Abstract
The circulating levels of soluble tumor necrosis factor receptor-1 (sTNF-R1) and sTNF-R2 are altered in numerous diseases, including several types of cancer. Correlations with the risk of progression in some cancers, as well as systemic manifestations of the disease and therapeutic side-effects, have been described. However, there is very little information on the levels of these soluble receptors in glioblastoma (GBM). Here, we report on an exploratory retrospective study of the levels of sTNF-Rs in the vascular circulation of patients with GBM. Banked samples were obtained from 112 GBM patients (66 untreated, newly-diagnosed patients and 46 with recurrent disease) from two institutions. The levels of sTNF-R1 in the plasma were significantly lower in patients with newly-diagnosed or recurrent GBM than apparently healthy individuals and correlated with the intensity of expression of TNF-R1 on the tumor-associated endothelial cells (ECs) in the corresponding biopsies. Elevated levels of sTNF-R1 in patients with recurrent, but not newly-diagnosed GBM, were significantly associated with a shorter survival, independent of age (p=0.02) or steroid medication. In contrast, the levels of circulating sTNF-R2 were significantly higher in recurrent GBM than healthy individuals and there was no significant correlation with expression of TNF-R2 on the tumor-associated ECs or survival time. The results indicate that larger, prospective studies are warranted to determine the predictive value of the levels of sTNF-R1 in patients with recurrent GBM and the factors that regulate the levels of sTNF-Rs in the circulation in GBM patients.
Keywords: Soluble TNF-R1, soluble TNF-R2, glioblastoma, age, survival, endothelial cell
Introduction
Soluble tumor necrosis factor receptor-1 (sTNF-R1) and sTNF-R2 are found in the vascular circulation and can modulate the activity of tumor necrosis factor α (TNFα) in a physiologically relevant manner [1]. The levels of sTNF-R1 and sTNF-R2 in the vascular circulation are increased significantly with age and altered in obese individuals [2–4] and chronic inflammatory conditions and diseases [5–8] as well as several cancers [9–15]. Although correlations with the clinical stage, risk of progression, and the risk of developing certain cancers have been described [9–16] there is very little information on the levels of these soluble receptors in glioblastoma and their potential clinical relevance in GBM. To our knowledge, one previous study, in which the levels of circulating sTNF-R1 and sTNF-R2 were measured in 17 patients with recurrent GBM, found higher levels of sTNF-R2 but no significant difference in sTNF-R1 levels as compared to normal controls [17].
Although TNF-R1 and TNF-R2 are closely related they differ in terms of their cellular distribution and effects on cell function [reviewed in 5, 6, 18]. TNF-R1 is expressed by almost all nucleated cells and TNF-R2 is expressed primarily by hematopoietic cells (including macrophages) and endothelial cells [5, 6, 18]. Neither the origins of the circulating sTNF-R1 and sTNF-R2 nor the factors that regulate their levels have been established. The possibility that the tumor can affect this systemic immunoregulatory mechanism and thus affect not only the local tumor microenvironment but distal sites is of considerable interest [19–21]. Our recent finding that the tumor-associated endothelial cells (ECs) in GBM express elevated levels of TNF-R1 and TNF-R2 as compared to ECs in normal brain [22] suggested a potential link.
In this exploratory retrospective study, we measured the levels of circulating sTNF-R1 and sTNF-R2 in blood samples from patients with newly-diagnosed GBM or recurrent GBM and apparently healthy controls, determined whether such differences were physiologically relevant in terms of correlation with survival of the patients and determined whether the levels of the circulating sTNFRs correlated with their expression on the tumor-associated ECs.
Patients and Methods
Study samples
Blood samples were obtained from blood banks at the Cleveland Clinic (CCF), University of Alabama at Birmingham (UAB), or University Hospital-Case Western Reserve University (UH-CWRU). The blood samples had been collected either from patients undergoing surgery for resection of GBM at the respective institutions or apparently healthy controls. The studies were carried out after review by, and in accordance with the rules and regulations of, the Institutional Review Board (IRB) of each institution. All subjects signed a written informed consent permitting the research represented here. Clinical information was obtained by review of the clinical records by a neuro-oncologist (MSA). All of the patients had GBM (WHO Grade IV astrocytoma) as diagnosed histopathologically according to the World Health Organization classification guidelines [23].
For this study, blood samples from a total of 112 patients (66 newly-diagnosed GBM and 46 recurrent GBM) and 58 apparently healthy controls were used. In five patients, samples were collected both when newly-diagnosed and at recurrence. Based on the results of a pilot study using the CCF1 cohort (45 patients), the number of patients was expanded through inclusion of a second cohort of CCF patients (CCF2) as well as the UAB cohort (20 patients: all newly-diagnosed) according to the study design shown in Figure 1. EDTA-plasma samples from a UH-CWRU cohort were obtained and analyzed solely for the purpose of exploring the impact of molecular subtype of GBM on the levels of circulating sTNF-R1 and sTNF-R2.
Figure 1. Study Flow Diagram.
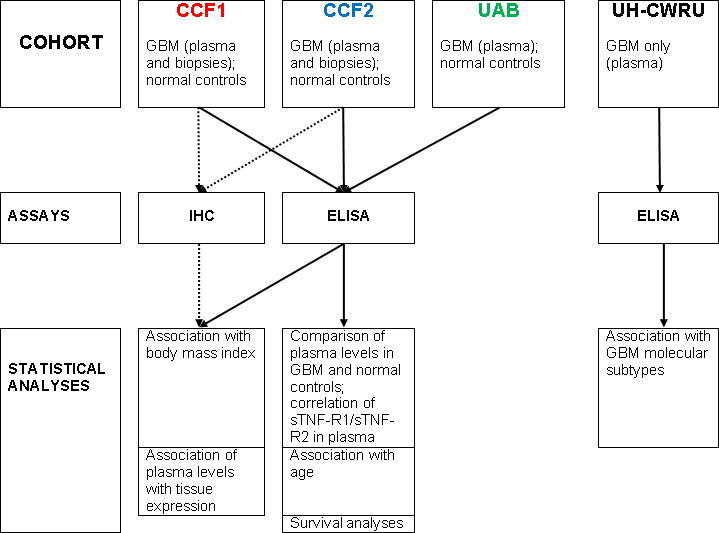
Depiction of the utilization of samples from each cohort in analysis by ELISA or IHC and subsequent statistical analysis.
The blood samples were collected in a standardized manner. Blood was collected into tubes with EDTA, placed on ice and processed within 2 h. Aliquots of plasma were snap frozen in liquid N2 and stored in the blood banks at −80°C. Blood samples from patients were collected at surgery. It has been reported that sTNF-R1/sTNF-R2 levels are stable under these conditions [24–26].
ELISA
ELISA kits were used to measure the levels of sTNF-R1 and sTNF-R2 (RayBiotech, Inc., Norcross, GA) and IL-10 (BD Biosciences, San Diego, CA). Concentrations were interpolated from standard curves prepared using standards analyzed in duplicate or triplicate on each plate. All samples were analyzed at least in duplicate.
Immunohistochemistry (IHC)
Formalin-fixed and paraffin-embedded blocks/tissue sections (3–4 sections/case) were stained with rabbit anti-TNF-R1, rabbit anti-TNF-R2, or mouse mAb anti-von Willebrand factor (vWf) as described previously [22] and in the Supplementary Methods. Staining in ECs in more than 5% of blood vessels was the threshold for positivity.
Survival Analysis
Overall survival was defined as time from diagnosis until date of death. Patients alive at the time of study were right censored at their date of last clinical follow-up.
Statistical Analysis
All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). The statistical design is outlined in the Supplementary Methods. P values < 0.05 were considered statistically significant.
Results
Demographic characteristics of the patients and healthy controls are shown in Table 1 and the clinicopathological characteristics and treatment regimens for the patients are shown in Supplemental Table 1A&B. At the time these patient samples were collected, routine analysis of IDH1 and MGMT status and the molecular subtype of GBM were not the standard-of-care.
Table 1.
Characteristics of the Study Population
| N | Age* (years) | Gender | ||||
|---|---|---|---|---|---|---|
| Median | Range | Male | Female | Unidentified | ||
| All GBM patients | 112 | 54 | 24–80 | 72 (64%) | 40 (36%) | |
| All controls | 58 | 49 | 24–79 | 30 (53%) | 27 (47%) | 1 |
| Newly-diagnosed | 66 | 55 | 27–80 | 41(62%) | 25 (38%) | |
| Recurrent | 46 | 53 | 24–80 | 31 (67%) | 15 (33%) | |
| CCF cohort 1 | 45 | 54 | 24–80 | 27 (60%) | 18 (40%) | |
| CCF cohort 1 controls | 14 | 39.5 | 26–63 | 9 (64%) | 4 (29%) | 1 |
| CCF cohort 2 | 47 | 52 | 27–80 | 35 (74%) | 12 (26%) | |
| CCF cohort 2 controls | 20 | 57 | 24–79 | 10 (50%) | 10 (50%) | |
| UAB cohort | 20 | 56.5 | 44–64 | 10 (50%) | 10 (50%) | |
| UAB cohort controls | 24 | 48.5 | 40–69 | 11(46%) | 13 (54%) | |
Age at sample collection
Levels of sTNF-R1 in the vascular circulation of GBM patients
The unadjusted values for the median, mean±SD, and range for sTNF-R1 and sTNF-R2 levels are provided in Supplemental Table 2. The mean levels of sTNF-R1 were significantly lower in the GBM patients than normal controls (cohort adjusted means 403.3c000B1;68.3 versus 1038±91.8, mean±SEM; p value<0.0001, Linear Regression adjusting for cohort) (Fig. 2a). This held true when the sTNF-R1 levels were compared to the controls after adjusting for cohort and age, or after eliminating the outlier in the controls (7877 pg/ml) (p value<0.001 and p value<0.001, respectively). The mean sTNF-R1 levels also were lower than healthy controls in newly-diagnosed (p value=0.0005) (Fig. 2b) and recurrent GBM (p value<0.0001) (Fig. 2c). There was not a significant difference in the sTNF-R1 levels between newly-diagnosed (520 pg/ml) and recurrent GBM (518 pg/ml) (p value=0.97; Linear regression adjusting for cohort). As a control, we measured the levels of circulating IL-10 and, consistent with the literature [27, 28], found a higher mean level of IL-10 in GBM patients (CCF1 and UAB cohorts) than the corresponding normal controls (cohort adjusted means 21.7 pg/ml ± 4.27 versus 7.76±5.28, mean±SEM, respectively) (p value=0.047, Linear Regression adjusting for cohort).
Figure 2. Analysis of circulating sTNF-R1 in patients with GBM.
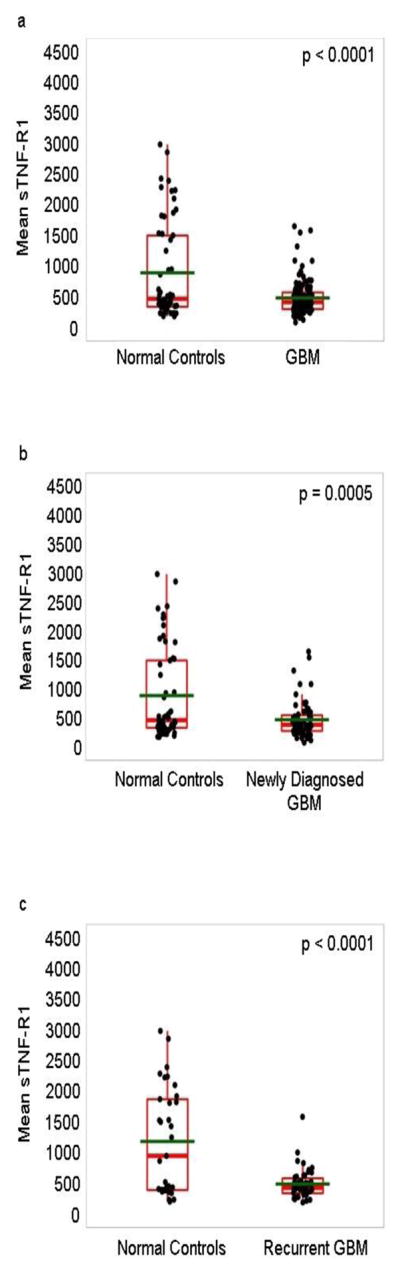
The levels of sTNF-R1 in the vascular circulation of patients undergoing surgery for GBM at initial diagnosis and recurrence (n=112; CCF1, CCF2 and UAB cohorts), for GBM at initial diagnosis (n=66; CCF1, CCF2 and UAB cohorts), or for recurrent GBM (n=46; CCF1 and CCF2 cohorts), and the normal controls (n=58; 14 for CCF1 cohort, 20 for CCF2 cohort, and 24 for UAB cohort) were determined by ELISA analysis. (Panel a) Plasma levels of sTNF-R1 in the combined group of GBM patients. (Panel b) Plasma levels of sTNF-R1 in the patients with newly diagnosed GBM. (Panel c) Plasma levels of sTNF-R1 in the patients with recurrent GBM. The data are plotted as Box and Whisker plots, green line denotes the mean. One outlier for sTNF-R1 in the control subjects (7877 pg/ml) was removed from panels a, b and c for graphing purposes only. Statistical analysis: Linear Regression adjusting for cohort.
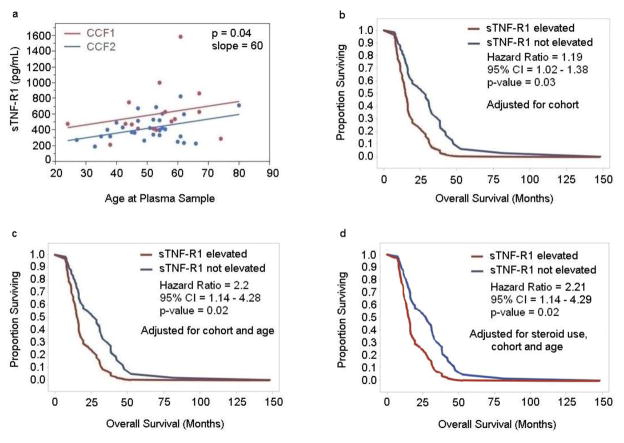
We did not find a significant difference in the sTNFR1 levels in patients who received steroid medications as compared to those who did not (Linear regression analysis). Nor did we find significant correlations between the levels of sTNF-R1 and gender in the entire group of GBM patients, the newly-diagnosed patients, or the patients with recurrent GBM (Linear correlation analysis). We did, however, find a significant correlation between the levels of sTNF-R1 and age at collection of blood samples in patients with newly-diagnosed GBM (p value=0.01, Linear regression analysis after adjusting for cohort) (SFig. 1) and patients with recurrent GBM (p value=0.04) (Fig. 3a). For every 10 year increase in age, the level of sTNF-R1 increased 90 pg/ml (Slope=90, 95% CI=2 to 16) in patients with newly-diagnosed GBM and 60 pg/ml (slope=60, 95% CI=0.5 to 12) in patients with recurrent GBM.
Figure 3. Analysis of correlation of circulating sTNF-R1 in patients with recurrent GBM and overall survival independent of age and steroid use.
(Panel a) The levels of sTNF-R1 in the vascular circulation of patients with recurrent GBM (n=46) were analyzed for an association with age. The correlation was assessed using Linear Regression analysis after adjusting for cohort (p value=0.04). (Panels b-d), The levels of sTNF-R1 in the vascular circulation of patients undergoing surgery for recurrent GBM (n=46; CCF1 and CCF2 cohorts) were determined by ELISA analysis and plotted against their overall survival in months. The correlation with overall survival was assessed using the Cox Proportional Hazards Model adjusting for cohort (p value=0.03) (Panel b), adjusting for cohort and age (p value=0.02) (Panel c), and adjusting for cohort, age and steroid use (Panel d). The data are plotted as Kaplan-Meier survival curves for elevated sTNF-R1 (red line) versus not elevated sTNF-R1 (blue line) (Panels b-d).
It has been reported that the levels of TNF-R1 mRNA are higher in the mesenchymal molecular subtype of GBM than the other molecular subtypes [29]. EDTA-plasma samples from 38 newly-diagnosed GBM patients (median age 66.9 years; range 36.3 to 83.7 years) for whom the molecular subtype of the tumor has been determined by TCGA (10 mesenchymal, 14 classical, 9 proneural, 3 neural, and 2 G-CIMP) were available. On analysis of these samples, we did not find an association between the circulating levels of sTNF-R1 or sTNF-R2 with the mesenchymal, and classical or proneural molecular subtypes of GBM (p value=0.25, and p value=0.60, respectively, Kruskal-Wallis test).
Independent of age, elevated levels of sTNF-R1 are associated with a shorter overall survival time in patients with recurrent GBM
We found that higher levels of sTNF-R1 were associated with a significantly shorter survival of patients with recurrent GBM after adjusting for cohort effect (p value=0.03) (Fig. 3b). The adjusted median survival for the group with elevated levels of sTNF-R1 was 15.2 months (95% CI: 12.6, 22.6), and the adjusted median survival for the group in which the levels of sTNF-R1 were not elevated was 28.7 months (95% CI: 17.9, 38.5) (Fig. 3b). For every 100 pg/ml increase in the level of sTNF-R1, the risk of death increased 19% (HR = 1.19, 95% CI =1.02 – 1.38). We did not, however, find a correlation between the level of sTNF-R1 and overall survival in the patients with newly-diagnosed GBM (p value=0.11, Cox proportional hazards model adjusting for cohort).
As age is associated with a shorter overall survival for GBM patients [30, 31] and as we had found that elevated levels of sTNF-R1 were associated with increasing age in the patients with recurrent GBM (Fig. 3a), the correlation between overall survival and elevated levels of sTNF-R1 in the patients with recurrent GBM was re-analyzed after adjusting for age at collection of blood sample as well as cohort. Higher levels of sTNF-R1 were associated with a significantly shorter overall survival in patients with recurrent GBM after adjusting for age and cohort (p value=0.02) (Fig. 3c) or after adjusting for age, use of steroid therapy and cohort (p value=0.02) (Fig. 3d). Patients with recurrent GBM with an elevated level of sTNF-R1 had a 2.2-fold higher risk of death (HR = 2.2, 95% CI = 1.14 to 4.28).
Levels of sTNF-R2 in the vascular circulation of GBM patients
We did not find a significant difference in the mean levels of circulating sTNF-R2 between healthy controls and the entire group of GBM patients (p value=0.81, Linear Regression adjusting for cohort) (SFig. 2a) or the newly-diagnosed patients (p value=0.66) (SFig. 2b). The levels of circulating sTNF-R2 were significantly higher in the patients with recurrent GBM as compared to normal controls (cohort adjusted means 1156±81.29 versus 774.9±92.61, respectively, mean±SEM) (p value=0.002) (SFig. 2c). The mean level of circulating sTNF-R2 in newly diagnosed GBM patients (1077 pg/ml) was not significantly different from the mean level in recurrent GBM (1134 pg/ml) (p value=0.78). The levels of sTNF-R2 did not correlate with age in the patients with newly-diagnosed (p value=0.88) or recurrent GBM (p value=0.35) or with gender. The levels of sTNF-R2 were significantly lower in the GBM patients who received steroids than those who did not (adjusted mean 920.4 versus 1211.1 respectively, p =0.02) but there was not a significant difference in the levels of sTNF-R2 between patients with recurrent GBM who received steroids and those who did not and no correlation between the levels of sTNF-R2 and the likelihood that they received steroid medications. We did not find a statistically significant association of overall survival with the circulating levels of sTNF-R2 in patients with recurrent GBM (p value=0.06) or newly-diagnosed GBM patients (p value=0.68, Cox proportional hazards model adjusting for cohort).
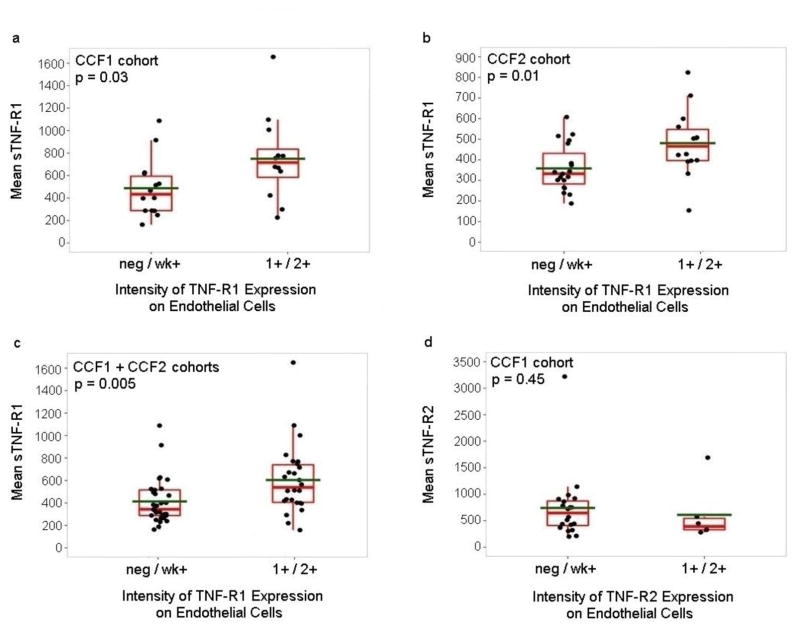
Association of the intensity of TNF-R1 expression on tumor-associated ECs in GBM biopsies with the levels of sTNF-R1 in the vascular circulation
In a previous study, we found significantly higher expression of TNF-R1 and TNF-R2 on the tumor-associated ECs than ECs in normal brain as determined by immunohistochemical analysis of biopsies of 49 patients with GBM and 38 normal brain samples [22]. The study included analysis of biopsies from 26 of the GBM patients in the CCF1 cohort included in the current study (20 newly-diagnosed and 6 recurrent). In the current study, we found that the intensity of TNF-R1 expression on the tumor-associated ECs correlated with the levels of circulating sTNF-R1 in these patients (p value=0.03) (Fig. 4a). Specifically, negative or weak intensity expression of TNF-R1 on the tumor-associated ECs was associated with lower levels of sTNF-R1 in the blood (Fig. 4a). Sufficient biopsy tissue was available for 33 of the 47 patients in the CCF2 cohort (14 newly-diagnosed and 19 recurrent) for immunohistochemical analysis of TNF-R1 and TNF-R2. Similar results were obtained using these samples, with a direct correlation between the intensity of TNF-R1 expression on the tumor-associated ECs and the levels of circulating sTNF-R1 (p value=0.01) (Fig. 4b). When results of the CCF1 and CCF2 cohorts were combined, sTNF-R1 levels were significantly higher when the intensity of TNF-R1 on the tumor-associated ECs in the GBM biopsies was higher (p value=0.005) (Fig. 4c). There was not, however, a significant correlation between the intensity of expression of TNF-R2 on the tumor-associated ECs and the circulating levels of sTNF-R2 in either the original 26 GBM biopsies (p value=0.45) (Fig. 4d) or the 33 biopsies from the CCF2 cohort (p value=0.31, Exact Wilcoxon rank-sum test).
Figure 4. Analysis of association of circulating sTNF-R1 with intensity of expression of the receptor on GBM tumor-associated endothelial cells.
(Panels a & d) The levels of circulating (Panel a) sTNF-R1 and (Panel d) sTNF-R2 in the 26 patients from the CCF1 cohort that had been included in a previous analysis of the expression of the receptors on the tumor-associated ECs in GBM biopsies were determined (Two-sided Exact Wilcoxon Rank-Sum Tests, p values=0.03 and 0.45, respectively). (Panel b) The levels of circulating sTNF-R1 in the 33 patients from the CCF2 cohort are plotted against the intensity of TNF-R1 protein expression on the tumor-associated ECs in their GBM biopsies, and an association determined using a Two-sided Exact Wilcoxon Rank-Sum Test (p value=0.01). (Panel c) The levels of circulating sTNF-R1 in the 59 patients from CCF1 and CCF2 cohorts are plotted against the intensity of TNF-R1 expression on the tumor-associated ECs in their GBM biopsies, and an association determined using Linear Regression analysis after adjusting for cohort (p value=0.005).
Discussion
The results of this exploratory study indicate that the levels of the sTNF-Rs in the vascular circulation are altered in GBM and identify a correlation between higher levels of sTNF-R1 in the vascular circulation in patients with recurrent GBM and a shorter overall survival time independent of age or steroid medication. These results are consistent with the reports that sTNF-R1 levels correlate with tumor grade and invasion and are an independent prognostic factor in colorectal cancer [9], and that the increase in the level of sTNF-R1 was in proportion to the clinical stage of the disease in adenocarcinoma of the cervix [10]. Our current studies also indicate a significant correlation between the levels of TNF-R1 on the tumor-associated ECs and the levels in the circulation suggesting that a tumor-driven process plays a key role in determining the levels of sTNF-R1 in the circulation. If this is the case, it would suggest an additional mechanism through which tumor cells can not only remodel their immediate microenvironment, but act systemically to modify responses at distal sites both within the brain and other organs [19–21].
Caution must be used in interpretation of the roles of specific cytokine interactions in driving GBM, however, due to the complexity and dynamism of the cytokine cascades, the diversity of the cellular targets and the interactions between tumor-driven mechanisms, therapeutic responses and intrinsic patient characteristics. Thus, the current findings do not exclude the potential effects of factors other than the expression on the tumor-associated ECs on the levels of circulating TNF-R1. Indeed, we found an association between the levels of sTNF-R1 and age in patients with GBM as has been reported in healthy individuals [2]. The extensive chemotherapy, radiation and surgery modalities [30, 31] that are used routinely to treat patients with GBM could also affect the levels of the sTNF-Rs [16, 19] in patients with recurrent GBM. However, the current results suggest that the tendency to discount alterations in cytokine levels as solely due to treatment should be avoided as we found that the levels of sTNF-R1 were significantly lower in blood samples obtained from the untreated, newly-diagnosed patients as compared to normal controls and that steroid medication did not affect the levels of sTNF-R1 in recurrent GBM although they did affect the levels of sTNF-R2 in newly-diagnosed GBM. Recently, elevated levels of sTNF-R2 have been with associated with neurocognitive dysfunction in patients with newly-diagnosed breast cancer prior to treatment [20] as well as with fatigue, depression and cognitive impairment presenting as side-effects of chemotherapy and radiation therapy [16, 19].
The current studies do not address the issue of the mechanisms that regulate the generation of the sTNFRs but do suggest differences in their regulation, which is consistent with their differential cell expression and function [reviewed in 5, 6, 18] and may reflect differences in the activities of proteases, including those secreted in the tumor microenvironment [32–36].
The potential mechanisms of action of sTNF-R1 and sTNF-R2 have not been fully elucidated and the significantly lower mean levels of sTNF-R1 in both the newly-diagnosed and recurrent GBM patients is consistent with the profound systemic immunosuppression, involving multiple different cellular and cytokine compartments including high levels of IL-10, which is a hallmark of GBM [37–40]. The sTNF-Rs may reduce the levels of TNFα in the circulation or block its activity [1, 5, 6, 18]. The levels of TNFα protein in the vascular circulation of patients with GBM have been variously reported to be lower [38, 41] and higher [28, 42] than in the vascular circulation of normal controls. On analysis of a subset of our samples (20) from GBM patients and normal controls (14), we did not find a significant difference in the mean levels of circulating TNFα as measured by ELISA (data not shown). TNF-R1 is expressed by glioma tumor cell lines [43, 44] whereas TNF-R2 is expressed by some, but not all glioma cell lines [43] and shedding of both TNF-R1 and TNF-R2 by glioma cell lines has been described [45]. Microrarray analysis indicates overexpression of TNF-R1 in some GBM tumors where RNA was extracted from whole tumors [29, 46]. Locally, in the tumor microenvironment, it has been suggested that sTNF-R1 and sTNF-R2 may act as decoy receptors to inhibit TNFα-induced apoptosis or play a role in inhibiting TNFα-stimulated chemotaxis of macrophages [47].
The correlation between higher levels of circulating sTNF-R1 and a shorter overall survival in recurrent GBM was found to be independent of the potential confounding factors of age and steroid medication in this exploratory study. The analysis of the other potential interactions was limited by the retrospective nature of the study. We did not find a correlation between the molecular subtype and levels of circulating sTNF-R1 in our one cohort of GBM patients for whom molecular subtyping was available. In terms of the potential effects of comorbid conditions, we did not find a significant difference in the sTNF-R levels between patients who had a body mass index indicating obesity and those who did not (data not shown). However, larger prospective studies are required to further investigate the potential effects of alterations in the levels of circulating sTNF receptors in obesity and other potentially co-morbid conditions, including diabetes, inflammatory diseases, and depression [3–8, 48, 49], which may impact survival or quality of life in GBM [16, 19, 20].
Supplementary Material
Acknowledgments
This work was supported by NIH grants CA512883 and CA175120 (CLG) and HL103553 and HL085324 (MAO), and the Rosa Ella Burkhardt Brain Tumor and Neuro-Oncology Center of the Cleveland Clinic. We thank the Melvin Burkhardt chair in neurosurgical oncology and the Karen Colina Wilson research endowment within the Brain Tumor and Neuro-Oncology Center at the Cleveland Clinic for support (to RJW). We thank Dr. Fiona Hunter for critical review of the manuscript, and Jessica Ray for assistance with organization of the samples.
Footnotes
Disclosure: The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–4. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 2.Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, et al. The origins of age-related proinflammatory state. Blood. 2005;105:2294–9. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamska A, Nikolajuk A, Karczewska-Kupczewska M, Kowalska I, Otziomek E, Gorska M, Straczkowski M. Relationships between serum adiponectin and soluble TNFα receptors and glucose and lipid oxidation in lean and obese subjects. Acta Diabetol. 2012;49:17–24. doi: 10.1007/s00592-010-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arismendi E, Rivas E, Agusti A, Rios J, Barreiro E, Vidal J, Rodriguez-Roisin R. The systemic inflammome of severe obesity before and after bariatric surgery. PLoS ONE. 2014;9:e107859. doi: 10.1371/journal.pone.0107859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119:651–665. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner D, Blaser H, Mak TW. Regulation of tumor necrosis factor signaling: live or let die. Nat Rev Immunol. 2015;15:362–74. doi: 10.1038/nri3834. [DOI] [PubMed] [Google Scholar]
- 7.Carrlsson AC, Nordquist L, Larsson TE, Carrero J-J, Larsson A, Lind L, Arnlov J. Soluble tumor necrosis factor receptor 1 is associated with glomerular filtration rate progression and incidence of chronic kidney disease in two community-based cohorts of elderly individuals. Cardiorenal Med. 2015;5:278–288. doi: 10.1159/000435863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma S, Purohit S, Sharma A, Hopkins D, Steed L, Bode B, Anderson SW, Caldwell R, She J-X. Elevated serum levels of soluble TNF receptors and adhesion molecules are associated with diabetic retinopathy in patients with type-1 diabetes. Mediators of Inflammation. 2015:2015. doi: 10.1155/2015/279393. Article ID 279393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaminska J, Nowacki MP, Kowalska M, Rysinska A, Chwalinski M, Fuksiewicz M, Michalski W, Chechlinska M. Clinical significance of serum cytokine measurements in untreated colorectal cancer patients: soluble tumor necrosis factor receptor type I--an independent prognostic factor. Tumour Biol. 2005;26:186–94. doi: 10.1159/000086951. [DOI] [PubMed] [Google Scholar]
- 10.Kotowicz B, Kaminska J, Fuksiewicz M, Kowalska M, Jonska-Gmyrek J, Gawrychowski K, Sobotkowski J, Skrzypczak M, Starzewski J, Bidzinski M. Clinical significance of serum CA-125 and soluble tumor necrosis factor receptor type I in cervical adenocarcinoma patients. Int J Gynecol Cancer. 2010;20:588–92. doi: 10.1111/IGC.0b013e3181d5c27a. [DOI] [PubMed] [Google Scholar]
- 11.Burger RA, Darcy KM, DiSaia PJ, Monk BJ, Grosen EA, Gatanaga T, Granger GA, Wang J, Tian C, Hanjani P, Cohn DE. Association between serum levels of soluble tumor necrosis factor receptors/CA 125 and disease progression in patients with epithelial ovarian malignancy: a gynecologic oncology group study. Cancer. 2004;101:106–15. doi: 10.1002/cncr.20314. [DOI] [PubMed] [Google Scholar]
- 12.Kaminska J, Kowalska M, Kotowicz B, Fuksiewicz M, Glogowski M, Wojcik E, Chechlinska M, Steffen J. Pretreatment serum levels of cytokines and cytokine receptors in patients with non-small cell lung cancer, and correlations with clinicopathological features and prognosis. M-CSF - an independent prognostic factor. Oncology. 2006;70:115–25. doi: 10.1159/000093002. [DOI] [PubMed] [Google Scholar]
- 13.Dossus L, Becker S, Rinaldi S, Lukanova A, Tjonneland A, Olsen A, Overvad K, Chabbert-Buffet N, Boutron-Ruault M-C, Clavel-Chapelon F, Teucher B, Chang-Claude J, Pischon T, et al. Tumor necrosis factor (TNF)-α, soluble TNF receptors and endometrial cancer risk: the EPIC study. Int J Cancer. 2011;129:2032–2037. doi: 10.1002/ijc.25840. [DOI] [PubMed] [Google Scholar]
- 14.Chan AT, Ogino S, Giovannucci EL, Fuchs CS. Inflammatory markers are associated with risk of colorectal cancer and chemopreventive response to anti-inflammatory drugs. Gastroenterology. 2011;140:799–808. doi: 10.1053/j.gastro.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heemann C, Kreuz M, Stoller I, Schoof N, von Bonin F, Ziepert M, Loffler M, Jung W, Pfreundschuh M, Trumper L, Kube D. Circulating levels of TNF receptor II are prognostic for patients with peripheral T-cell non-Hodgkin lymphoma. Clin Cancer Res. 2012;18:3637–47. doi: 10.1158/1078-0432.CCR-11-3299. [DOI] [PubMed] [Google Scholar]
- 16.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 17.Ammirati M, Rao S, Granger G. Detection of TNF inhibitors (soluble receptors) in the sera and tumor cyst fluid of patients with malignant astrocytoma of the brain. Frontiers in Bioscience. 2001;6:b17–24. doi: 10.2741/ammirat. [DOI] [PubMed] [Google Scholar]
- 18.Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328:222–5. doi: 10.1016/j.canlet.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganz PA, Bower JE, Kwan L, Castellon SA, Silverman DHS, Geist C, Breen EC, Irwin MR, Cole SW. Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun. 2013;30:S99–S108. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel SK, Wong AL, Wong FL, Breen EC, Hurria A, Smith M, Kinjo C, Paz IB, Kruper L, Somlo G, Mortimer JE, Palomares MR, Irwin MR, Bhatia S. Inflammatory biomarkers, comorbidity, and neurocognition in women with newly diagnosed breast cancer. J Natl Cancer Inst. 2015;107:djv131. doi: 10.1093/jnci/djv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misri S, Papagiannakopoulos T, Kinouchi K, Liu Y, Cervantes M, Baldi P, Jacks T, Sassone-Corsi P. Lung adenocarcinoma distally rewires hepatic circadian homeostasis. Cell. 2016;165:896–909. doi: 10.1016/j.cell.2016.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang P, Rani MR, Ahluwalia MS, Bae E, Prayson RA, Weil RJ, Nowacki AS, Hedayat H, Sloan AE, Lathia JD, Rich JN, Tipps R, Gladson CL. Endothelial expression of TNF receptor-1 generates a proapoptotic signal inhibited by integrin alpha6beta1 in glioblastoma. Cancer Res. 2012;72:1428–37. doi: 10.1158/0008-5472.CAN-11-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016 doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 24.Hardikar S, Song X, Kratz M, Anderson GL, Blount PL, Reid BJ, Vaughan TL, White E. Intraindividual variability over time in plasma biomarkers of inflammation and effects of long-term storage. Cancer Causes Control. 2014;25:969–976. doi: 10.1007/s10552-014-0396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pai JK, Curhan GC, Cannuscio CC, Rifai N, Ridker PM, Rimm EB. Stability of novel plasma markers associated with cardiovascular disease: Processing within 36 hours of specimen collection. Clin Chem. 2002;48(10):1781–1784. [PubMed] [Google Scholar]
- 26.Aziz N, Nishanian P, Mitsuyasu R, Detels R, Fahey JL. Variables that affect assays for plasma cytokines and soluble activation markers. Clin Diag Lab Immunol. 1999;6(1):89–95. doi: 10.1128/cdli.6.1.89-95.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar R, Kamdar D, Madden L, Hills C, Crooks D, O’Brien D, Greenman J. Th1/Th2 cytokine imbalance in meningioma, anaplastic astrocytoma and glioblastoma multiforme patients. Oncol Rep. 2006;15:1513–6. doi: 10.3892/or.15.6.1513. [DOI] [PubMed] [Google Scholar]
- 28.Albulescu R, Codrici E, Popescu ID, Mihai S, Necula LG, Petrescu D, Teodoru M, Tanase CP. Cytokine patterns in brain tumor progression. Mediators of Inflammation 2013. 2013 doi: 10.1155/2013/979748. Article ID: 979748 org/10.1155/2013/979748. [DOI] [PMC free article] [PubMed]
- 29.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahluwalia MS, Gladson CL. Progress on antiangiogenic therapy for patients with malignant glioma. J Oncol. 2010 doi: 10.1155/2010/689018. Article ID 689018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weller M, van den Bent M, Hopkins K, Tonn JC, Stupp R, Falini A, Cohen-Jonathan-Moyal E, Frappaz D, Henriksson R, Balana C, Chinot O, Ram Z, Reifenberger G, Soffietti R, Wick W. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15:e395–403. doi: 10.1016/S1470-2045(14)70011-7. [DOI] [PubMed] [Google Scholar]
- 32.Wu B, Sha L, Wang Y, Xu W, Yu Y, Feng F, Sun C, Xia L. Diagnostic and prognostic value of a disintegrin and metalloproteinase-17 in patients with gliomas. Oncol Lett. 2014;8:2616–20. doi: 10.3892/ol.2014.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin J, Lemke C, Redies C, Yan X, Mix E, Rolfs A, Luo J. ADAM17 overexpression promotes angiogenesis by increasing blood vessel sprouting and pericyte number during brain microvessel development. Int J Dev Biol. 2011;55:961–8. doi: 10.1387/ijdb.103210jl. [DOI] [PubMed] [Google Scholar]
- 34.Zheng X, Jiang F, Katakowski M, Lu Y, Chopp M. ADAM17 promotes glioma cell malignant phenotype. Mol Carcinog. 2012;51:150–64. doi: 10.1002/mc.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Chen L, Chen J, Hu W, Gao H, Xie B, Wang X, Yin Z, Li S. ADAM17 promotes U87 glioblastoma stem cell migration and invasion. Brain Res. 2013;1538:151–8. doi: 10.1016/j.brainres.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 36.Rose-Johns S. ADAM17, shedding, TACE as therapeutic targets. Pharmacol Res. 2013;17:19–22. doi: 10.1016/j.phrs.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 37.See AP, Parker JJ, Waziri A. The role of regulatory T cells and microglia in glioblastoma-associated immunosuppression. J Neurooncol. 2015;123:405–412. doi: 10.1007/s11060-015-1849-3. [DOI] [PubMed] [Google Scholar]
- 38.Gousias K, Markou M, Arzoglou V, Voulgaris S, Vartholomatos G, Kostoula A, Voulgari P, Polyzoidis K, Kyritsis AP. Frequent abnormalities of the immune system in gliomas and correlation with the WHO grading system of malignancy. J Neuroimmunol. 2010;226:136–42. doi: 10.1016/j.jneuroim.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 39.Nduom EK, Weller M, Heimberger AB. Immunosuppressive mechanisms in glioblastoma. Neuro-Oncology. 2015;17:vii9–vii14. doi: 10.1093/neuonc/nov151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silver DJ, Sinyuk M, Vogelbaum MA, Ahluwalia MS, Lathia JD. The intersection of cancer, cancer stem cells, and the immune system: therapeutic opportunities. Neuro-Oncol. 2016;18:153–159. doi: 10.1093/neuonc/nov157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zisakis A, Piperi C, Themistocleous MS, Korkolopoulou P, Boviatsis EI, Sakas DE, Patsouris E, Lea RW, Kalofoutis A. Comparative analysis of peripheral and localised cytokine secretion in glioblastoma patients. Cytokine. 2007;39:99–105. doi: 10.1016/j.cyto.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Reynes G, Vila V, Martin M, Parada A, Fleitas T, Reganon E, Martinez-Sales V. Circulating markers of angiogenesis, inflammation and coagulation in patients with glioblastoma. J Neurooncol. 2011;102:35–41. doi: 10.1007/s11060-010-0290-x. [DOI] [PubMed] [Google Scholar]
- 43.Kato T, Sawamura Y, Tada M, Sakuma S, Sudo M, Abe H. p55 and p75 tumor necrosis factor receptor expression on human glioblastoma cells. Neurol Med Chir (Tokyo) 1995;35:567–74. doi: 10.2176/nmc.35.567. [DOI] [PubMed] [Google Scholar]
- 44.Haghikia A, Ladage K, Lafenetre P, Hinkerohe D, Smikalla D, Haase CG, Dermietzel R, Faustmann PM. Intracellular application of TNF-alpha impairs cell to cell communication via gap junctions in glioma cells. J Neurooncol. 2008;86:143–52. doi: 10.1007/s11060-007-9462-8. [DOI] [PubMed] [Google Scholar]
- 45.Chen TC, Hinton DR, Sippy BD, Hofman FM. Soluble TNF-alpha receptors are constitutively shed and downregulate adhesion molecule expression in malignant gliomas. J Neuropathol Exp Neurol. 1997;56:541–50. doi: 10.1097/00005072-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Jain R, Poisson L, Narang J, Scarpace L, Rosenblum ML, Rempel S, Mikkelsen T. Correlation of perfusion parameters with genes related to angiogenesis regulation in glioblastoma: a feasibility study. AJNR Am J Neuroradiol. 2012;33:1343–8. doi: 10.3174/ajnr.A2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rego SL, Swamydas M, Kidiyoor A, Helms R, De Piante A, Lance AL, Mukherjee P, Dreau D. Soluble tumor necrosis factor receptors shed by breast tumor cells inhibit macrophage chemotaxis. J Interferon Cytokine Res. 2013;33:672–681. doi: 10.1089/jir.2013.0009. [DOI] [PubMed] [Google Scholar]
- 48.Bai Y-M, Su T-P, Li CT, Tsai SJ, Chen M-H, Tu P-C, Chiou W-F. Comparison of pro-inflammatory cytokines among patients with bipolar disorder and unipolar depression and normal controls. Bipolar Disorders. 2015;17:269–277. doi: 10.1111/bdi.12259. [DOI] [PubMed] [Google Scholar]
- 49.Yamamori H, Ishima T, Yasuda Y, Fujimoto M, Kudo N, Ohi K, Hashimoto K, Takeda M, Hashimoto R. Assessment of a multi-assay biological diagnostic test for mood disorders in a Japanese population. Neuroscience Letters. 2016;612:167–171. doi: 10.1016/j.neulet.2015.12.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.