Abstract
Erectile dysfunction (ED) associated with type 2 diabetes mellitus (T2DM) involves dysfunctional nitric oxide (NO) signaling and increased oxidative stress in the penis. However, the mechanisms of endothelial NO synthase (eNOS) and neuronal NO synthase (nNOS) dysregulation, and the sources of oxidative stress, are not well defined, particularly at the human level. The objective of this study was to define whether uncoupled eNOS and nNOS, and NADPH oxidase upregulation, contribute to the pathogenesis of ED in T2DM men. Penile erectile tissue was obtained from 9 T2DM patients with ED who underwent penile prosthesis surgery for ED, and from 6 control patients without T2DM or ED who underwent penectomy for penile cancer. The dimer-to-monomer protein expression ratio, an indicator of uncoupling for both eNOS and nNOS, total protein expressions of eNOS and nNOS, as well as protein expressions of NADPH oxidase catalytic subunit gp91phox (an enzymatic source of oxidative stress) and 4-hydroxy-2-nonenal [4-HNE] and nitrotyrosine (markers of oxidative stress) were measured by Western blot in this tissue. In the erectile tissue of T2DM men, eNOS and nNOS uncoupling and protein expressions of NADPH oxidase subunit gp91phox, 4-HNE- and nitrotyrosine-modified proteins were significantly (p<0.05) increased compared to control values. Total eNOS and nNOS protein expressions were not significantly different between the groups. In conclusion, mechanisms of T2DM-associated ED in the human penis may involve uncoupled eNOS and nNOS and NADPH oxidase upregulation. Our description of molecular factors contributing to the pathogenesis of T2DM-associated ED at the human level is relevant for advancing clinically therapeutic approaches to restore erectile function in T2DM patients.
Keywords: eNOS uncoupling, nNOS uncoupling, human, oxidative stress
INTRODUCTION
347 million people worldwide, and nearly 26 million Americans (8.3% of the population), have diabetes mellitus (DM), with numbers projected to double by 2030 (Wild et al., 2004). Type 2 DM (T2DM) accounts for 90-95% of the diabetic population. ED is reported in 75% of men with T2DM, occurs at an earlier age in T2DM men than nondiabetic men, and increases in incidence with the duration of T2DM (Kalter-Leibovici et al., 2005).
Basic scientific studies indicate that increased oxidative stress and reduced nitric oxide (NO) bioavailability are the major derangements underlying the development and progression of vasculogenic and neurogenic ED associated with DM (Long et al., 2012; Vernet et al., 1995; Luttrell et al., 2008; Carneiro et al., 2010; Elliati et al., 2013; Albersen et al., 2011; Chiou et al., 2010; Oger et al., 2014; Kataoka et al. 2014; Musicki et al., 2016; Xie et al., 2007; Kovanecz et al., 2009; Chitaley 2009; Nunes et al., 2015; Wingard et al., 2007; Sanchez et al., 2012). In diabetic patients ED is similarly related to reduced NOS activity, as evidenced by elevated arginase activity (Bivalacqua et al., 2001) and decreased nNOS protein expression (Dashwood et al., 2011), nitrate/nitrite (Tuncayengin et al., 2003) and cGMP content (Angulo et al., 2010). Penile tissue (Tuncayengin et al., 2003) and blood (El-Latif et al., 2006; Hamden & Al-Matubsi 2009; Burnett et al., 2009) from T2DM men with ED exhibit increased oxidative stress and reduced antioxidant reserve compared with that of nondiabetic men.
Despite understanding that impaired NO signaling and increased oxidative stress are major factors in T2DM-associated ED at both the animal and human level, the mechanisms of impaired NO signaling and the sources of oxidative stress in T2DM human penis are not well undersood. We hypothesized that constitutive NOS uncoupling in the erectile tissue of T2DM men with ED may contribute to NOS dysfunction. Under physiologic conditions, NOSs are homodimeric heme-containing enzymes that catalyze the conversion of L-arginine to NO. However, under pathologic conditions resulting in a loss of functional enzyme's dimerization, NOSs can transform into prooxidants, generating predominantly superoxide anion rather than NO (‘NOS uncoupling’) (Fostermann & Sessa 2012). We further hypothesized that NADPH oxidase (NOX) may be upregulated in the erectile tissue of T2DM men with ED, contributing to both the decreased NO bioavailability and increased oxidative stress. NADPH oxidases are major sources of oxidative stress. They are multi-subunits complexes that transfer electrons from NADPH to oxygen, generating superoxide (Brandes et al., 2014). In this study we compared erectile tissue of T2DM with ED to that of nondiabetic men without ED.
MATERIALS AND METHODS
Study Population and Tissue Collection
Tissue collection and clinical history review were performed with approval by the Institutional Review Board of the Johns Hopkins Medical Institutions, and written informed consent was obtained from all patients. Human penile specimens (erectile tissue) were obtained from a diabetic group comprising 9 patients with T2DM and ED, mean age of 58.4 years (range 52 to 71), who underwent penile prosthesis surgery, and a control group comprising 6 patients with a mean age of 57 years (range 48 to 73) without severe ED (based on erectile function questionnaire, Rosen et al., 1999) or diabetes histories who underwent penectomy for penile cancer (n=5) and urethral cancer (n=1). Penile tissue from these groups was unassociated with malignancy. All surgeries were performed by the same surgeon. One control and one diabetic patient had hypertension, and one diabetic patient had hypercholesterolemia. Penile erectile tissue samples were collected at surgery, immediately placed in cold saline, and then frozen in liquid nitrogen. Due to limited amount of samples, not all samples could be used for all measurements, as detailed in the Results section.
Western blot
Minced penile tissue was homogenized as described (Hurt et al., 2002). Homogenates were resolved on 4–20% Tris gels and transferred to polyvinylidene difluoride membrane. Membranes were probed with polyclonal rabbit anti-4-hydroxy-2-nonenal (4-HNE) antibody (Alpha Diagnostic International, San Antonio, TX, USA) at 1:2,000, rabbit anti-nitrotyrosine antibody (Abcam Inc, Cambridge, MA) at 1:2,000, mouse anti-gp91phox antibody at 1:1,000 (BD Transduction Laboratories, San Diego, CA, USA), mouse anti-eNOS antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:1,000, or rabbit anti-nNOS antibody (BD Transduction Laboratories) at 1:1,000 dilutions (Musicki et al.,2014). Signals were standardized to β-actin (Sigma Chemical, St. Louis, MO). For the analysis of the dimeric and monomeric forms of eNOS and nNOS, low-temperature sodium dodecyl sulfate (SDS)-gel electrophoresis was used with partially purified penile homogenates, as described previously (Musicki et al., 2008). Membranes were then probed with anti-eNOS or anti nNOS antibodies at 1:1,000 dilutions. Bands were detected by horseradish peroxidase conjugated anti-mouse or anti-rabbit antibodies (GE Healthcare, Piscataway, NJ, USA), and analyzed using National Institutes of Health Image software. The analysis of 4-HNE and nitrotyrosine is a densitometric composite of all proteins in each lane. eNOS and nNOS uncoupling were represented inversely as a ratio of active dimers to inactive monomers. All results were expressed relative to nondiabetic men.
Statistical Analysis
The data are expressed as the mean ± SEM. Statistical analysis was performed using modified Student's t-test. P less than 5% was considered significant.
RESULTS
eNOS and nNOS uncoupling in the erectile tissue of T2DM men
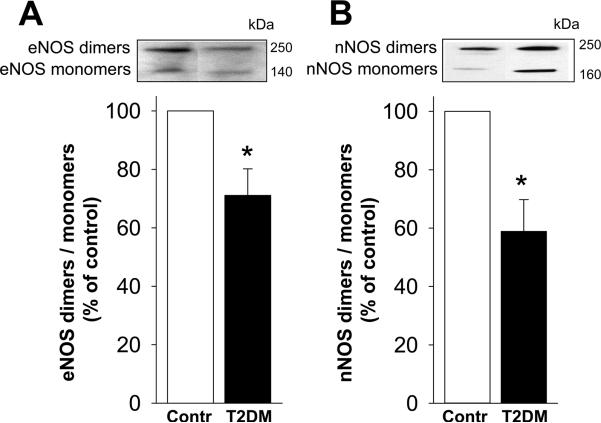
The ratio of eNOS dimers (functional eNOS)/monomers (nonfunctional eNOS), inversely related to eNOS uncoupling, was significantly (p<0.05) decreased in the erectile tissue of diabetic compared to that of control men (Figure 1A). Similarly, the ratio of nNOS dimers/monomers was significantly (p<0.05) decreased in the erectile tissue of diabetic men relative to levels found in control human erectile tissue (Figure 1B). These results indicate eNOS and nNOS uncoupling in the erectile tissue of diabetic men with ED.
Figure 1.
eNOS uncoupling (A) and nNOS uncoupling (B) are increased in the erectile tissue of T2DM men with ED compared to nondiabetic men without ED. Upper panels are representative western immunoblots. Lower panels represent quantitative analyses of eNOS and nNOS dimers and monomers in the same groups. NOS uncoupling is represented inversely as a ratio of active NOS dimers to inactive NOS monomers. Each bar represents the mean ± SEM. *p < 0.05. n=5 control and 9 diabetic samples for eNOS uncoupling; n= 6 control and 7 diabetic samples for nNOS uncoupling.
eNOS and nNOS protein expression in the erectile tissue of T2DM men
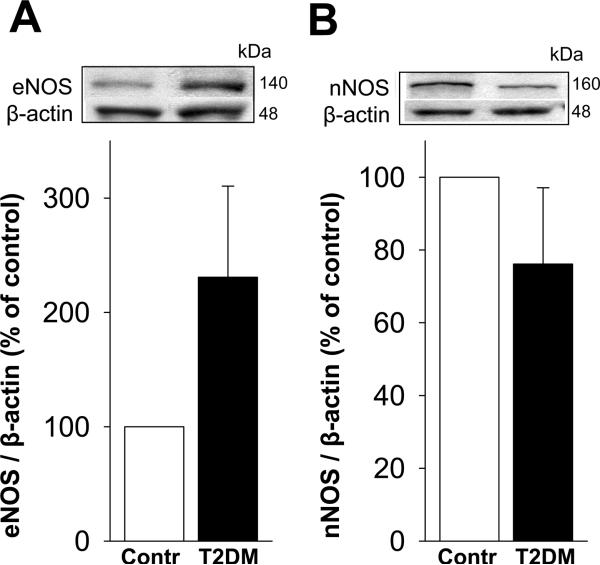
Protein expressions of eNOS (Figure 2A) and nNOS (Figure 2B) in the erectile tissue of diabetic patients did not differ from the values in control men (p=0.731 and 0.491, respectively).
Figure 2.
Total eNOS (A) and nNOS (B) protein expressions in the erectile tissue are not different between T2DM men with ED compared to nondiabetic men without ED. Upper panels are representative western immunoblots. Lower panels represent quantitative analyses of eNOS and nNOS in the same groups. Each bar represents the mean ± SEM. n=6 control and 8 diabetic samples for eNOS; n= 6 control and 7 diabetic samples for nNOS.
Upregulation of NADPH oxidase subunit gp91phox and oxidative stress in the erectile tissue of T2DM men
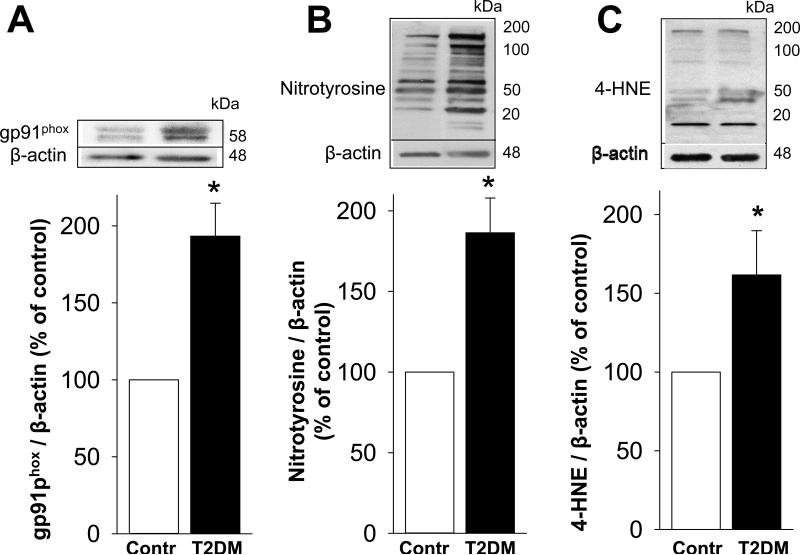
Protein expression of gp91phox, a catalytic subunit of an ROS-producing enzyme NADPH oxidase, was increased (p<0.05) in the erectile tissue of diabetic men with ED compared to that of control men (Figure 3A). 3-nitrotyrosine, a biomarker for peroxynitrite (the reaction product of NO with superoxide, Souza et al., 2008), was increased (p<0.05) in the erectile tissue of diabetic compared to that of control men (Figure 3B). 4-HNE, a product of lipid peroxidation and a marker and inducer of oxidative stress (Cohen et al., 2013), was also increased (p<0.05) in the erectile tissue of diabetic compared to that of control men (Figure 3C). These results indicate upregulation of NADPH oxidase and increased oxidative stress in diabetic human erectile tissue.
Figure 3.
Protein expressions of NADPH oxidase subunit gp91phox (A), nitrotyrosine (B), and 4-HNE (C) are increased in the erectile tissue of T2DM men with ED compared to nondiabetic men without ED. Upper panels are representative western immunoblots. Lower panels represent quantitative analyses of the proteins in the same groups. The analyses of nitrotyrosine and 4-HNE are densitometric composite of all proteins in each lane. Each bar represents the mean ± SEM. *p < 0.05. n=4 control and 8 diabetic samples for gp91phox; n= 5 control and 8 diabetic samples for nitrotyrosine; n=4 control and 6 diabetic samples for 4-HNE.
DISCUSSION
The results of this study demonstrate that eNOS and nNOS regulatory mechanisms associated with penile erection are altered in the penis of T2DM patients with ED, affirming that this condition features functionally inactivated constitutive NOSs. Here we show loss of functional, dimeric states of eNOS and nNOS by uncoupling and upregulation of NADPH oxidase with increase in oxidative stress markers. These findings provide a molecular basis for chronically reduced NO bioavailability and oxidative stress in the penis by T2DM. Our findings also identify these sources of ROS as potential molecular targets for pharmacologic therapies for diabetic ED, although more mechanistic studies will be required to demonstrate how they can be therapeutically targeted and modulated.
ED associated with T2DM involves impairments in endothelial and neurogenic components of penile erection, mediated by dysfunctional constitutive NOS isoenzymes (Musicki & Burnett 2007). Proper catalytic function of eNOS and nNOS requires dimerization. Within the NOS enzymes, electrons flow from one monomer to the heme on the other monomer to produce NO (Fostermann & Sessa 2012). eNOS and nNOS uncoupling, measured indirectly as a decreased ratio of active dimers to inactive monomers, refers to a switch in the enzyme's activity from NO-producing to a predominantly superoxide-producing enzyme (Fostermann & Sessa 2012). Previous studies demonstrated eNOS uncoupling in the penis of T2DM rats (Musicki et al., 2016) and nNOS uncoupling in the rat penile arteries under conditions of insulin resistance (Sanchez et al., 2012). We now extend these findings to the erectile tissue of T2DM men with ED, showing that both eNOS and nNOS are uncoupled. Uncoupled eNOS and nNOS conceivably contribute to increased oxidative stress, reduced NO bioavailability, and ED. The precise mechanism by which eNOS and nNOS become uncoupled in the human diabetic penis is not known at this time and requires further mechanistic studies.
Oxidative stress plays a significant role in diabetic ED (Musicki & Burnett 2007). Increased oxidative stress reduces NO bioavailability by promoting NO inactivation and by decreasing NO production (Vanhoutte et al., 2016). The NADPH oxidases are a family of enzymes that catalyze electron transfer from cytosolic NADPH to molecular oxygen to generate superoxide as its primary product. The prototype NADPH oxidase NOX2/gp91phox possesses cytosolic subunits (p47phox, p67phox, or homologues) and membrane-bound subunits (gp91phox and p22phox), which form a functional enzyme complex upon activation (Brandes et al., 2014). Increased protein expression of NOX2 subunits has been demonstrated in the penis of T2DM rats (Long et al., 2012; Musicki et al., 2016) and mice (Nunes et al., 2015). We now report upregulated NADPH oxidase catalytic subunit gp91phox in the human T2DM penis, implying activated NADPH oxidase as a source of oxidative stress. Increased oxidative stress is evident as increased protein expression of 4-HNE and 3-nitrotyrosine. 4-HNE is a relatively stable end product of lipid peroxidation which binds to histidine, lysine, and cysteine residues of proteins forming adducts (Cohen et al., 2013). Nitrotyrosine is a product of tyrosine nitration mediated by reactive nitrogen species such as peroxynitrite. During NOS uncoupling, when NO and superoxide anions are simultaneously produced, their reaction is extremely rapid to form peroxynitrite (Souza et al., 2008). eNOS and nNOS uncoupling, together with increased oxidative/nitrosative stress, conceivably translates to decreased total NO production in the penis of T2DM men with ED.
In spite of dysfunctional eNOS, decreased NO bioavailability, and increased oxidative stress associated with T2DM, eNOS mRNA and protein levels are often maintained or even increased in the diabetic vasculature (Li et al., 2002). In our study, we found maintained, even slightly elevated levels of eNOS protein expression in the T2DM human penis. These findings indicate that sufficient expression of eNOS protein alone does not guarantee adequate eNOS function required for proper endothelial function, because the up-regulated eNOS is uncoupled. In our study, nNOS protein expression in the human penis was not affected by diabetes. Several animal studies (Albersen et al., 2011; Garcia et al., 2010; Chiou et al., 2010) and one human study (Dashwood et al., 2011) reported decreased nNOS protein expression in T2DM penile tissue. Decreased expression of nNOS and its impairment by uncoupling conceivably contribute to deterioration of nitrergic neurons in the penis and neurogenic ED in T2DM. While the mechanism of nNOS loss is not fully understood, inactive nNOS monomers have been shown to be susceptible to ubiquitination and subsequent proteasomal degradation (Sharma et al., 2013). Further studies are needed to confirm reduced protein levels of nNOS and the mechanism of its depletion in human diabetic erectile tissue.
We acknowledge several limitations of our study. First, the study population alltogether comprised a small number of patients. Second, it is recognized that the T2DM patients had severe ED in line with their proceeding with penile prosthesis implantation, and a T2DM patient group with minimal or no ED may have offered an interesting comparative group. It should be noted that obtaining tissue from such a group, however, is practically unfeasible. Finally, our study was focused on several select signaling pathways. Future studies at the human level may include other possible molecular mechanisms associated with the pathophysiology of ED in T2DM, such as the RhoA/Rho-kinase contractile pathway.
In conclusion, penile tissue from T2DM men with ED exhibits eNOS and nNOS uncoupling and NADPH oxidase upregulation, along with overall increased oxidative stress, which conceivably provide a molecular basis for chronically reduced endothelial and neuronal NO bioavailability in the penis contributing to T2DM-associated ED. Elucidation of the molecular mechanisms involved in the pathogenesis of T2DM-associated ED, with confirmation at the human level, will conceivably offer new avenues for future treatment of T2DM-associated ED,
Acknowledgements
This work was supported by a grant from the National Institutes of Health, USA (NIH/NIDDK grant R01DK067223 to ALB)
Footnotes
Author's contributions
All authors take full responsibility for the content of the manuscript. The authors BM and ALB contributed to the conception and design, analysis and interpretation of data, and drafting and revising the manuscript content, and BM wrote the paper. The final version of the manuscript was approved by all authors. All the authors agree to be accountable for all aspects of the work.
Disclosures
The authors have nothing to disclose.
REFERENCES
- Albersen M, Lin G, Fandel TM, Zhang H, Qiu X, Lin CS, Lue TF. Functional, metabolic, and morphologic characteristics of a novel rat model of type 2 diabetes-associated erectile dysfunction. Urology. 2011;78:476, e1–8. doi: 10.1016/j.urology.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo J, González-Corrochano R, Cuevas P, Fernández A, La Fuente JM, Rolo F, Allona A, Sáenz de Tejada I. Diabetes exacerbates the functional deficiency of NO/cGMP pathway associated with erectile dysfunction in human corpus cavernosum and penile arteries. J Sex Med. 2010;7:758–768. doi: 10.1111/j.1743-6109.2009.01587.x. [DOI] [PubMed] [Google Scholar]
- Bivalacqua TJ, Hellstrom WJ, Kadowitz PJ, Champion HC. Increased expression of arginase II in human diabetic corpus cavernosum: in diabetic-associated erectile dysfunction. Biochem Biophys Res Commun. 2001;283:923–927. doi: 10.1006/bbrc.2001.4874. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Weissmann N, Schröder K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radic Biol Med. 2014;76:208–226. doi: 10.1016/j.freeradbiomed.2014.07.046. [DOI] [PubMed] [Google Scholar]
- Burnett AL, Strong T, Trock BJ, Jin L, Bivalacqua TJ, Musicki B. Serum biomarker measurements of endothelial function and oxidative stress after daily dosing of sildenafil in type 2 diabetic men with erectile dysfunction. J Urol. 2009;181:245–251. doi: 10.1016/j.juro.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Carneiro FS, Giachini FR, Carneiro ZN, Lima VV, Ergul A, Webb RC, Tostes RC. Erectile dysfunction in young non-obese type II diabetic Goto-Kakizaki rats is associated with decreased eNOS phosphorylation at Ser1177. J Sex Med. 2010;40:273–279. doi: 10.1111/j.1743-6109.2010.02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou WF, Liu HK, Juan CW. Abnormal protein expression in the corpus cavernosum impairs erectile function in type 2 diabetes. BJU Int. 2010;105:674–680. doi: 10.1111/j.1464-410X.2009.08852.x. [DOI] [PubMed] [Google Scholar]
- Chitaley K. Type 1 and Type 2 Diabetic-Erectile Dysfunction: Same Diagnosis (ICD-9), Different Disease? J Sex Med. 2009;6:262–268. doi: 10.1111/j.1743-6109.2008.01183.x. [DOI] [PubMed] [Google Scholar]
- Cohen G, Riahi Y, Sunda V, Deplano S, Chatgilialoglu C, Ferreri C, Kaiser N, Sasson S. Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes. Free Radic Biol Med. 2013;65:978–987. doi: 10.1016/j.freeradbiomed.2013.08.163. [DOI] [PubMed] [Google Scholar]
- Dashwood MR, Crump A, Shi-Wen X, Loesch A. Identification of neuronal nitric oxide synthase (nNOS) in human penis: a potential role of reduced neuronally-derived nitric oxide in erectile dysfunction. Curr Pharm Biotechnol. 2011;12:1316–1321. doi: 10.2174/138920111798280965. [DOI] [PubMed] [Google Scholar]
- El-Latif MA, Makhlouf AA, Moustafa YM, Gouda TE, Niederberger CS, Elhanbly SM. Diagnostic value of nitric oxide, lipoprotein(a), and malondialdehyde levels in the peripheral venous and cavernous blood of diabetics with erectile dysfunction. Int J Impot Res. 2006;18:544–549. doi: 10.1038/sj.ijir.3901473. [DOI] [PubMed] [Google Scholar]
- Ellati RT, Dokun AO, Kavoussi PK, Steers WD, Annex BH, Lysiak JJ. Increased phosphodiesterase type 5 levels in a mouse model of type 2 diabetes mellitus. J Sex Med. 2013;10:362–369. doi: 10.1111/j.1743-6109.2012.02854.x. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MM, Fandel TM, Lin G, Shindel AW, Banie L, Lin CS, Lue TF. Treatment of erectile dysfunction in the obese type 2 diabetic ZDF rat with adipose tissue-derived stem cells. J Sex Med. 2010;7:89–98. doi: 10.1111/j.1743-6109.2009.01541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan FB, Al-Matubsi HY. Assessment of erectile dysfunction in diabetic patients. Int J Androl. 2009;32:176–185. doi: 10.1111/j.1365-2605.2008.00873.x. [DOI] [PubMed] [Google Scholar]
- Hurt KJ, Musicki B, Palese MA, Crone JC, Becker RE, Moriarty JL, Snyder SH, Burnett AL. Akt-dependent phosphorylation of endothelial nitric oxide synthase mediated penile erection. Proc Natl Acad Sci USA. 2002;99:4061–4066. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalter-Leibovici O, Wainstein J, Ziv A, Harman-Bohem I, Murad H, Raz I, Israel Diabetes Research Group (IDRG) Investigators Clinical, socioeconomic, and lifestyle parameters associated with erectile dysfunction among diabetic men. Diabetes Care. 2005;28:1739–1744. doi: 10.2337/diacare.28.7.1739. [DOI] [PubMed] [Google Scholar]
- Kataoka T, Hotta Y, Maeda Y, Kimura K. Assessment of androgen replacement therapy for erectile function in rats with type 2 diabetes mellitus by examining nitric oxide-related and inflammatory factors. J Sex Med. 2014;11:920–929. doi: 10.1111/jsm.12447. [DOI] [PubMed] [Google Scholar]
- Kovanecz I, Nolazco G, Ferrini MG, Toblli JE, Heydarkhan S, Vernet D, Rajfer J, Gonzalez-Cadavid NF. Early onset of fibrosis within the arterial media in a rat model of type 2 diabetes mellitus with erectile dysfunction. BJU Int. 2009;103:1396–1404. doi: 10.1111/j.1464-410X.2008.08251.x. [DOI] [PubMed] [Google Scholar]
- Li H, Wallerath T, Munzel T, Forstermann U. Regulation of endothelial-type NO synthase expression in pathophysiology and in response to drugs. Nitric Oxide. 2002;7:149–164. doi: 10.1016/s1089-8603(02)00111-8. [DOI] [PubMed] [Google Scholar]
- Long T, Liu G, Wang Y, Chen Y, Zhang Y, Qin D. TNF-α, Erectile dysfunction, and NADPH oxidase-mediated ROS generation in corpus cavernosum in high-fat diet/streptozotocin-induced diabetic rats. J Sex Med. 2012;9:1801–1814. doi: 10.1111/j.1743-6109.2012.02739.x. [DOI] [PubMed] [Google Scholar]
- Luttrell IP, Swee M, Starcher B, Parks WC, Chitaley K. Erectile dysfunction in the type II diabetic db/db mouse: Impaired venoocclusion with altered cavernosal vasoreactivity and matrix. Am J Physiol Heart Circ Physiol. 2008;294:H2204–H2211. doi: 10.1152/ajpheart.00027.2008. [DOI] [PubMed] [Google Scholar]
- Musicki B, Burnett AL. Endothelial dysfunction in diabetic erectile dysfunction. Int J Impot Res. 2007;19:129–138. doi: 10.1038/sj.ijir.3901494. [DOI] [PubMed] [Google Scholar]
- Musicki B, Liu T, Strong T, Jin L, Laughlin MH, Turk JR, Burnett AL. Low-fat diet and exercise preserve eNOS regulation and endothelial function in the penis of early atherosclerotic pigs: A molecular analysis. J Sex Med. 2008;5:552–561. doi: 10.1111/j.1743-6109.2007.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musicki B, Bivalacqua TJ, Champion HC, Burnett AL. Sildenafil promotes eNOS activation and inhibits NADPH oxidase in the transgenic sickle cell mouse penis. J Sex Med. 2014;11:424–430. doi: 10.1111/jsm.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musicki B, Hannan JL, Lagoda G, Bivalacqua TJ, Burnett AL. Mechanistic link between erectile dysfunction and systemic endothelial dysfunction in type 2 diabetic rats. Andrology. 2016 doi: 10.1111/andr.12218. e-pub ahead of print 6 May 2016; doi: 10.1111/andr.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes KP, Teixeira CE, Priviero FB, Toque HA, Webb RC. Beneficial effect of the soluble guanylyl cyclase stimulator BAY 41-2272 on impaired penile erection in db/db-/- type II diabetic and obese mice. J Pharmacol Exp Ther. 2015;353:330–339. doi: 10.1124/jpet.114.220970. [DOI] [PubMed] [Google Scholar]
- Oger-Roussel S1, Behr-Roussel D, Caisey S, Kergoat M, Charon C, Audet A, Bernabé J, Alexandre L, Giuliano F. Bladder and erectile dysfunctions in the Type 2 diabetic Goto-Kakizaki rat. Am J Physiol Regul Integr Comp Physiol. 2014;306:R108–117. doi: 10.1152/ajpregu.00033.2013. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–326. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- Sánchez A, Contreras C, Martínez MP, Climent B, Benedito S, García-Sacristán A, Hernández M, Prieto D. Role of neural NO synthase (nNOS) uncoupling in the dysfunctional nitrergic vasorelaxation of penile arteries from insulin-resistant obese Zucker rats. PLoS One. 2012;7:e36027. doi: 10.1371/journal.pone.0036027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma NM, Llewellyn TL, Zheng H, Patel KP. Angiotensin II-mediated posttranslational modification of nNOS in the PVN of rats with CHF: role for PIN. Am J Physiol Heart Circ Physiol. 2013;305:H843–H855. doi: 10.1152/ajpheart.00170.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza JM, Peluffo G, Radi R. Protein tyrosine nitration: functional alteration or just a biomarker? Free Radic Biol Med. 2008;45:357–366. doi: 10.1016/j.freeradbiomed.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Tuncayengin A, Biri H, Onaran M, Sen I, Tuncayengin O, Polat F, Erbaş D, Bozkirli I. Cavernosal tissue nitrite, nitrate, malondialdehyde and glutathione levels in diabetic and non-diabetic erectile dysfunction. Int J Androl. 2003;26:250–254. doi: 10.1046/j.1365-2605.2003.00427.x. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Zhao Y, Xu A, Leung SW. Thirty Years of Saying NO: Sources, Fate, Actions, and Misfortunes of the Endothelium-Derived Vasodilator Mediator. Circ Res. 2016;119:375–396. doi: 10.1161/CIRCRESAHA.116.306531. [DOI] [PubMed] [Google Scholar]
- Vernet D, Cai L, Garban H, Babbit ML, Murray FT, Rajfer J, Gonzalez-Cadavid NF. Reduction of penile nitric oxide synthase in diabetic BB/WORdp (type I) and BBZ/WORdp (type II) rats with erectile dysfunction. Endocrinology. 1995;136:5709–5717. doi: 10.1210/endo.136.12.7588327. [DOI] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global Prevalence of Diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Wingard C, Fulton D, Husain S. Altered penile vascular reactivity and erection in the Zucker obese-diabetic rat. J Sex Med. 2007;4:348–362. doi: 10.1111/j.1743-6109.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- Xie D, Odronic SI, Wu F, Pippen A, Donatucci CF, Annex BH. Mouse model of erectile dysfunction due to diet-induced diabetes mellitus. Urology. 2007;70:196–201. doi: 10.1016/j.urology.2007.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]