Abstract
In prior studies, we have identified the ability of low-level lysosomal photodamage to potentiate the phototoxic effect of subsequent photodamage to mitochondria. The mechanism involves calpain-mediated cleavage of the autophagy-associated protein ATG5 to form a pro-apoptotic fragment (tATG5). In this report, we explore the permissible time lag between the two targeting procedures along with the effect of simultaneously targeting both lysosomes and mitochondria. This was found to be as effective as the sequential protocol with no gap between the irradiation steps. Inhibition of calpain reversed the enhanced efficacy of the ‘simultaneous’ protocol. It appears that even a minor level of lysosomal photodamage can have a significant effect on the efficacy of subsequent mitochondrial photodamage. We propose that these results may explain the efficacy of Photofrin, a photosensitizing product that also targets both lysosomes and mitochondria for photodamage.
Graphical Abstract
A very low level of prior or simultaneous lysosomal photodamage (NPe6) can convert a 10% photokilling effect from mitochondrial photodamage (BPD) into an LD50 effect.
INTRODUCTION
In 1996, it was reported that a sequential PDT protocol involving two photosensitizing agents could eradicate large (~1 cm) sarcomas in the mouse. A comparable degree of cancer control could not be achieved with either sensitizer alone (1). The combination involved the phenothiazinium photosensitizer termed EtNBS and ‘benzoporphyrin derivative’ (Verteporfin, BPD), agents now known to target lysosomes and mitochondria, respectively (2,3) although this selectivity was not appreciated at the time. The order of irradiation was important: activation of EtNBS needed to occur before BPD to achieve an optimal effect.
Studies in cell culture have provided evidence for a mechanism that appears to explain the results (4–7). Simon’s group has shown that the autophagy-associated protein termed ATG5 can be cleaved by the protease calpain to form a pro-apoptotic product [8]. We propose that release of calcium ions from photodamaged lysosomes is the ‘trigger’ for the enhanced efficacy of the combination targeting protocol, i.e., by providing the signal that leads to calpain activation. In examining the results by Cincotta et al (1), we initially used the chlorin NPe6, an agent that selectively targets lysosomes for photodamage (9). Results obtained with NPe6 or EtNBS were later found to be essentially identical (5).
In this report, we explore the persistence of the effect whereby lysosomal photodamage potentiates the apoptotic response to PDT directed at mitochondria. The efficacy of simultaneous vs. sequential targeting was also examined. The results of the latter study may explain the efficacy of Photofrin, a commonly-used photosensitizer that targets both lysosomes and mitochondria.
MATERIALS AND METHODS
Chemicals and supplies
NPe6 was obtained from Dr. Kevin M. Smith, Louisiana State University. BPD (benzoporphyrin derivative, Verteporfin) was provided by VWR (Cat No 1711461). Other reagents were obtained from Sigma-Aldrich and were of the highest available purity. Fluorescent probes used in this study were purchased from Life Technologies, Inc.
Cell culture and clonogenic assays
Procedures for the growth of murine hepatoma 1c1c7 cells and for carrying out clonogenic assays have been described (10). To maintain pH in the absence of CO2, irradiations were carried out in media buffered to pH 7.2 with HEPES. Viability was determined by colony counting using an Oxford Optronix GelCount device. This device can identify groups of 30 cells or more as colonies. Although the GelCount can detect colonies without additional staining, crystal violet was used as a colony marker. All experiments were performed in triplicate.
DEVDase assays
Cells were collected 2 h after irradiation and assayed for DEVDase activity as described in Ref. 10. This procedure monitors activation of caspases 3/7, an index of apoptosis. A kit provided by Invitrogen was used for this purpose (cat. no. E13184). Caspase levels are reported in terms of nmol product/min/mg protein. Each assay was performed in triplicate. The Micro Lowry assay was used to determine protein concentrations in cell lysates using bovine serum albumin as the standard.
PDT protocols
Cell cultures were prepared using 30 mm cover slips in sterile plastic dishes. These were incubated at 37°C with 20 µM NPe6 + 0.5 µM BPD for one h as specified. The medium was then replaced and the dishes irradiated with a 600-watt quartz-halogen source. The light beam was passed through a 10 cm layer of water to remove IR and the bandwidth was further confined by interference filters (Oriel, Stratford CT) to 660 ± 10 nm or 690 ± 10 nm to initiate photodamage catalyzed by NPe6 or BPD, respectively. Other studies were carried out where both wavelengths were simultaneously provided so that both lysosomal and mitochondrial photodamage occurred over the same interval. This involved use of a combination of low- and high-pass filters to provide 645–700 nm light. The total light doses were 23 mJ/sq cm at 660 nm and, 20 mJ/sq cm at 690 nm. While the total broad-band irradiation (600 – 750 nm) was 126 mJ/sq cm, we estimate from absorbance profiles that this closely approximated the pertinent light flux produced by the individual interference filters.
In a second study, we assessed the effects of a much lower PDT dose directed at lysosomes on subsequent photokilling by BPD. For this study, cells were treated with 20 µM NPe6 + 0.5 µM for 1 h, then sequentially irradiated at 660 nm (10 mJ/sq cm) and at 690 nm (20 mJ/sq cm). Additional studies were carried out using only 660 nm or 690 nm irradiation. In some studies involving BPD + NPe6, a 10 µM concentration of the calpain antagonist ALLN (11) was present for 1 hr prior to irradiation.
Microscopy
Effects of photodamage on maintenance of the mitochondrial membrane potential (ΔΨm) were assessed by fluorescence microscopy using the probe mitotracker orange (MTO) as described in Ref 4. Cells were labeled with MTO for 10 min at 37°C directly after irradiation. Images were acquired with a Nikon E-600 microscope using a Rolera EM-CCD camera and MetaMorph software (Molecular Devices, Sunnyvale CA). Fluorescence was detected using a Nikon ‘B’ filter combination (excitation 510–560 nm, emission at wavelengths >590 nm). A 650 nm low-pass filter was placed in the emission beam to prevent photosensitizer fluorescence from reaching the CCD camera. At least 3 images were acquired for each sample, typical representations are shown.
RESULTS and DISCUSSION
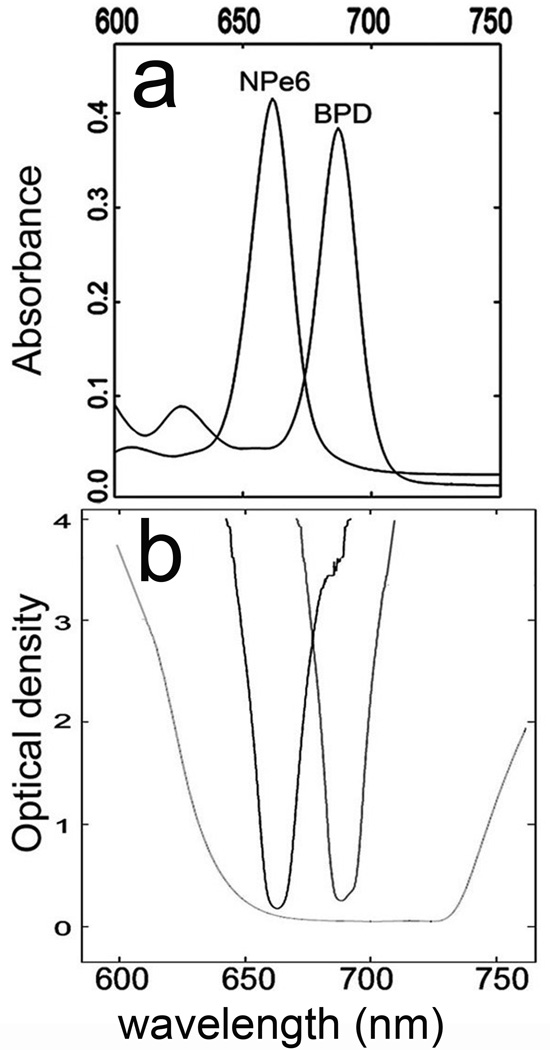
Absorbance profiles of the two photosensitizers used in this study, together with spectral properties of the light sources are shown in Fig. 1. Transmission profiles of the interference filters closely mimic the absorbance spectra of the individual agents. The combination of low-pass and high-pass filters is shown to provide irradiation that includes the absorbance spectra of both NPe6 and BPD.
Figure 1.
Spectral properties of light sources and photosensitizers. A, absorbance spectra of NPe6 and BPD between 600 and 750 nm (solvent = ethanol). B, optical transmission of 660 and 690 nm bandpass filters and of a combination of a 640 nm high-pass and a 725 low-pass filter.
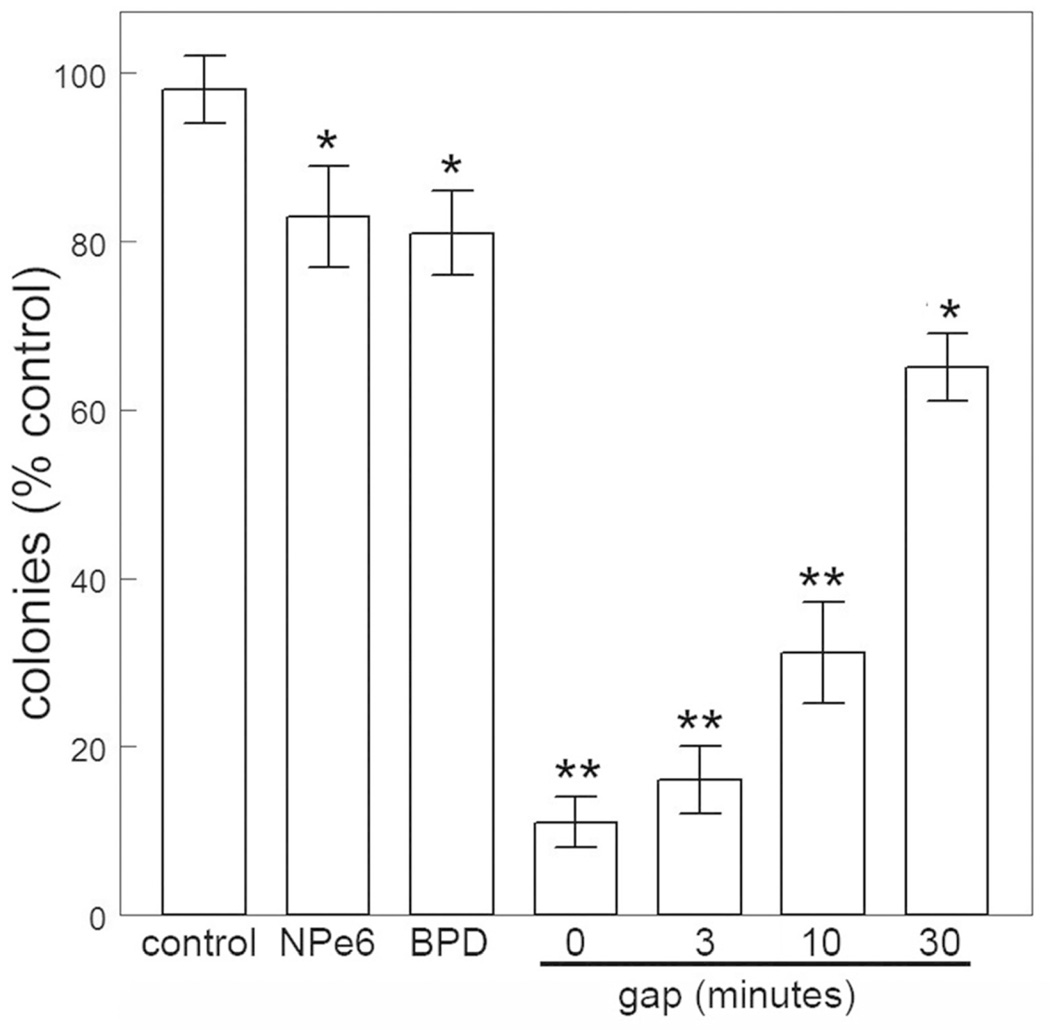
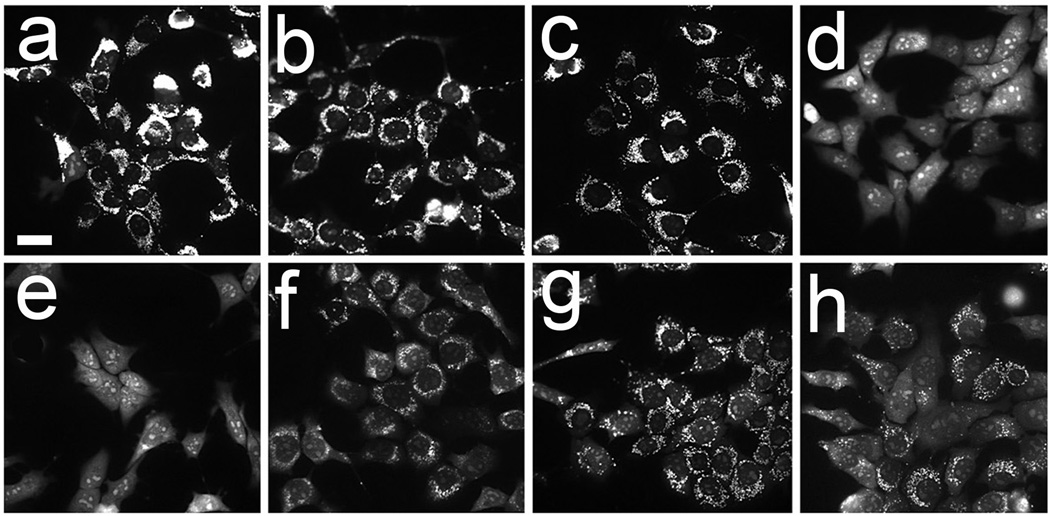
In prior studies involving the sequential PDT protocol, irradiations were carried out in sequence, with no gap between exposure of photosensitized cells to 660 nm and 690 nm light. When we held the cells at 37°C for varying intervals between irradiations, the ability of lysosomal photodamage to sensitize cells to the phototoxic effects of subsequent mitochondrial photodamage was gradually lost (Fig. 2). This effect was also reflected when we examined effects of these protocols on ΔΨm (Fig. 3) and on caspase activation (Table 1). The latter correlation is consistent with the proposal that photokilling by these protocols involves apoptotic cell death. When cells were held at 15°C between irradiations loss of ΔΨm was partially maintained (Fig. 3 panel h). These data indicate that the loss of efficacy enhancement is temperature-sensitive, likely reflecting the instability of the ATG5 fragment. Further studies will be designed to explore this possibility.
Figure 2.
Photokilling by NPe6 or BPD individually and sequentially (NPe6 →BPD) with the gap between irradiations varying from 0 to 30 min. Data represent mean ± SD for three determinations. Symbols: * = differs from control p < 0.01; ** p < 0.005.
Figure 3.
Loss of ΔΨm directly after photodamage: a, untreated control; b, BPD alone; c, NPe6 alone; d, sequential PDT NPe6 → BPD, no gap, e, 3 min gap, f, 10 min gap, g, 30 min gap; h, 30 min gap with cells held at 15°C. Light and drug doses are specified in the text. These images represent results of experiments that were repeated 3 times. White bar in panel a = 20 µm.
Table 1.
DEVDase activity associated with photodamage
| Conditions | DEVDase activity |
|---|---|
| Control | 0.07 ± 0.01 |
| BPD alone | 0.21 ± 0.04 |
| NPe6 alone | 0.11 ± 0.03 |
| BPD → NPe6 sequential irradiation, no gap | 2.6 ± 0.15* |
| BPD → NPe6 | 3' gap | 2.3 ± 0.11* |
| BPD → NPe6 | 10' gap | 1.8 ±0.15* |
| BPD → NPe6 | 30' gap | 0.9 ± 0.10 |
| BPD → NPe6 | 30‘ gap (cells held at 15°C) | 1.4 ± 0.18* |
| BPD/NPe6 | simultaneous irradiation | 2.8 ± 0.14* |
1c1c7 cultures were treated with 20 µM NPe6 and 0.5 µM BPD for 1 h, before irradiation as indicated in the text. DEVDase activity was measured 2 h after irradiation.
DEVDase units = nmol product/min/mg protein.
Values represent mean ± SD for three determinations.
Statistically different from control values (P < 0.05).
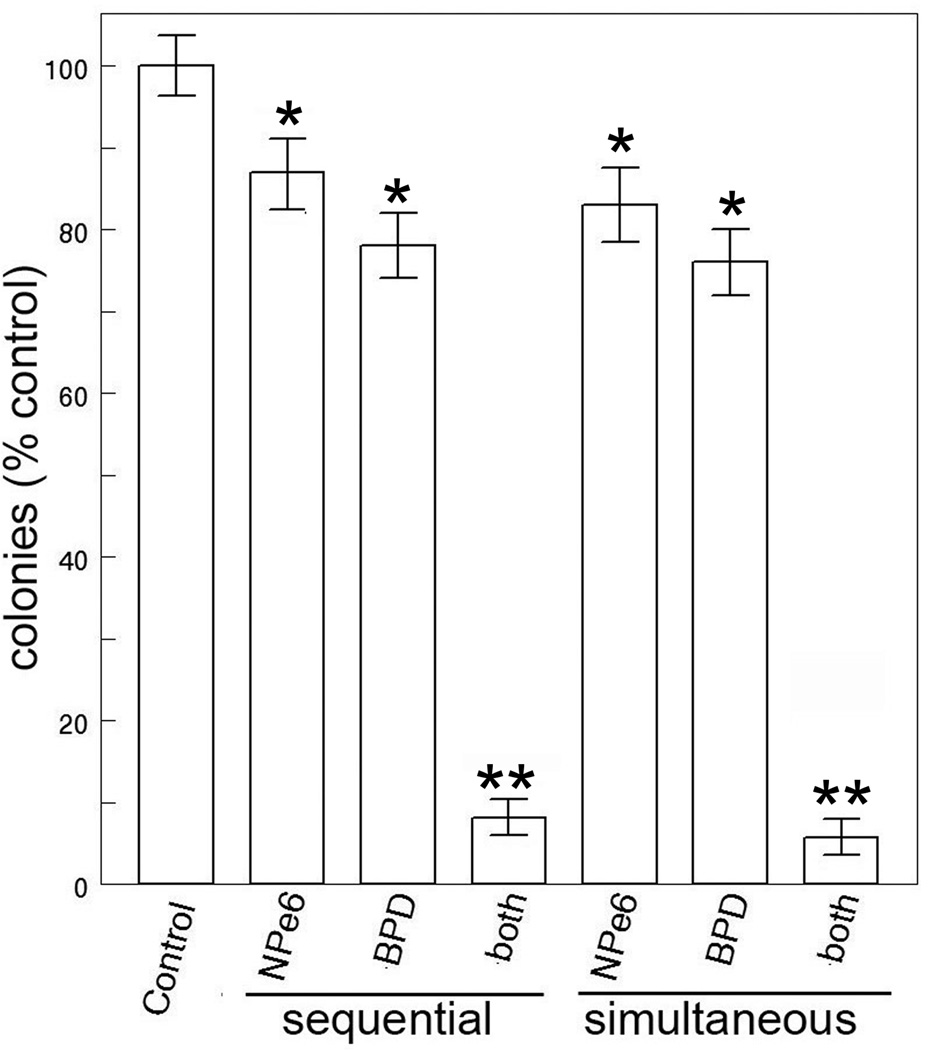
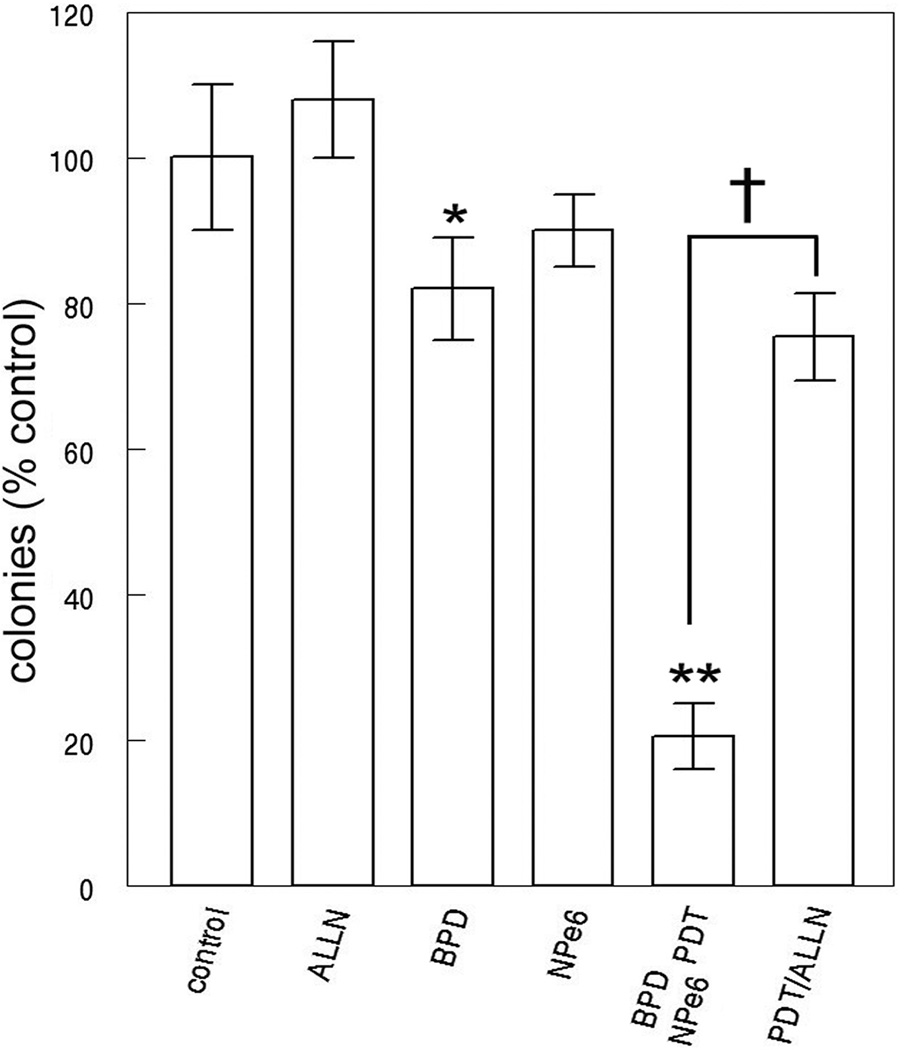
Using broad-band irradiation, it was feasible to simultaneously excite both NPe6 and BPD. Clonogenic studies indicated that the ‘simultaneous’ protocol was as effective as sequential irradiation where NPe6 photodamage occurred first (Fig. 4). In these experiments, individual interference filters were used to provide activating light for all results termed ‘sequential’ while 625–755 nm light was used to acquire results termed ‘simultaneous’. In the presence of the calpain antagonist ALLN, the level of photokilling by the NPe6/BPD combination was substantially reduced (Fig. 5). We attribute this to impairment of the calpain-mediated cleavage of ATG5.
Figure 4.
Photokilling of 1c1c7 cells in culture by a combination of NPe6 (20 µM) + BPD (0.5 µM) with irradiation proceeding sequentially (cells irradiated at 660 ± 10 nm, then at 690 ± 10 nm) or simultaneously using the 625–725 nm light. Data represent mean ± SD for three determinations. Symbols: * = differs from control p < 0.03; ** = < 0.005.
Figure 5.
Clonogenic studies showing the effect of the calpain antagonist ALLN on photokilling by simultaneous irradiation using NPe6 + BPD. These data represent the average ± SD for 3 experiments. Statistics: * = statistically different from control (p = 0.07); ** (P < 0.002). Bracketed values were different (p < 0.001). Photokilling induced by PDT using BPD or NPe6 only is also shown.
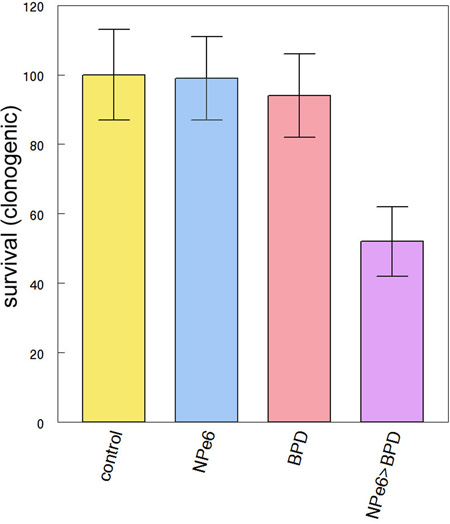
Even a minor level of lysosomal photodamage can have a significant effect on the subsequent efficacy of photodynamic effects directed toward mitochondria. In Table 2, we show the effect of 10 mJ/sq cm irradiation at 660 nm on subsequent photokilling mediated by BPD. Although the loss of viability after 660 nm irradiation was not statistically significant, this level of photodamage could significantly increase the loss of viability resulting from subsequent mitochondrial photodamage.
Table 2.
Effect of low-dose lysosomally-directed PDT on subsequent photokilling directed at mitochondria.
| Conditions | Clonogenicity (% control) |
|---|---|
| No additions | 100 ± 13 |
| NPe6/BPD, 660 nm (10 mJ/sq cm) | 99 ± 12 |
| NPe6/BPD 690 nm (20 mJ/sq cm) | 94 ± 12 |
| NPe6/BPD 660 nm then 690 nm | 52 ± 10* |
Cells were incubated with both photosensitizing agents and irradiated at 660 nm or 690 nm only, or sequentially at 660 and 690 nm. Data represent mean ± SD for three experiments.
Significantly different from untreated controls (p < 0.05).
In this regard, it must be remembered that our studies involve use of PDT for eradication of monolayers in cell culture, not treatment of large solid tumors in animal tumor models or in a clinical setting. It has been estimated from optical properties of typical tissues (12) that of 1000 photons at 690 nm falling on a 1 cm thickness of tissue, approx. 180 will penetrate 1 cm. With 650 nm, the number falls to 110 and at 630 nm, ~50. While these numbers will depend on the specific tissue being examined, the relative values should be valid.
CONCLUSIONS
In a prior publication, we reported that Photofrin, a porphyrin-based photosensitizing product, could be used for either step in the sequential protocol (7). This was attributed to the ability of Photofrin to target both lysosomes and mitochondria, as demonstrated in Ref. 7. Use of Photofrin as a photosensitizing agent essentially replicates the ‘simultaneous’ protocol involving NPe6 + BPD.
The sequential PDT approach has been shown to eradicate large (1 cm) tumors in an animal model (1), something not feasible with Photofrin, where the 630 nm exciting wavelength limits light penetration to < 5 mm (13). Although the efficacy of Photofrin is likely promoted by its multi-targeting ability, use of a 630 nm wavelength and a sensitizer with a relatively weak absorbance band in the red appears to limit the depth of tumor eradication even when both mitochondria and lysosomes are PDT targets. Data shown in Table 2 indicate that even a minor level of lysosomal photodamage can promote the lethality of PDT directed at mitochondria. Use of the combination PDT protocol with agents that have better absorbance profiles permits the eradication of tumor at a greater depth and/or volume. In some protocols, insertion of interstitial fibers is used to provide a sufficient light flux to treat larger tumors. A combination protocol using long-wavelength photosensitizers could also simplify this procedure, with fewer fibers being required for adequate cancer control.
Acknowledgments
This study was partially supported by a grant CA23378 from the NIH. Ann Marie Santiago provided excellent technical assistance during the course of these studies. Brian Pogue was helpful in interpreting data relating to optical properties of tissues.
REFERENCES
- 1.Cincotta L, Szeto D, Lampros E, Hasan T, Cincotta AH. Benzophenothiazine and benzoporphyrin derivative combination phototherapy effectively eradicates large murine sarcomas. Photochem Photobiol. 1996;63:229–237. doi: 10.1111/j.1751-1097.1996.tb03019.x. [DOI] [PubMed] [Google Scholar]
- 2.Peng TI, Chang CJ, Guo MJ, Wang YH, Yu JS, Wu HY, Jou MJ. Mitochondrion-targeted photosensitizer enhances the photodynamic effect-induced mitochondrial dysfunction and apoptosis. Ann. N. Y. Acad. Sci. 2005;1042:419–428. doi: 10.1196/annals.1338.035. [DOI] [PubMed] [Google Scholar]
- 3.Harvey EH, Webber J, Kessel D, Fromm D. Killing tumor cells: the effect of photodynamic therapy using mono-L-aspartyl chlorine and NS-398. Am. J. Surg. 2005;189:302–305. doi: 10.1016/j.amjsurg.2004.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessel D, Reiners JJ., Jr Enhanced efficacy of photodynamic therapy via a sequential targeting protocol. Photochem. Photobiol. 2014;90:889–895. doi: 10.1111/php.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessel D, Reiners JJ., Jr Promotion of proapoptotic signals by lysosomal photodamage. Photochem. Photobiol. 2015;91:931–936. doi: 10.1111/php.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessel D, Evans CL. Promotion of Pro-Apoptotic Signals by Lysosomal Photodamage: Mechanistic Aspects and Influence of Autophagy. Photochem. Photobiol. 2016;92:620–623. doi: 10.1111/php.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kessel D. Photodynamic therapy: Promotion of efficacy by a sequential protocol. J Porph. Phthalo. J Porph. Phthalo. 2016;20:302–306. doi: 10.1142/S1088424616500073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU. Calpain-mediated cleavage of ATG5 switches autophagy to apoptosis. Nat. Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 9.Roberts WG, Liaw LH, Berns MW. In vitro photosensitization II. An electron microscopy study of cellular destruction with mono-L-aspartyl chlorin e6 and photofrin II. Lasers Surg. Med. 1989;9:102–108. doi: 10.1002/lsm.1900090204. [DOI] [PubMed] [Google Scholar]
- 10.Andrzejak M, Price M, Kessel DH. Apoptotic and autophagic responses to photodynamic therapy in 1c1c7 murine hepatoma cells. Autophagy. 2011;7:979–984. doi: 10.4161/auto.7.9.15865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang KKW, Yuen P. Calpain inhibition: an overview of its therapeutic potential. Trends Pharmacol. Sci. 1994;15:412–419. doi: 10.1016/0165-6147(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 12.Sandell JL, Zhu TC. A review of in-vivo optical properties of human tissues and its impact on PDT. J. Biophotonics. 2011;4:773–787. doi: 10.1002/jbio.201100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson BC. Photodynamic therapy: light delivery and dosage for second-generation photosensitizers. In: Bock G, Harnett S, editors. Photosensitizing compounds: their chemistry, biology and clinical use. Ciba Foundation Symposium 146. Chichester, Toronto: John Wiley & Sons; 1989. pp. 60–77. [DOI] [PubMed] [Google Scholar]