Abstract
In Hungary the incidence of tuberculosis among the homeless population was 676 per 100,000 in 2002. Sixty-nine percent (140 patients) of all homeless tuberculosis patients were notified in Budapest (the capital). Therefore, a retrospective study that included 66 homeless tuberculosis patients notified in Budapest in 2002 was conducted to determine the rate of recent transmission of the disease and medical risk factors and to identify transmission pathways by means of conventional and molecular epidemiologic methods. IS6110 DNA fingerprinting revealed that 71.2% of the isolates could be clustered. Thirty-four (51.5%) patients belonged to five major clusters (size, from 4 to 11 individuals), and 13 (19.7%) belonged to six smaller clusters. Additional analysis of patient records found that 2 (18%) of the 11 patients in cluster A, 3 (37.5%) of the 8 patients in cluster B, and 2 (33%) of the 6 patients in cluster C were residents of the same three homeless shelters during the diagnosis of tuberculosis. Review of the database of the National Tuberculosis Surveillance Center (NTSC) revealed that 21.2% of the cases have not been reported to the NTSC. These findings indicate that the screening and treatment of tuberculosis among the homeless need to be strengthened and also warrant the review of environmental control steps in public shelters. Improvement of adherence of clinicians to surveillance reporting regulations is also necessary.
In Hungary the incidence of tuberculosis has been gradually decreasing since 1996 (6). The incidence of the disease was 42.6 cases per 100,000 people (4,278 cases) in 1996 and 29.6 cases per 100,000 people (3,007 cases) in 2002 (6, 9, 13). However, tuberculosis notification rates by county showed significant geographic differences in particular parts of the country (13). In 2002 the highest notification rates, representing 53% of all reported cases (1,594 patients) were observed in Budapest and the capital region and in three northeastern counties (13). The higher incidence in the eastern counties reflects the influence of seasonal workers from the neighboring countries with high tuberculosis incidence and the poorer socioeconomic conditions of this region. In Budapest one of the most important tuberculosis-related factors is homelessness. Although the absolute number of notified homeless tuberculosis patients in the country was not very high in 2002 (203 patients, 6.8% of notified cases) the incidence of the disease in this at-risk population (an estimated 30,000 adult Hungarians being homeless) was 676 cases per 100,000 people (4, 13). Sixty-nine percent (140 patients) of all homeless tuberculosis patients were notified in Budapest, representing 21% of all cases detected in the capital (13).
Based on these epidemiologic findings, a retrospective population-based study was organized to determine the rate of tuberculosis cases due to recent transmission and medical risk factors related to tuberculosis and to identify the pathways of transmission among homeless tuberculosis patients notified in Budapest in 2002 by means of conventional and molecular epidemiologic methods.
Conventional epidemiologic data were obtained from the database of the National Tuberculosis Surveillance Center (NTSC). The NTSC database contains information on the tuberculosis patients concerning history of Mycobacterium bovis BCG vaccination, results of contact investigations, extrinsic and intrinsic risk factors (being a resident, foreign born, immigrant, or homeless; having close contact with patients with tuberculosis; being a health care worker or resident of a congregate setting; or having medical conditions known to increase the risk for tuberculosis, e.g., alcohol or drug abuse, human immunodeficiency virus [HIV] infection, steroid or other immunosuppressive therapy, diabetes), acid-fast smear and culture results, and drug susceptibility test results. Risk factors are listed on the notification form, and patients are asked about each to determine which factors are relevant to them. In addition, physicians are required to test all patients for these risk factors and confirm their presence or absence by adequate diagnostic methods (9). The NTSC requires reporting on its standard form by the physician, hospital, and laboratory of all newly diagnosed and reactivated tuberculosis cases to the 160 regional respiratory medicine dispensaries within a week after the diagnosis or the start of antituberculosis treatment. The regional institutes send the data to the district chest clinics for primary care and/or to the NTSC. The district chest clinics enter the data into their local surveillance databases, and at the end of each month, an update is sent to the NTSC (9).
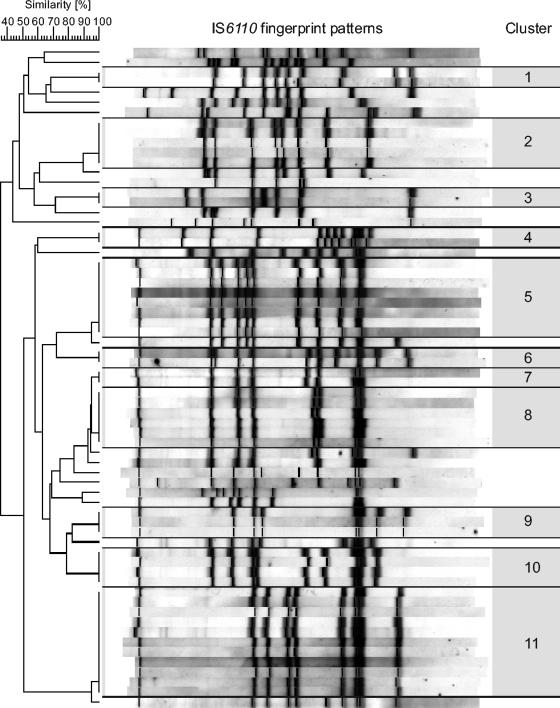
DNA fingerprinting using the insertion sequence IS6110 as a probe has become the internationally standardized method for comparison of Mycobacterium tuberculosis complex isolates at the strain level in epidemiologic studies. Previous IS6110 fingerprinting studies have shown that polymorphism of IS6110 restriction fragment length patterns among unrelated isolates is high, while epidemiologically linked strains show identical fingerprint patterns. Therefore, detection of identical M. tuberculosis fingerprint patterns likely indicates recent disease transmission. Extraction of DNA from mycobacterial strains and IS6110 DNA fingerprinting were performed in line with a standardized protocol as described previously (5, 12, 15). The IS6110 fingerprint patterns of the examined strains were analyzed using the Bionumerics version 2.5 software (Applied Maths, Kortrijk, Belgium) as described previously (5, 12, 15). Clusters were defined as groups of patients with M. tuberculosis strains showing identical restriction fragment length polymorphism patterns (same number of IS6110 bands at identical positions [position tolerance, 1.3%]). Clusters were considered to have arisen from recent transmission, and the cluster rate was used as a measure of the amount of recent transmission in the study population.
All mycobacterial strains were isolated from homeless patients in the Hungarian Reference Laboratory for Mycobacteria at Koranyi Institute for Tuberculosis and Respiratory Medicine during 2002. The 66 isolates of 66 newly diagnosed patients were representing 47.1% of all homeless patients diagnosed in Budapest during the study period (13). All cultures were identified as M. tuberculosis complex by means of the AccuProbe culture identification test (Gen-Probe Inc., San Diego, Calif.) and conventional biochemical tests (8, 14). Susceptibility testing of all 66 isolates for isoniazid (INH), rifampin (RMP), pyrazinamid (PZA), ethambutol (EMB), and streptomycin (STR) was carried out by the proportion method on Löwenstein-Jensen medium as described by Canetti et al. (3). The critical concentrations of INH, RMP, PZA, EMB, and STR were 0.2, 40, 200, 1.0, and 10 μg/ml, respectively. RMP resistance-associated mutations of the 81-bp core region of the rpoB gene were also determined by applying the commercially available, PCR-based reverse hybridization line probe rapid test (Inno-LiPA Rif. TB Test; Innogenetics N.V., Ghent, Belgium) as described elsewhere (2).
The characteristics of the patients are summarized in Table 1. In the study population there were 61 men (92.4%) and 5 women (7.6%), and the male-to-female ratio was 12.2:1. The patients were from 31 to 73 years old, and the mean age was 47.8 years (men, 48.2 years; women, 44.4 years) (Table 1). All patients were born in Hungary and were HIV negative. Medical risk factors could be identified in 23 (34.8%) patients, of which 22 (33.3%) were alcohol abusers and 2 (3.0%) had diabetes mellitus (Table 1). Of the 66 isolates, 10 (15.2%) were resistant to at least one antituberculosis drug (Table 1). Four (6.0%) patients were infected by polyresistant strains, and two (3.0%) of these isolates showed resistance to both INH and RMP and thus were classified as multidrug resistant (Table 1). The Inno-LiPA Rif. TB Test analysis of the three RMP-resistant strains revealed the common mutation S531L and two rare mutation patterns (ΔS1+ΔS4 with R4a positivity and ΔS4). As regards the DNA fingerprinting results, 47 (71.2%) isolates could be classified into 11 different clusters (Fig. 1). Thirty-four (51.5%) patients belonged to five major clusters (size, from 4 to 11 individuals), and 13 (19.7%) belonged to six smaller clusters with three or fewer individuals (Fig. 1). None of the patients were infected by strains of the Beijing genotype.
TABLE 1.
Characteristics of the 66 homeless tuberculosis patients
| Characteristic | Value for group |
|---|---|
| Male | 61 (92.4%) |
| Female | 5 (7.6%) |
| Male/female | 12.2/1 |
| Mean age (yr) | |
| Male | 48.2 |
| Female | 44.4 |
| All | 47.8 |
| Age distribution | |
| 30-59 yr | 59 (89.3%) |
| 60+ yr | 7 (10.7%) |
| Medical risk factors | |
| Alcohol abuse | 22 (33.3%) |
| Diabetes mellitus | 2 (3.0%) |
| No. and proportion of resistant cases | |
| Resistant to any drug | 10 (15.2%) |
| Polyresistanta | 4 (6.0%) |
| INH + RMP + PZA + EMB | 1 (1.5%) |
| INH + RMP + PZA | 1 (1.5%) |
| INH + EMB | 1 (1.5%) |
| EMB + STR | 1 (1.5%) |
| RMP monoresistant | 1 (1.5%) |
| EMB monoresistant | 1 (1.5%) |
| STR monoresistant | 4 (6.0%) |
Including cases that are multidrug resistant (resistant to both INH and RMP).
FIG. 1.
IS6110 fingerprint patterns and clusters of the 66 homeless tuberculosis patients.
A retrospective review of the database of the NTSC, the National Reference Laboratory for Mycobacteria, and patient charts revealed that 14 (21.2%) cases had not been reported to the NTSC by the responsible clinician. One of these patients harbored a drug-resistant strain. Only 16 (24.2%) patients were cured, while 16 (24.2%) patients disappeared and 20 (30.3%) patients were still on treatment due to therapeutic failure or default. Additional analysis of the records found that 2 (18%) of the 11 patients in cluster A, 3 (37.5%) of the 8 patients in cluster B, and 2 (33%) of the 6 patients in cluster C were residents of the same three homeless shelters during the diagnosis of tuberculosis.
The incidence (676 cases per 100,000 people) of tuberculosis among the homeless in Hungary is alarming, since none of the patients was known to be HIV positive. In comparison, the rate of the disease among the homeless was 221 cases per 100,000 people in the highest-incidence district in New York City and 270 cases per 100,000 people in San Francisco in the peak year (1992) of the tuberculosis resurgence in the United States, although HIV-related tuberculosis was also a problem by that time (7, 10). The high rate (69.7%) of clustered cases indicates that the vast majority of the tuberculosis in the homeless resulted from recent transmission (Fig. 1). The rate of clustered cases was similar to previous findings on homeless tuberculosis patients by Barnes et al. in Los Angeles and Moss et al. in San Francisco (1, 10). Traditional contact tracing of the homeless is extremely complicated since these individuals are mobile and often uncooperative. However, in our retrospective study DNA fingerprinting in conjunction with conventional epidemiologic methods was able to reveal that 14.9% of all clustered patients were staying in the same three homeless shelters at the time of diagnosing the disease. It is also noteworthy that the disease was cured in only 24.2% of the patients. These findings indicate that the screening and treatment of tuberculosis among homeless need to be strengthened, and they also warrant the review of environmental control steps in public shelters. At present, treatment of tuberculosis in Hungary is self administered; thus, the number of patients who actually complete treatment is unknown (9). The introduction of directly observed therapy programs is indispensable to reduce tuberculosis in the homeless. Since the most common medical risk factor for tuberculosis of these patients was alcohol abuse, the alcohol addiction counseling of these patients should also be organized.
In a previous study that was examining a significant number of resistant M. tuberculosis strains, it was demonstrated that in Hungary less common or novel mutations of the rpoB gene occur more frequently than in other countries in the world (2). Similar to those results, the occurrence of two rare mutations among the three RMP-resistant isolates in the present study may also suggest the local generation of RMP-resistant clones due to inadequate treatment or patient compliance. However, this needs to be confirmed with a larger number of RMP-resistant homeless patients from Budapest.
One of the most troubling findings of this study was that 21.2% of the cases were not reported to the NTSC. This nonadherence of clinicians to national regulations could contribute to the increase of treatment failures, relapses, development of drug resistance, and transmission of tuberculosis.
At present, tuberculosis surveillance in Hungary is based only on conventional epidemiologic methods (9). In line with previous observations, this first Hungarian molecular epidemiologic study indicates that the routine application of DNA fingerprinting would be desirable to determine tuberculosis transmission rates and links in at-risk populations, especially in drug-resistant cases (11).
Acknowledgments
We thank I. Radzio, T. Ubben, and P. Vock for their excellent technical assistance.
Á. Somoskövi and J. Mester were supported by grants 1D43TW00915 and 2D43TW00233 from the Fogarty International Center, National Institutes of Health, Bethesda, Md.
REFERENCES
- 1.Barnes, P. F., H. el-Hajj, S. Preston-Martin, M. D. Cave, B. E. Jones, M. Otaya, J. Pogoda, and K. D. Eisenach. 1996. Transmission of tuberculosis among the urban homeless. JAMA 275:305-307. [PubMed] [Google Scholar]
- 2.Bartfai, Z., A. Somoskovi, C. Kodmon, N. Szabo, E. Puskas, L. Kosztolanyi, E. Farago, J. Mester, L. M. Parsons, and M. Salfinger. 2001. Molecular characterization of rifampin-resistant isolates of Mycobacterium tuberculosis from Hungary by DNA sequencing and the line probe assay. J. Clin. Microbiol. 39:3736-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canetti, G., W. Fox, A. Khomenko, N. Mahler, N. K. Menon, D. A. Mitchison, N. Rist, and N. A. Smeley. 1969. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull. W. H. O. 41:21-43. [PMC free article] [PubMed] [Google Scholar]
- 4.David, B., J. Oross, and M. Vecsei. 1998. Homelessness and tuberculosis. Report of the Soros Foundation Health System Development Programme. Soros Foundation, Budapest, Hungary.
- 5.Diel, R., S. Schneider, K. Meywald-Walter, C. M. Ruf, S. Rusch-Gerdes, and S. Niemann. 2002. Epidemiology of tuberculosis in Hamburg, Germany: long-term population-based analysis applying classical and molecular epidemiological techniques. J. Clin. Microbiol. 40:532-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EuroTB (inVS/KNCV) and the National Coordinators for Tuberculosis Surveillance in the WHO European Region. 2003. Surveillance of tuberculosis cases in Europe. Report on tuberculosis cases notified in 2001. Institut de Veille Sanitaire, Saint-Maurice, France.
- 7.Frieden, T. R. 1994. Tuberculosis control and social change. Am. J. Public Health 84:1721-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kent, P. T., and G. P. Kubica. 1985. Public health mycobacteriology. A guide for a level III laboratory. Centers for Disease Control, Atlanta, Ga.
- 9.Mester, J., I. Vadasz, G. Pataki, L. Parsons, T. Fodor, M. Salfinger, and A. Somoskovi. 2002. Analysis of tuberculosis surveillance in Hungary in 2000. Int. J. Tuberc. Lung Dis. 6:966-973. [PubMed] [Google Scholar]
- 10.Moss, A. R., J. A. Hahn, J. P. Tulsky, C. L. Daley, P. M. Small, and P. C. Hopewell. 2000. Tuberculosis in the homeless. A prospective study. Am. J. Respir. Crit. Care Med. 162:460-464. [DOI] [PubMed] [Google Scholar]
- 11.Niemann, S., E. Richter, S. Rusch-Gerdes, H. Thielen, and H. Heykes-Uden. 1999. Outbreak of rifampin and streptomycin-resistant tuberculosis among homeless in Germany. Int. J. Tuberc. Lung Dis. 3:1146-1147. [PubMed] [Google Scholar]
- 12.Niemann, S., S. Rusch-Gerdes, and E. Richter. 1997. IS6110 fingerprinting of drug-resistant Mycobacterium tuberculosis strains isolated in Germany during 1995. J. Clin. Microbiol. 35:3015-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pataki, G., Á. Megyesi, and I. Fehér. 2003. Annual report of the Hungarian medical care centers in respiratory medicine, 2002. National Korányi Institute for Tuberculosis and Respiratory Medicine, Budapest, Hungary.
- 14.Somoskovi, A., J. E. Hotaling, M. Fitzgerald, V. Jonas, D. Stasik, L. M. Parsons, and M. Salfinger. 2000. False-positive results for Mycobacterium celatum with the AccuProbe Mycobacterium tuberculosis complex assay. J. Clin. Microbiol. 38:2743-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Embden, J. D., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, et al. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]