 343 (46) | 80 (23) | 56 (16) | 62 (18) | 30 (9) | 749 |
| Total | 1,429 (53) | 146 (10) | 132 (9) | 610 (43) | 63 (4) | 1,247 (47) | 267 (21) | 256 (20) | 228 (18) | 99 (8) | 2,676 |
KES, Klebsiella spp., Enterobacter spp., and Serratia spp.
CNS, coagulase-negative staphylococci.
Enterobacteria were the most frequent cause of BSI, accounting for 39% of the cases (n = 1,054), including 44% of the community-acquired (629 of 1,429) and 34% of the nosocomial (425 of 1,247) cases (Table 1). The proportion of community-acquired infections caused by enterobacteria decreased steadily during the study period, from 54% (154 of 283) in 2000 to 34% (139 of 406) in 2003 (P < 0.001), whereas no significant change was observed in the proportion of nosocomial cases caused by these bacteria.
S. aureus was responsible for 15% of the BSI cases during this period (n = 413), including 10% of the community-acquired cases (146 of 1,429) and 21% of the nosocomial cases (267 of 1,247) (Table 1). The mean incidence of nosocomial S. aureus BSI was 0.11 cases per 1,000 days of hospitalization (0.09 to 0.13 cases, depending on the year). During the study period, the proportion of S. aureus BSIs that were community-acquired cases increased slightly, from 32% (21 of 66) in 2000 to 38% (48 of 128) in 2003.
S. aureus antibiotic susceptibility and BSI characteristics. (i) Prevalence of methicillin resistance in S. aureus BSI strains.
One-third (33%) of the 413 cases of BSI due to S. aureus were caused by an MRSA strain (n = 136) (Table 2). The proportion of MRSA strains depended on the origin of the BSI: 25 of the 166 community-acquired cases (15%) and 113 of the 268 nosocomial cases (42%). The strains responsible for 360 of the 413 S. aureus BSI cases were successfully sent to our reference laboratory. The microbiological study was thus conducted with these 360 S. aureus strains, which comprised 236 of the 277 MSSA strains (85%) and 124 of the 136 MRSA strains (91%).
TABLE 2.
Classification of S. aureus BSIs and mortality rates according to antibiogroup and origin of BSI
| BSI origin | S. aureus | No. (%) of BSIs in yr
|
Mortality rateb | ||||
|---|---|---|---|---|---|---|---|
| 2000 | 2001 | 2002 | 2003 | Total | |||
| All | MSSA | 43 (65) | 62 (74) | 89 (66) | 83 (65) | 277 (66) | 28 (14) |
| CMSSA | 40 (96) | 51 (89) | 63 (88) | 51 (77) | 205 (87) | 22 (13) | |
| EMSSA | 1 (4) | 6 (11) | 9 (12) | 15 (23) | 31 (13) | 6 (25) | |
| NKa | 2 | 5 | 17 | 17 | 41 | 80 | |
| MRSA | 23 (35) | 22 (26) | 46 (34) | 45 (35) | 136 (33) | 29 (26) | |
| CMRSA | 20 (87) | 18 (95) | 32 (82) | 37 (90) | 108 (87) | 25 (27) | |
| NORSA | 3 (13) | 1 (5) | 7 (18) | 4 (10) | 16 (13) | 4 (27) | |
| NK | 3 | 7 | 4 | 12 | 27 | ||
| Community acquired | MSSA | 18 (86) | 26 (90) | 49 (84) | 48 (83) | 141 (85) | 13 (15) |
| CMSSA | 17 (100) | 23 (92) | 23 (77) | 24 (83) | 87 (86) | 10 (13) | |
| EMSSA | 2 (8) | 7 (23) | 5 (17) | 14 (14) | 3 (25) | ||
| NK | 1 | 1 | 19 | 19 | 40 | 55 | |
| MRSA | 3 (14) | 3 (10) | 9 (16) | 10 (17) | 25 (15) | 2 (11) | |
| CMRSA | 3 (100) | 3 (100) | 9 (100) | 9 (100) | 24 (100) | 2 (11) | |
| NORSA | |||||||
| NK | 1 | 1 | 6 | ||||
| Nosocomial | MSSA | 25 (56) | 36 (62) | 49 (57) | 45 (56) | 155 (58) | 15 (13) |
| CMSSA | 23 (96) | 28 (88) | 40 (95) | 27 (73) | 118 (87) | 12 (12) | |
| EMSSA | 1 (4) | 4 (12) | 2 (5) | 10 (27) | 17 (13) | 3 (23) | |
| NK | 1 | 4 | 7 | 8 | 20 | 44 | |
| MRSA | 20 (44) | 21 (38) | 37 (43) | 35 (44) | 113 (42) | 27 (30) | |
| CMRSA | 17 (85) | 14 (88) | 25 (78) | 28 (87) | 84 (84) | 23 (31) | |
| NORSA | 3 (15) | 2 (12) | 7 (22) | 4 (13) | 16 (16) | 4 (27) | |
| NK | 5 | 5 | 3 | 13 | 23 | ||
NK, not known.
Values are percentages. Values in parentheses are percentages of MRSA (or MSSA) from all S. aureus strains, according to the origin of the BSI.
(ii) Phenotypes of MSSA strains causing BSI.
We found that 205 of the 236 MSSA strains (87%) were susceptible to multiple antibiotics (Table 2). We refer to these strains as CMSSA. Eighteen MSSA strains were resistant to at least two antibiotics (not including penicillin), and 13 MSSA strains showed decreased susceptibility or resistance to fluoroquinolones. The proportion of MSSA strains resistant to antibiotics increased steadily during the study period, from 1 of 41 in 2000 (4%) to 15 of 66 (23%) in 2003; we refer to these strains as EMSSA.
(iii) Phenotypes of MRSA strains causing BSI.
We found that 108 of the 124 MRSA strains (87%) displayed typical resistance to multiple antibiotics (Table 2); these strains are referred to as CMRSA. The 108 CMRSA strains were divided into four groups on the basis of antibiotic resistance (antibiogroups): 45 MRSA strains (42%) were resistant to kanamycin and fluoroquinolones (antibiogroup 1); 32 strains (30%) were resistant to kanamycin, erythromycin, lincomycin, and fluoroquinolones (antibiogroup 2); 18 strains (17%) were resistant to gentamicin, kanamycin, erythromycin, lincomycin, and fluoroquinolones (antibiogroup 3); and 4 strains (4%) were resistant to kanamycin, erythromycin, and fluoroquinolones (antibiogroup 4). Sixteen of the 124 MRSA strains (13%) were resistant to no more than three antibiotics (excluding penicillin and methicillin); these strains are referred to as NORSA. During the study period, the proportion of NORSA strains among MRSA strains causing BSI varied from 5 to 18%, according to the year. All the NORSA strains were associated with nosocomial BSI, and none of the 24 cases of community-acquired infection due to MRSA were associated with a NORSA strain; this difference was significant (P = 0.023).
(iv) Antibiotic susceptibility and portal of entry for S. aureus strains causing BSI.
Clinicians identified the portal of entry of the strain responsible for BSI in 311 of the 413 BSI cases (75%), comprising 204 of the 277 MSSA cases (74%) and 107 of the 136 MRSA cases (79%) (Table 3). For both MSSA and MRSA, the major portals of entry were the skin and intravascular devices. The skin served as the portal of entry for 25 of the 107 MRSA (23%) and 53 of the 204 MSSA (26%) cases. Intravascular devices were associated with 25 out of 107 MRSA (23%) and 49 out of 204 MSSA (24%) BSI cases. The urinary tract was the third major portal of entry for MRSA strains (22 of 108 [20%]).
TABLE 3.
Classification of S. aureus BSI according to portal of entry, antibiogroup, and origin of BSI
| BSI origin | S. aureus | No. (%) of BSIs at portal of entry
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Skin | Vascular devices | Urinary | Pulmonary | Surgical site | Digestive | Others | Total | ||
| All | MRSA | 25 (23) | 25 (23) | 21 (20) | 14 (13) | 10 (9) | 4 (4) | 8 | 107 |
| CMRSA | 20 (22) | 19 (21) | 19 (21) | 14 (15) | 9 (10) | 4 (4) | 6 | 91 | |
| NORSA | 5 (31) | 6 (37) | 2 (12) | 1 (6) | 2 | 16 | |||
| MSSA | 53 (26) | 49 (24) | 25 (12) | 22 (11) | 21 (10) | 8 (4) | 26 | 204 | |
| CMSSA | 45 (25) | 43 (24) | 23 (13) | 19 (11) | 17 (10) | 7 (4) | 23 | 177 | |
| EMSSA | 8 (30) | 6 (22) | 2 (7) | 3 (11) | 4 (15) | 1 (4) | 3 | 27 | |
| Total | 78 (25) | 74 (24) | 46 (15) | 36 (12) | 31 (10) | 12 (4) | 34 | 311 | |
| Community acquired | MRSA | 7 (39) | 5 (28) | 3 (17) | 1 (6) | 2 | 18 | ||
| CMRSA | 7 (39) | 5 (28) | 3 (17) | 1 (6) | 2 | 18 | |||
| NORSA | |||||||||
| MSSA | 36 (43) | 1 (1) | 1 (1) | 8 (10) | 10 (12) | 5 (6) | 22 | 83 | |
| CMSSA | 31 (45) | 1 (1) | 5 (7) | 8 (12) | 5 (7) | 19 | 69 | ||
| EMSSA | 5 (36) | 1 (7) | 3 (21) | 2 (14) | 13 | 14 | |||
| Total | 43 (43) | 1 (1) | 6 (6) | 11 (11) | 10 (10) | 6 (6) | 24 | 101 | |
| Nosocomial | MRSA | 18 (20) | 25 (28) | 16 (18) | 11 (12) | 10 (11) | 3 (3) | 6 | 89 |
| CMRSA | 13 (18) | 19 (26) | 14 (19) | 11 (15) | 9 (12) | 3 (4) | 4 | 73 | |
| NORSA | 5 (31) | 6 (37) | 2 (12) | 1 (6) | 2 | 16 | |||
| MSSA | 17 (14) | 48 (40) | 24 (20) | 14 (12) | 11 (9) | 3 (2) | 4 | 121 | |
| CMSSA | 14 (13) | 42 (39) | 23 (21) | 14 (13) | 9 (8) | 2 (2) | 4 | 108 | |
| EMSSA | 13 (23) | 6 (46) | 1 (8) | 2 (15) | 1 (8) | 13 | |||
| Total | 35 (17) | 73 (35) | 40 (19) | 25 (12) | 21 (10) | 6 (3) | 10 | 210 | |
(v) S. aureus antibiotic susceptibility and rate of mortality.
The vital status during the 7 days following BSI diagnosis was known for 306 of the 413 cases of BSI due to S. aureus (74%) (Table 2). Fifty-eight deaths were identified: 15 of the 105 community-acquired (14%) cases and 42 of the 202 nosocomial cases (21%). The mortality rate was significantly higher for MRSA (29 of 110 [26%]) than for MSSA (28 of 197 [14%]) (P = 0.009).
(vi) S. aureus antibiotic susceptibility and virulence genes.
Given the clonal diffusion of PVL-producing MRSA strains in France since 2002 and the major increase in the number of EMSSA strains in 2003 associated with an increase in mortality rates (Table 2), we used PCR to test 69 of the 128 S. aureus strains isolated from BSI cases in 2003 for sequences corresponding to the lukS-PV, lukF-PV, and tst genes. This set of strains included all 15 EMSSA strains, 39 of the 45 MRSA strains, and 15 of the 51 CMSSA strains. The tst gene, which encodes the virulence factor TSST-1, was identified in two of the CMSSA strains tested, one of the EMSSA strains, and none of the MRSA strains. The lukS-PV and lukF-PV genes, encoding PVL, were not detected in any of the strains studied.
PFGE typing, occurrence of virulence genes, and BSI characteristics. (i) Genotyping.
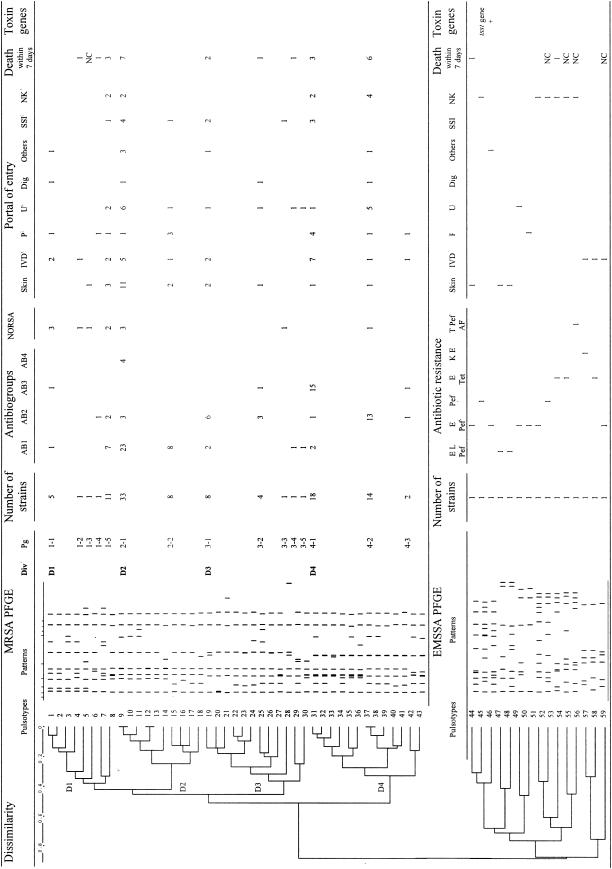
Of the 124 MRSA strains sent to the central laboratory of the RHC, 109 were available for genotyping. PFGE revealed 43 different pulsotypes (1 to 43). The relationships among the pulsotypes are shown on a dendrogram in Fig. 1. This analysis made it possible to identify four equilibrated divisions—D1 (19 strains; 17%), D2 (41 strains; 38%), D3 (15 strains; 14%); and D4 (34 strains; 31%)—in which strains were at least 60% similar and 15 pulsogroups in which strains displayed at least 80% similarity. The four major pulsogroups, 1-5, 2-1, 4-1, and 4-2, accounted for 70% of the MRSA strains (76 of 109).
FIG. 1.
Schematic representation of PFGE restriction patterns with SmaI and the genetic relationships among 109 MRSA and 15 EMSSA strains. Div, division; Pg, pulsogroup; IVD, intravascular device; P, pulmonary; U, urinary tract; Dig, digestive portal of entry; SSI, surgical site infection; E, erythromycin; L. lincomycin; Pef, pefloxacin; Tet, tetracyclin; K, kanamycin; T, tobramycin; AF, fusidic acid; NK, not known. Antibiogroup 1 consists of strains resistant to tobramycin, kanamycin, and pefloxacin (Pef); antibiogroup 2 consists of strains resistant to tobramycin, kanamycin, erythromycin, lincomycin, and pefloxacin; antibiogroup 3 consists of strains resistant to gentamicin, tobramycin, kanamycin, erythromycin, lincomycin, and pefloxacin; antibiogroup 4 consists of strains resistant to tobramycin, kanamycin, erythromycin, and pefloxacin.
Given the major increase in the frequency of EMSSA strains in 2003, with as many of these strains isolated in 2003 alone as in the first 3 years of the study together (Table 2), we genotyped the 15 EMSSA strains isolated in 2003 to assess the possibility of clonal emergence of EMSSA. The population of isolates obtained was highly heterogeneous (Fig. 1), ruling out such clonal diffusion. Indeed, 15 different pulsotypes (pulsotypes 44 to 58) and 15 pulsogroups, according to the criteria used for MRSA strains, were obtained for these 15 strains. All of the EMSSA pulsotypes were well separated from the MRSA pulsotypes. Most of the EMSSA strains were >60% dissimilar (Fig. 1).
(ii) PFGE and antibiogroups.
We assessed the relationship between antibiogroups and pulsogroups. We found a significant association between some MRSA pulsogroups and antibiogroups. Twenty-three of the 33 MRSA strains from pulsogroup 2-1 (70%) and all eight of the MRSA strains from pulsogroup 2-2 (100%) belonged to antibiogroup 1, which contained a total of 45 strains (69%). There is thus a significant association between division D2, which comprises pulsogroups 2-1 and 2-2, and antibiogroup 1 (P < 0.001). Six of the eight strains from pulsogroup 3-1 (75%) and 13 of the 14 strains from pulsogroup 4-2 (93%) belonged to antibiogroup 2, which contained 30 strains, revealing a significant association between pulsogroups 3-1 and 4-2 and antibiogroup 2 (P < 0.001). Fifteen of the 18 strains from pulsogroup 4-1 (83%) belonged to antibiogroup 3, which contained 18 strains (83%), revealing a significant association (P < 0.001).
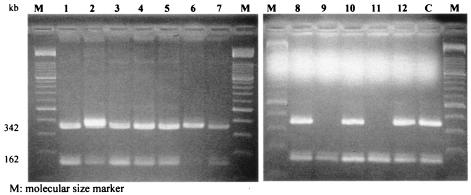
In addition, NORSA strains appeared to be specifically associated with division D1 and pulsogroup 1-1 of this division. Indeed, 7 of the 12 NORSA strains (58%) belonged to division 1 (P < 0.001) and 3 of the 5 pulsogroup 1-1 strains (60%) were NORSA strains. Multiplex PCR showed that 10 of these 12 NORSA strains carried SCCmec type IV (Fig. 2).
FIG. 2.
Multiplex PCR assay for typing of major SCCmec elements of the 12 NORSA strains. Lanes 1 to 12 show the SSCmec types of the 12 NORSA strains (Fig. 1). Lane C shows the SSCmec type IV of the PVL-producing MRSA strain that emerged in France in 2000 (15).
With EMSSA, we obtained no evidence of a link between the antibiotype and the PFGE pattern, probably due to the heterogeneity of these strains and because too few strains were studied.
(iii) PFGE and the portal of entry of S. aureus strains causing BSI.
We assessed the relationship between the portal of entry and the pulsogroup (Fig. 1).
A cutaneous portal of entry was found with 11 of the 30 MRSA strains (37%) belonging to pulsogroup 2-1. Eleven of the 22 BSIs (50%) in which the skin was the portal of entry were associated with MRSA strains belonging to pulsogroup 2-1 (P = 0.014). Vascular devices served as the portal of entry for 7 of the 16 MRSA strains (44%) belonging to pulsogroup 4-1. Seven of the 22 BSIs (32%) in which a vascular device served as the portal of entry were associated with MRSA strains belonging to pulsogroup 4-1 (P = 0.039). A pulmonary portal of entry was found for 3 of the 8 MRSA strains (38%) belonging to pulsogroup 2-2 and 4 of the 16 MRSA strains (25%) belonging to pulsogroup 4-1. Seven of the 13 BSIs (54%) associated with a pulmonary portal of entry were caused by MRSA strains belonging to pulsogroup 2-2 or 4-1 (P = 0.010). A urinary portal of entry was found for 5 of the 10 MRSA strains (50%) belonging to pulsogroup 4-2. Five of the 19 BSIs (26%) associated with a urinary portal of entry were caused by MRSA strains belonging to pulsogroup 4-2 (P = 0.017). The chi-square test revealed a trend toward an association between some portals of entry and some genetic groups of MRSA strains. Indeed, given the need to apply a correction factor for multiple comparisons, links can be considered significant if the P value is <0.01.
Despite the limited number of EMSSA strains (15 strains), the dendrogram obtained showed two populations of isolates that were 80% dissimilar to each other. One population was composed of only three EMSSA strains isolated from BSIs in which vascular devices served as the portal of entry, and the other population consisted of 12 isolates for which the portal of entry was the skin (four cases) or had not been identified (six cases).
(iv) PFGE and mortality rate.
The mortality rate within 7 days of diagnosis varied according to the MRSA pulsogroup only, from 0% for pulsogroup 2-2 to 43% for pulsogroup 4-2 (Fig. 1). More patients infected with strains from pulsogroup 3-1 (2 strains; 40%) and pulsogroup 4-2 (6 strains; 43%) died than those infected with other strains (17 strains; 23%) (P = 0.032).
DISCUSSION
This work establishes a comprehensive picture of the epidemiology of severe S. aureus infections in hospitals in the Centre region of France during a period covering 2,365,067 patient days. Our data confirm several major findings of previously published regional BSI surveys (10, 13). First, the incidences of nosocomial BSI (0.53 cases per 1,000 days of hospitalization) and nosocomial S. aureus BSI (0.11 cases per 1,000 days of hospitalization), the high prevalence of methicillin resistance in S. aureus strains isolated from the bloodstream (33%), and the mortality rate within 7 days of diagnosis of S. aureus BSI (21% of the nosocomial cases) were similar to those recently reported in European and U.S. studies (12, 36). Second, our results confirm the dissemination in the region of three well-described epidemic clones of MRSA that are currently spreading throughout France (pulsogroups 2-1, 4-1, and 4-2) (Fig. 1) (16, 22, 28). The two gentamicin-susceptible clones (pulsogroups 2-1 and 4-2) disseminating endemically in France were also found in the Centre region (4, 27). However, the gentamicin-resistant clone (pulsogroup 4-1), which has been reported to be decreasing in prevalence, is still common in our region. Third, our results confirm that antibiotyping and PFGE delimit similar MRSA strain populations (6). Therefore, antibiotyping may be considered a first step in the study of epidemic and endemic S. aureus strains that has to be completed by PFGE, which provides more discriminatory results that allow the establishment of a regional typing network.
This work adds several new elements to the available epidemiological data on S. aureus BSI: (i) the increase in number of EMSSA strains causing BSI with unusual clinical and genetic features, (ii) the implication of NORSA strains in sporadic nosocomial cases of BSI only within the HCI of the region, and (iii) a correlation between the major MRSA PFGE clones and BSI cases classified according to the portal of entry.
Increase in EMSSA strains causing BSI.
The proportion of EMSSA strains increased from 4% in 2000 to 23% of the MSSA strains in 2003. Genetic analysis (PFGE) revealed considerable diversity among these strains (Fig. 1), ruling out the possibility of the dissemination of an epidemic clone in the region. This population of EMSSA strains seems to be associated with more severe cases of BSI, often with a pulmonary portal of entry when community acquired and with a higher mortality rate within 7 days of BSI diagnosis (27%) than is observed with CMSSA (13%). Thus, these EMSSA strains are more likely to have originated from the selection of strains by previous and prolonged antibiotic treatments in individual patients rather than from the clonal diffusion of possibly selected virulent strains in the community. The emergence of such strains has not yet been reported.
Implication of NORSA strains only in sporadic nosocomial cases of BSI.
There are increasing numbers of reports of NORSA infections in individuals with no risk factors for MRSA acquisition. These strains are often described as community-acquired MRSA (18, 33). In this study, limited to BSI, NORSA strains were found to be exclusively associated with nosocomial infections. This suggests that the NORSA strains responsible for superficial and localized diseases encountered within the community can induce invasive diseases such as BSI only if risk factors resulting in hospitalization are also present.
NORSA strains have recently been implicated in outbreaks occurring in the community (3, 5, 32), including a few cases of BSI (29). These NORSA strains produced toxins, especially enterotoxin C (25), Panton-Valentine leukocidin (15), and TSST-1 (38), and carried SCCmec type IV (15, 25). In our cases of nosocomial BSI, the NORSA strains lacked virulence genes encoding these toxins. This also provides evidence that a deficiency in the host is more likely to be required for strong expression of the pathogenicity of such strains than a large array of virulence factors. In these BSI cases, most NORSA strains were also carriers of SCCmec type IV. Therefore, SCCmec type IV in NORSA strains is not exclusively related to community-acquired infections.
Nosocomial outbreaks of NORSA infection have also occurred in The Netherlands (40), Germany (41, 42), Australia (32, 37), and Norway (2). In these countries, NORSA strains displayed high levels of epidemicity, possibly due to their ability to multiply much faster than hospital-associated multiresistant MRSA strains, as demonstrated in vitro (34). In this study, the nosocomial NORSA strains were not related epidemiologically, suggesting that they did not reflect the spread of a single NORSA clone in the region. It has been suggested that NORSA strains have a low capacity to spread and to persist in the hospital environment, which is dominated by multidrug-resistant MRSA clones, because of their limited antibiotic resistance (14). The incidence of MRSA is much lower in Germany and The Netherlands than in France. Thus, the present predominance of multidrug-resistant MRSA clones in the HCI in our French region is probably preventing the occurrence of outbreaks due to NORSA strains.
Correlation between major MRSA PFGE clones and portal of entry.
S. aureus strains colonize and establish infection in a wide range of body sites, including the blood, indwelling biomaterials, mucosal surfaces, bone, and other tissues. The mechanisms underlying the tropism of S. aureus for specific infection sites are unclear. Associations have been demonstrated between the disease type, the pattern of toxin genes, and the genetic backgrounds of particular S. aureus strains (23). For example, a correlation has been found between unrelated cases of S. aureus infectious diseases and a single subset of strains, such as a clone producing TSST-1 responsible for most epidemiologically unrelated cases of urogenital toxic shock syndrome (31), a clone producing PVL and causing necrotic pneumonia (19), and clones producing TSST-1 and enterotoxin C in neonatal toxic shock syndrome-like exanthematous disease (26). For MRSA strains, a correlation has been reported between a particular clone and respiratory tract infections (8). Nevertheless, comparison of the virulence determinants harbored by S. aureus strains isolated from sterile sites and from healthy individuals indicated that seven determinants are significantly more common in invasive isolates, but no combinations of the seven genes were either more or less likely to cause disease than others with the same number of virulence-associated genes (35). We investigated the relationship between the portal of entry and the pulsogroup (Fig. 1) and found that some portals of entry tended to be associated with particular MRSA pulsogroups. These data support the concept developed by Falkow (17), according to which “the basic unit of bacterial pathogenicity is the clone or lineage that expands due to the possession of unique combinations of virulence and regulatory genes in the appropriate background.” If particular lineages of S. aureus are found to be significantly associated within populations of clinical isolates, it will be important to identify the phenotypic and/or genotypic traits accounting for their overrepresentation in particular groups of isolates. This should make it possible to identify the S. aureus strains most likely to cause infectious disease according to the specific state of each patient.
Acknowledgments
This work was supported by the Centre de Coordination de la Lutte contre les Infections Nosocomiales of West France (CCLIN Ouest), the Agence Régionale de l'Hospitalisation de Centre, and the Centre Hospitalier Universitaire de Tours.
We are grateful to H. de Lencastre and C. Oliviera for helpful discussions concerning the interpretation of the SSCmec-typing results and to B. Giraudeau, a biostatistician from the Centre Hospitalier Universitaire de Tours, for his help with the statistical analysis.
The contributing members of the Bloodstream Infection Study Group of the Relais d'Hygiène du Centre were M.-N. Adam, J. Akli, P. Amirault, M.-N. Bachelier, M. Beigneux, H. Berjon, D. Bloc, M. Boyer, M. Cahiez, B. Cattier, M. Chabaud-Meyer, F. Cotty, F. Coulomb, G. Courouble, M.-C. Courtin, L. Danse, P. de Mauregard, B. Faucqueur, C. Fièvre, P. Foloppe, F. Fongauffier, A.-M. Gingras-Roux, F. Guinard, I. Goard, V. Gorin, J.-L. Graveron, P. Harriau, C. Hombrouck-Alet, D. Imbault, F. Jacqmin, J.-F. Jamet, P. Laudat, O. Lehiani, J. Loulergue, E. Morin, C. Naudion, L. Ollivier, K. Opsomer, F. Petit, S. Petit, S. Picault, D. Poisson, J.-P. Pourrat, D. Ratovohery, S. Rossard, A. Secher, and J.-F. Theron le Gargasson.
REFERENCES
- 1.Albertini, M. T., C. Benoit, L. Berardi, Y. Berrouane, A. Boisivon, P. Cahen, C. Cattoen, Y. Costa, P. Darchis, E. Deliere, D. Demontrond, F. Eb, F. Golliot, G. Grise, A. Harel, J. L. Koeck, M. P. Lepennec, C. Malbrunot, M. Marcollin, S. Maugat, M. Nouvellon, B. Pangon, S. Ricouart, M. Roussel-Delvallez, A. Vachee, A. Carbonne, L. Marty, V. Jarlier, and the Microbiology Surveillance Network of Northern France. 2002. Surveillance of methicillin-resistant Staphylococcus aureus (MRSA) and Enterobacteriaceae producing extended-spectrum beta-lactamase (ESBLE) in Northern France: a five-year multicentre incidence study. J. Hosp. Infect. 52:107-113. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, B. M., K. Bergh, M. Steinbakk, G. Syversen, B. Magnaes, H. Dalen, and J. N. Bruun. 1999. A Norwegian nosocomial outbreak of methicillin-resistant Staphylococcus aureus resistant to fusidic acid and susceptible to other antistaphylococcal agents. J. Hosp. Infect. 41:123-132. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 2003. Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections—Los Angeles County, California, 2002-2003. Morb. Mortal. Wkly. Rep. 52:88. [PubMed] [Google Scholar]
- 4.Blanc, D. S., P. Francioli, A. Le Coustumier, L. Gazagne, E. Lecaillon, P. Gueudet, F. Vandenesch, and J. Etienne. 2001. Reemergence of gentamicin-susceptible strains of methicillin-resistant Staphylococcus aureus in France: a phylogenetic approach. J. Clin. Microbiol. 39:2287-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanc, D. S., C. Petignat, P. Moreillon, J. M. Entenza, M. Eisenring, H. Kleiber, A. Wenger, N. Troillet, C. Blanc, and P. Francioli. 1999. Unusual spread of a penicillin-susceptible methicillin-resistant Staphylococcus aureus clone in a geographic area of low incidence. Clin. Infect. Dis. 29:1512-1518. [DOI] [PubMed] [Google Scholar]
- 6.Blanc, D. S., C. Petignat, P. Moreillon, A. Wenger, J. Bille, and P. Francioli. 1996. Quantitative antibiogram as a typing method for the prospective epidemiological surveillance and control of MRSA: comparison with molecular typing. Infect. Control Hosp. Epidemiol. 17:654-659. [DOI] [PubMed] [Google Scholar]
- 7.Bland, J. M., and D. G. Altman. 1995. Multiple significance tests: the Bonferroni method. BMJ 310:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booth, M. C., L. M. Pence, P. Mahasreshti, M. C. Callegan, and M. S. Gilmore. 2001. Clonal associations among Staphylococcus aureus isolates from various sites of infection. Infect. Immun. 69:345-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bronzwaer, S. L., U. Buchholz, J. L. Kool, J. Monen, and P. Schrijnemakers. 2001. EARSS activities and results: update. Euro Surveill. 6:2-5. [DOI] [PubMed] [Google Scholar]
- 10.Bussy Malgrange, V., O. Bajolet Laudinat, M. Gerdeaux, G. Laplatte, B. Mulin, J. C. Reveil, and S. Gayet. 1998. Epidemiology of hospital bacteremias in eastern France. Eastern CCLIN Network. Pathol Biol. 46:403-407. [PubMed] [Google Scholar]
- 11.Decousser, J. W., P. Pina, F. Picot, C. Delalande, B. Pangon, P. Courvalin, P. Allouch, and the ColBVH Study Group. 2003. Frequency of isolation and antimicrobial susceptibility of bacterial pathogens isolated from patients with bloodstream infections: a French prospective national survey. J. Antimicrob. Chemother. 51:1213-1222. [DOI] [PubMed] [Google Scholar]
- 12.Diekema, D. J., S. E. Beekmann, K. C. Chapin, K. A. Morel, E. Munson, and G. V. Doern. 2003. Epidemiology and outcome of nosocomial and community-onset bloodstream infection. J. Clin. Microbiol. 41:3655-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diekema, D. J., M. A. Pfaller, F. J. Schmitz, J. Smayevsky, J. Bell, R. N. Jones, M. Beach, and the SENTRY Partcipants Group. 2001. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S114-S132. [DOI] [PubMed] [Google Scholar]
- 14.Dominguez, M. A., H. de Lencastre, J. Linares, and A. Tomasz. 1994. Spread and maintenance of a dominant methicillin-resistant Staphylococcus aureus (MRSA) clone during an outbreak of MRSA disease in a Spanish hospital. J. Clin. Microbiol. 32:2081-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dufour, P., Y. Gillet, M. Bes, G. Lina, F. Vandenesch, D. Floret, J. Etienne, and H. Richet. 2002. Community-acquired methicillin-resistant Staphylococcus aureus infections in France: emergence of a single clone that produces Panton-Valentine leukocidin. Clin. Infect. Dis. 35:819-824. [DOI] [PubMed] [Google Scholar]
- 16.Enright, M. C., D. A. Robinson, G. Randle, E. J. Feil, H. Grundmann, and B. G. Spratt. 2002. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc. Natl. Acad. Sci. USA 99:7687-7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falkow, S. 1997. What is a pathogen? ASM News 63:359-370. [Google Scholar]
- 18.Fey, P. D., B. Said-Salim, M. E. Rupp, S. H. Hinrichs, D. J. Boxrud, C. C. Davis, B. N. Kreiswirth, and P. M. Schlievert. 2003. Comparative molecular analysis of community- or hospital-acquired methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillet, Y., B. Issartel, P. Vanhems, J. C. Fournet, G. Lina, M. Bes, F. Vandenesch, Y. Piemont, N. Brousse, D. Floret, and J. Etienne. 2002. Association between Staphylococcus aureus strains carrying a gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359:753-759. [DOI] [PubMed] [Google Scholar]
- 20.Grundmann, H., S. Hori, M. C. Enright, C. Webster, A. Tami, E. J. Feil, and T. Pitt. 2002. Determining the genetic structure of the natural population of Staphylococcus aureus: a comparison of multilocus sequence typing with pulsed-field gel electrophoresis, randomly amplified polymorphic DNA analysis, and phage typing. J. Clin. Microbiol. 40:4544-4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartstein, A. I., M. A. Denny, V. H. Morthland, A. LeMonte, and M. A. Pfaller. 1995. Control of methicillin-resistant Staphylococcus aureus in a hospital and an intensive care unit. Infect. Control Hosp. Epidemiol. 16:405-411. [DOI] [PubMed] [Google Scholar]
- 22.Heym, B., M. Le Moal, L. Armand-Lefevre, and M. H. Nicolas-Chanoine. 2002. Multilocus sequence typing (MLST) shows that the ‘Iberian’ clone of methicillin-resistant Staphylococcus aureus has spread to France and acquired reduced susceptibility to teicoplanin. J. Antimicrob. Chemother. 50:323-329. [DOI] [PubMed] [Google Scholar]
- 23.Jarraud, S., C. Mougel, J. Thioulouse, G. Lina, H. Meugnier, F. Forey, X. Nesme, J. Etienne, and F. Vandenesch. 2002. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun. 70:631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarraud, S., G. Cozon, F. Vandenesch, M. Bes, J. Etienne, and G. Lina. 1999. Involvement of enterotoxins G and I in staphylococcal toxic shock syndrome and staphylococcal scarlet fever. J. Clin. Microbiol. 37:2446-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones, T. F., M. E. Kellum, S. S. Porter, M. Bell, and W. Schaffner. 2002. An outbreak of community-acquired foodborne illness caused by methicillin-resistant Staphylococcus aureus. Emerg. Infect. Dis. 8:82-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kikuchi, K., N. Takahashi, C. Piao, K. Totsuka, H. Nishida, and T. Uchiyama. 2003. Molecular epidemiology of methicillin-resistant Staphylococcus aureus strains causing neonatal toxic shock syndrome-like exanthematous disease in neonatal and perinatal wards. J. Clin. Microbiol. 41:3001-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lelievre, H., G. Lina, M. E. Jones, C. Olive, F. Forey, M. Roussel-Delvallez, M. H. Nicolas-Chanoine, C. M. Bebear, V. Jarlier, A. Andremont, F. Vandenesch, and J. Etienne. 1999. Emergence and spread in French hospitals of methicillin-resistant Staphylococcus aureus with increasing susceptibility to gentamicin and other antibiotics. J. Clin. Microbiol. 37:3452-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemaitre, N., W. Sougakoff, A. Masmoudi, M. H. Fievet, R. Bismuth, and V. Jarlier. 1998. Characterization of gentamicin-susceptible strains of methicillin-resistant Staphylococcus aureus involved in nosocomial spread. J. Clin. Microbiol. 36:81-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyashita, T., Y. Shimamoto, H. Nishiya, Y. Koshibu, H. Sugiyama, Y. Ono, T. Satoh, H. Haraoka, J. Nakano, K. Ohta, T. Sato, N. Morinaga, and M. Noda. 2002. Destructive pulmonary embolism in a patient with community-acquired staphylococcal bacteremia. J. Infect. Chemother. 8:99-102. [DOI] [PubMed] [Google Scholar]
- 30.Murchan, S., M. E. Kaufmann, A. Deplano, R. de Ryck, M. Struelens, C. E. Zinn, V. Fussing, S. Salmenlinna, J. Vuopio-Varkila, N. El Solh, C. Cuny, W. Witte, P. T. Tassios, N. Legakis, W. van Leeuwen, A. van Belkum, A. Vindel, I. Laconcha, J. Garaizar, S. Haeggman, B. Olsson-Liljequist, U. Ransjo, G. Coombes, and B. Cookson. 2003. Harmonization of pulsed-field gel electrophoresis protocols for epidemiological typing of strains of methicillin-resistant Staphylococcus aureus: a single approach developed by consensus in 10 European laboratories and its application for tracing the spread of related strains. J. Clin. Microbiol. 41:1574-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musser, J. M., P. M. Schlievert, A. W. Chow, P. Ewan, B. N. Kreiswirth, V. T. Rosdahl, A. S. Naidu, W. Witte, and R. K. Selander. 1990. A single clone of Staphylococcus aureus causes the majority of cases of toxic shock syndrome. Proc. Natl. Acad. Sci. USA 87:225-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Brien, F. G., J. W. Pearman, M. Gracey, T. V. Riley, and W. B. Grubb. 1999. Community strain of methicillin-resistant Staphylococcus aureus involved in a hospital outbreak. J. Clin. Microbiol. 37:2858-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliveira, C., and H. de Lencastre. 2002. Multiplex PCR strategy for rapid identification of structural types and variants of the mes element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 46:2155-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okuma, K., K. Iwakawa, J. D. Turnidge, W. B. Grubb, J. M. Bell, F. G. O'Brien, G. W. Coombs, J. W. Pearman, F. C. Tenover, M. Kapi, C. Tiensasitorn, T. Ito, and K. Hiramatsu. 2002. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J. Clin. Microbiol. 40:4289-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peacock, S., C. Moore, A. Justice, M. Kantzanou, L. Story, K. Mackie, G. O'Neill, and N. Day. 2002. Virulent combinations of adhesins and toxin genes in natural populations of Staphylococcus aureus. Infect. Immun. 70:4987-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pittet, D., and R. P. Wenzel. 1995. Nosocomial bloodstream infections. Secular trends in rates, mortality, and contribution to total hospital deaths. Arch. Intern. Med. 155:1177-1184. [DOI] [PubMed] [Google Scholar]
- 37.Saiman, L., M. O'Keefe, P. L. Graham III, F. Wu, B. Said-Salim, B. Kreiswirth, A. LaSala, P. M. Schlievert, and P. Della-Latta. 2003. Hospital transmission of community-acquired methicillin-resistant Staphylococcus aureus among postpartum women. Clin. Infect. Dis. 37:1313-1319. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi, N. 2003. Neonatal toxic shock syndrome-like exanthematous disease (NTED). Pediatr. Int. 45:233-237. [DOI] [PubMed] [Google Scholar]
- 39.van der Mee-Marquet, N., G. Lina, R. Quentin, H. Yaouanc-Lapalle, C. Fievre, N. Takahashi, and J. Etienne. 2003. Staphylococcal exanthematous disease in a newborn due to a virulent methicillin-resistant Staphylococcus aureus strain containing the TSST-1 gene in Europe: an alert for neonatologists. J. Clin. Microbiol. 41:4883-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wannet, W. 2002. Spread of an MRSA clone with heteroresistance to oxacillin in the Netherlands. Euro Surveill. 7:73-74. [DOI] [PubMed] [Google Scholar]
- 41.Witte, W., C. Braulke, C. Cuny, D. Heuck, and M. Kresken. 2001. Changing pattern of antibiotic resistance in methicillin-resistant Staphylococcus aureus from German hospitals. Infect. Control Hosp. Epidemiol. 22:683-686. [DOI] [PubMed] [Google Scholar]
- 42.Witte, W., C. Braulke, D. Heuck, and C. Cuny. 2000. Methicillin-resistant Staphylococcus aureus in German hospitals develop narrower patterns of antimicrobial resistance. Euro Surveill. 5:31-34. [DOI] [PubMed] [Google Scholar]