Abstract
To overcome limiting factors in mass spectrometry-based screening methods such as automation while still facilitating the screening of complex mixtures such as botanical extracts, magnetic microbead affinity selection screening (MagMASS) was developed. The screening process involves immobilization of a target protein on a magnetic microbead using a variety of possible chemistries, incubation with mixtures of molecules containing possible ligands, a washing step that removes non-bound compounds while a magnetic field retains the beads in the microtiter well, and an organic solvent release step followed by LC-MS analysis. Using retinoid X receptor-α (RXRα) as an example, which is a nuclear receptor and target for anti-inflammation therapy as well as cancer treatment and prevention, a MagMASS assay was developed and compared with an existing screening assay, pulsed ultrafiltration (PUF)-MS. Optimization of MagMASS involved evaluation of multiple protein constructs and several magnetic bead immobilization chemistries. The full-length RXRα construct immobilized with amylose beads provided optimum results. Additional enhancements of MagMASS were the application of 96-well plates to enable automation, use of UHPLC instead of HPLC for faster MS analyses, and application of metabolomics software for faster, automated data analysis. Performance of MagMASS was demonstrated using mixtures of synthetic compounds and known ligands spiked into botanical extracts.
TOC graphic
Introduction
Approximately 50% of the cancer drugs used in the last 50 years have been inspired by natural products [1]. The main sources of discovery of these therapeutic natural product compounds have been ethnomedicine and biological screening [2, 3]. While these sources have historically been valuable for drug discovery, modern methods using reverse pharmacology drug discovery techniques (also known as target-based drug discovery) have been underutilizing natural products.
Instead of the slow process of testing for changes in a living cell or organism in response to a compound, knowledge of the disease-relevant receptor-ligand interactions allows for interrogation of a specific pathway. For example, a recombinant protein can be exposed to a single compound or mixture of compounds, and any receptor-ligand interactions can be detected by using fluorescence, enzymatic product formation, thermal stability change, or another method. After a hit is detected, the individual compound must be identified, isolated and retested for activity, and eventually developed as a drug lead. However, mixtures of natural products such as botanical extracts are rarely used in high-throughput screening primarily because of the extra expertise and time required to identify active constituents [3].
As with any reverse pharmacology drug discovery screen, biological relevance depends upon the choice of target. In this investigation, the retinoid X receptor-α (RXRα) was used as the target protein because it is an important nuclear receptor in the cancer protein network, is a known target for multiple chemotherapy agents, and unlike most other nuclear receptors, few ligands are known [4, 5]. The endogenous ligand for RXRα is 9-cis retinoic acid, a derivative of vitamin A [6]. A knock-out mouse of RXRα develops a phenotype similar to vitamin A deficient mice with characteristic developmental morphology, differentiation, and cellular growth [7]. One major mechanism of cell death linking RXRα activity with cancer is through its inhibition of NF-E2 P45-related factor 2 (Nrf2) [8]. Treatment with an RXRα ligand can modulate the genes regulated by Nrf2, including critical cytoprotective genes implicated in cancer. Several RXRα ligands, such as bexarotene, have received FDA approval to treat lymphoma [4, 9], but these compounds have serious toxic side effects. Therefore, less toxic RXRα ligands are needed for therapeutic use.
RXRα has several structural requirements for biological activity and use in high-throughput screening. After binding a ligand, RXRα monomers change conformation and dimerize to form an active confirmation that can bind to DNA [10, 11]. Because RXRα dimerizes with one-third of all nuclear receptors including itself, the challenge in targeting RXRα is obtaining specificity [12, 13]. The ligand binding domain (LBD) of RXRα is relatively independent, both structurally and functionally, and has been used instead of the full-length construct to study many structural and functional aspects of RXRα [14, 15]. Both full-length RXRα and its LBD were considered in this paper.
Pulsed ultrafiltration mass spectrometry (PUF-MS) was invented in 1997 to address the need for screening mixtures of compounds such as synthetic combinatorial libraries and complex natural product mixtures such as botanical extracts as a first step in drug discovery [16]. Similar to other reverse pharmacology methods, PUF-MS begins with the incubation of small molecules with the target protein of interest (Figure 1A). Protein and ligand are free in solution, and protein-free compounds are separated from the protein-bound fraction by filtration through an ultrafiltration membrane. PUF-MS is sensitive and reliable [17], but speed of the assay is limited by the ultrafiltration step. To increase the throughput of reverse pharmacology natural product mixture screening, we developed Magnetic Microbead Affinity Selection Screening (MagMASS), in which the target of interest is tethered to magnetic beads instead of free in solution [18]. To separate ligands from unbound compounds, the receptor-bound fraction is retained in a well of a microtiter plate using a magnet while the unbound fraction is removed (Figure 1B). Magnetic beads are often used for affinity isolation of proteins from complex mixtures [19], but they are less often used to isolate small molecule ligands to a target for discovery [20].
Figure 1. Comparison of PUF-MS and MagMASS.
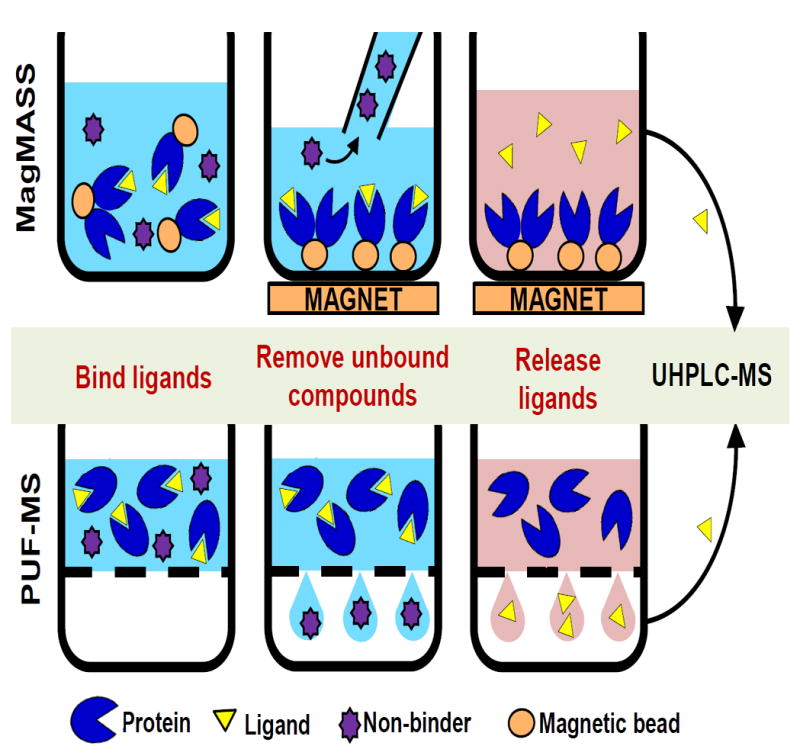
During affinity selection screening, ligands (yellow triangles) but not other low molecular weight compounds (purple stars) bind to a macromolecular target (RXRα, blue). A) In PUF-MS, the ligand-receptor complexes are separated in solution from non-binding compounds by filtration through an ultrafiltration membrane (grey dashed line). Ligands are released by denaturing the receptor and recovered in the ultrafiltrate for LC-MS analysis. B) During MagMASS, an external magnetic force is applied to secure the magnetic beads (orange ovals) containing immobilized receptor and affinity bound ligand while the unbound compounds are washed away. Ligands are released using organic solvent and/or a pH change and separated from the magnetic beads for UHPLC-MS analysis.
With the goal of increasing the throughput of affinity mass spectrometric-based screening, a new MagMASS assay was developed using RXRα as the target protein, which enabled direct comparison with a previously developed PUF-MS RXRα assay. Although both full-length and the ligand binding domain of RXRα were active in the PUF-MS assays, only the full-length form of RXRα was active after immobilization for MagMASS. Both MS screening methods were effective at affinity isolation of RXRα ligands from complicated matrices such as botanical extracts. The throughput of both approaches benefited equally from the substitution of UHPLC for HPLC during MS analyses and from the application of metabolomics software for automated UHPLC-MS data analysis. However, MagMASS provided several distinct advantages over PUF-MS, including 6-fold faster separation of bound ligand from unbound compounds and compatibility with 96-well automation.
Experimental Section
Chemicals and Reagents
Ketoconazole, LG100268, 9-cis-retinoic acid, and 13-cis-retinoic acid were purchased from Sigma-Aldrich (St. Louis MO, USA). Polypropylene 2-mL conical bottom 96-well plates were purchased from Fisher Scientific (Hanover Park, IL). Amicon Ultra Centrifugal Filters 10K and 30K were purchased from Millipore (Billerica, MA, USA). Pierce N-hydroxy-succinamide (NHS)-activated magnetic beads were purchased from Thermo Scientific (Rockford, IL, USA), and amylose-functionalized magnetic beads were purchased from New England Biolabs (Ipswich, MA, USA). NanoOrange kits (Life Technologies, Hanover Park, IL) were used to measure protein concentrations. Deionized water was prepared using a PureLab Option-Q purification system (Elga, Woodridge, IL).
A 20-compound equimolar mixture (10 µM each in methanol) of non-RXRα ligands was prepared from an in-house library and was tested for purity using a Shimadzu (Kyoto, Japan) ion trap-time-of-flight high resolution mass spectrometer: PA452, peroxicam, indomethacin, thiamine HCl, custom steroid II-39, custom steroid II-17-1, melatonin, oxomate, acetaminophen, spirobrassinin, yangonin, harmaline, flavone, N-methylserotonin, custom carbolene, isoliquiretigenin, tolbutamide, eridictual, formononetin, and naringenin. The compounds were selected to simulate the chemical diversity of both combinatorial libraries and natural product libraries without introducing the matrix associated with a botanical extract. Aerial plant parts (leaf, stem and inflorescence) of Proserpinaca palustris L. (mermaid weed) and Stenaria nigricans (Lam.) Terrell (diamond flowers) were obtained from the Chicago Botanic Garden (Chicago, IL) where they were taxonomically identified. Voucher specimens (herbarium voucher numbers 13706 and 18630) were deposited in the Chicago Botanical Garden Herbarium. Extracts were prepared via cold percolation in methanol and dried by rotary evaporation. The extracts were reconstituted at 40 mg/mL in methanol.
RXRα, RXRα ligand binding domain (LBD), and maltose binding protein (MBP)
The plasmid for recombinant RXRα was generously provided by Prof. Matthew Redinbo (University of North Carolina, Chapel Hill, NC). Protein was expressed and purified as described previously. Briefly, both MBP (N-terminus, 43 kDa) and the SRC-1 co-activator peptide (C-terminus, 2 kDa) are present on the protein construct and required for enhanced stability and solubility of full-length RXRα, totaling a 96 kDa protein [19]. The RXRα construct was transformed into BL21(DE3) cells for over-expression and grown at 37°C in LB media until reaching A600>0.6, upon which isopropyl β-D-1-thiogalactopyranoside was added to a final concentration of 1 mM. The temperature was reduced to 20°C, and the cells were grown for an additional 20 h. After expression, cells were harvested by centrifugation and lysed by using an Emulsiflex C5 (Avestin, Ottowa, ON). The lysate was cleared by centrifugation at 30,000×g, and the soluble material was passed over NTA-Sepharose beads (Life Technologies, Grand Island, NY). The NTA-Sepharose beads were washed with 50 column volumes of 50 mM Tris (2-carboxyethyl)phosphine (pH 7.5) containing 500 mM NaCl and 25 mM imidazole, and the protein was eluted from the column in this buffer with the imidazole concentration increased to 500 mM.
Half of the purified protein was exchanged into 25 mM Tris (pH 7.5) 250 mM NaCl, 20% glycerol, and 0.5 mM TCEP (tris(2-carboxyethyl)phosphine) by overnight dialysis or by gel-filtration using an SD75 column (GE Healthcare, Pittsburgh, PA) to produce pure MBP-RXRα with a final concentration of 15 µM. The remaining starting material was digested with a 1:50 ratio (mol/mol) of TEV protease overnight at 4°C while being dialyzed into the low imidazole buffer to remove RXRα. After TEV protease digestion, the His6-MBP protein was re-purified using NTA-Sepharose beads and again dialyzed into the same buffer as MBP-RXRα. Purified MBP alone was used as a control for full-length MBP-RXRα screening experiments. An SDS-PAGE gel was used to confirm the molecular weight and purity of MBP-RXRα and MBP, which showed that MBP was present as a single band of 43 kDa and that MBP-RXRα appeared at 96 kDa and showed some minimal degradation at lower molecular weight bands (Supplemental Figure 1).
The LBD of RXRα (expressed in E. coli), corresponding to amino acids 223 – 463 and an apparent molecular weight of 27 kDa, was purchased from Active Motif (Carlsbad, CA) at 6.17 mg/mL in 50 mM Tris (pH 8.0), 150 mM NaCl, 1mM dithiothreitol, and 50% glycerol. SDS-PAGE was used to confirm the molecular weight and purity of RXRα LBD, which showed only minimal degradation and minor dimerization (Supplemental Figure 1). Denatured LBD was prepared by heating at 90°C for 10 min and was used as a negative control for RXRα LBD experiments.
Immobilization of proteins on magnetic beads
N-Hydroxy-succinimide (NHS)-activated magnetic beads (20 µL per well, 10 mg/mL beads, approximate binding capacity 20-50 µg protein/mg of bead) were used to immobilize MBP-RXRα, control MBP, LBD, or denatured LBD. A 60-lb magnetic plate consisting of 12 neodymium rare earth magnets (N45 2×1×1/8" NdFeB) (CMS Magnetics, Garland, TX) was used to retain the beads while storage buffer was removed. The beads were washed with 1 mM HCl, and the beads were resuspended in 300 μL 50 mM borate buffered to pH 7.5. Protein was immobilized by incubating 100 pmol protein with the beads at room temperature for 1 h with gentle shaking every 5 min. Proteins remaining in the supernatant were saved for quantification using a NanoOrange kit. Beads were washed twice with 0.1 M glycine buffer (pH 2.0) and washed once with water. Unreacted NHS sites were saturated by incubating with excess 3 M ethanolamine at pH 9.0 for 1 h. The beads were washed once with water and resuspended in 300 μL TBS buffer containing 50 mM Tris-buffered saline and 500 mM NaCl for the binding assays.
For immobilization on amylose magnetic beads, the beads were washed with water and resuspended in TBS buffer containing 50 mM Tris-buffered saline and 0.5 M NaCl. Beads (20 μL, 10 mg/mL) were incubated with the target protein, RXRα-MBP or MBP (100 pmol), and a ligand (10 pmol). The amylose magnetic beads will bind with the MBP and the ligand will bind with the RXRα. The incubation solution was removed from the amylose beads, and unbound protein was quantified using the NanoOrange kit.
RXRα assays
All screening experiments contained the same incubation buffer (0.2 M NaCl and 20 mM Tris-HCl adjusted to pH 7.4) at the same incubation volume (300 μL), and identical amounts of ligand(s) (10 pmol each) and protein (100 pmol). Experiments testing the matrix effects of botanical extracts contained P. palustris extract or S. nigricans extract at 133 μg/mL. Experiments evaluating possible interference from combinatorial libraries screened 20-compound mixtures (10 μM each in methanol).
PUF-MS was carried out as described previously [17] with minor alterations. Briefly, the ultrafiltration membrane (10 KDa cut-off for RXRα LBD or 30 KDa cut-off for MBP-RXRα and MBP) was pre-washed with 150 μL binding buffer by centrifugation at 13,000×g for 10 min at 4°C. Protein and ligand mixtures were incubated in the dark for 1 h at room temperature. Then, the unbound fraction was removed by centrifugation at 13,000×g for 10 min at 4°C. Protein was washed three times with 300 μL portions of 30 mM ammonium acetate (pH 7.5). Ligand mixtures were eluted with two washes of 100 μL 90% methanol in water. The released ligands were evaporated to dryness and then reconstituted in 50 μL 80% aqueous methanol containing 100 nM ketoconazole internal standard immediately prior to LC–MS analysis.
MagMASS was carried out in 2-mL 96-well plates with conical bottoms. Beads (20 μL containing 100 pmol protein) and ligand were added to 279 μL incubation buffer, and incubated at 4°C for 1 h. Beads were resuspended every 15 min using a multichannel pipette. Beads were retained on the magnetic plate while the unbound fraction was removed by washing 3 times with 900 μL aliquots of binding buffer and one last wash with water. Ligands were eluted by treating the beads with 100 μL methanol containing 50 nM ketoconazole (internal standard for UHPLC-MS) and transferred to clean wells. After evaporation to dryness, each ligand sample was reconstituted in 50 μL of 80% methanol.
Quantification of proteins was performed using NanoOrange Protein Quantitation Kits as follows. Standard protein samples of MBP-RXRα, MBP, and LBD were prepared by dilution with NanoOrange diluent (2 µM sodium azide) to 20 µg/mL, 15 µg/mL, 10 µg/mL, 5 μg/mL, 2 μg/mL, and 0 μg/mL. The supernatants remaining after protein immobilization on magnetic beads were normalized to the background fluorescence due to the respective buffers. Regression equations were calculated from the protein dilutions normalized to fluorescence from diluent alone. Using this limit of detection and the theoretical amount of protein present in solution if no protein was bound to beads after the immobilization step, the LOD of percent unbound was calculated for each protein in each buffer.
Ligand analyses were carried out using a Shimadzu LCMS-8040 mass spectrometer equipped with electrospray and a Shimadzu Nexera UHPLC system. Separations were carried out at room temperature using an in-line filter and a Waters Xterra C18 HPLC column (2.1 mm × 50 mm, 3 μm) or a BEH UHPLC column (2.1 mm × 50 mm, 1.7 μm). The mobile phase consisted of a 3-min linear gradient from 30% to 100% methanol in 0.1% formic acid in water at a flow rate of 0.6 mL/min. Each library compound, internal standard (ketoconazole), and ligand were measured using UHPLC-MS/MS with collision-induced dissociation and selected reaction monitoring (SRM) using optimized electrospray parameters (nebulizing gas flow 3 L/min, desolvation line 250 °C, heat block 400 °C, drying gas flow 15 L/min), collision energies and SRM transitions. A total of 50-transitions were monitored using a 2 ms dwell time for library screening or a 13 ms dwell time for quantitative analyses of ligands. The SRM transitions for quantitation included m/z 364 to 294 for LG100268, m/z 301 to 123 for 9-cis retinoic acid, m/z 301 to 81 for 13-cis retinoic acid, and m/z 531 to 244 for ketoconazole (see chemical structures in Supplemental Figure 2). All the compounds were analyzed in a single method using the fast polarity switching (5 ms) of the Shimadzu LCMS-8040. The software used for collecting and viewing data was Shimadzu Lab Solutions Version 5.65.
Standards were prepared over the range 10 nM to 1000 nM containing ketoconazole at 100 nM as an internal standard. The upper limit of detection corresponded to 100% retention of a positive control ligand by the target protein. Specific binding was determined based on an increase in peak area in the UHPLC–MS chromatogram of a ligand relative to the corresponding negative control incubation with denatured protein. Statistics on the MagMASS and PUF-MS dataset were performed in Excel for Mac version 14.5.1. A one-way paired t-test was performed on the data for each experimental condition with replicates over four days.
Results and Discussion
To identify ligands of a target protein most efficiently, the MagMASS technique was optimized and validated at every step, from protein immobilization to the evaluation of matrix effects. Two magnetic bead functional group chemistries were evaluated for immobilizing the target protein: covalent tethering by NHS and immobilization by amylose-MBP affinity. A 10-fold excess of theoretical bead capacity was used to ensure efficient protein immobilization, and untethered protein remaining in solution after immobilization was measured. Both NHS and amylose magnetic beads retained MBP-RXRα or MBP alone with nearly 100% efficiency (Supplemental Table 1).
To verify the functionality of immobilized RXRα compared with solution-phase protein, affinity capture of the ligand LG100268 was compared using the established PUF-MS approach [17] and MagMASS using NHS beads (covalent attachment) or amylose-MBP beads (non-covalent immobilization) (Figure 2). Using identical amounts of protein and ligand, LG100268 was detected as a specific ligand for RXRα using all three approaches with MBP as a negative control (Figure 2). Relative to the internal standard (ketoconazole), PUF-MS showed the strongest signal for the affinity recovery of LG100268 (79% recovery; p<0.05, N=6) followed by NHS immobilization (33% recovery; p<0.001, N=9) and then amylose (21% recovery; p<1×10-4, N=8).
Figure 2. Comparison of MagMASS and pulsed ultrafiltration LC-MS (PUF-MS) to test ligands for binding to MBP-RXRα.
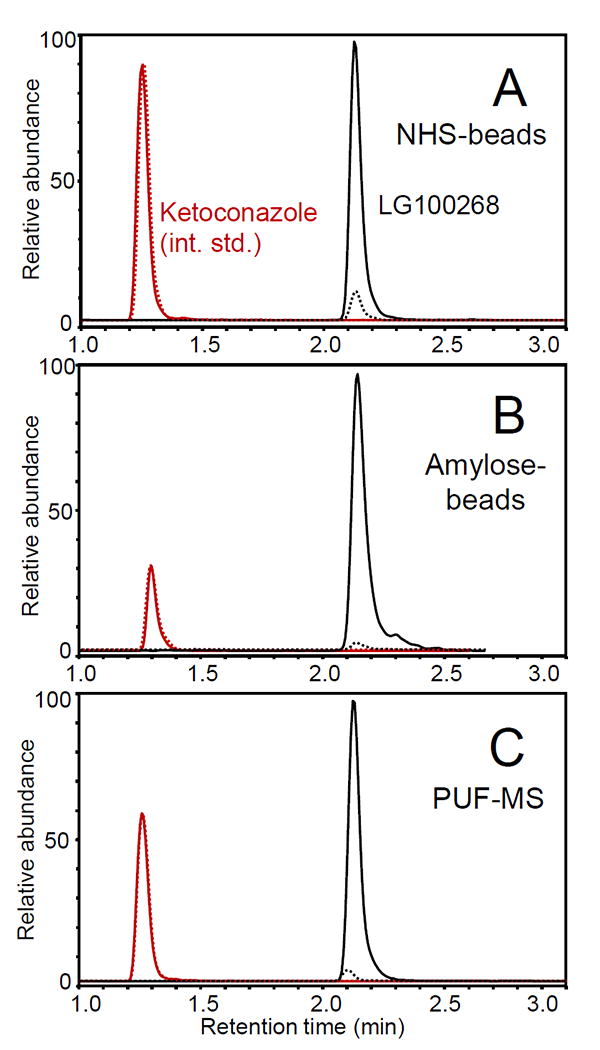
The UHPLC-MS/MS SRM chromatograms show the RXRα ligand LG100268 (SRM transition m/z 364 to 294, positive ion electrospray) retained by MBP-RXRα (solid line) compared with the MBP negative control (dashed line). Each incubation contained 100 pmol protein and 100 nM LG100268. The chromatograms were normalized to the internal standard, ketoconazole (100 nM; positive ion electrospray SRM transition m/z 531 to m/z 244). A) MagMASS using MBP-RXRα immobilized on NHS beads; B) MagMASS using amylose beads to retain the MBP-containing protein; and C) PUF LC-MS using the MBP-RXRα protein in solution. Note that MBP is maltose binding protein (43 kDa) and that MBP-RXRα (96 kDa) is a stable construct of RXRα containing MPB on the N-terminus and SRC-1 co-activator peptide (2 kDa) on the C-terminus.
The covalent NHS mechanism of protein immobilization employs a N-succinamide functional group on the magnetic bead, which reacts with primary amines mostly on lysines and arginines of proteins. Although LG100268 was retained in high abundance during MagMASS using NHS-immobilized MBP-RXRα, the immobilization process (priming the beads, immobilizing the protein, and deactivating the unreacted sites on the beads) required ~4 h to complete. Furthermore, immobilization through random lysine and arginine residues, especially through multipoint attachment to the support, can change the functionality of the protein. Although immobilization of receptors and enzymes in some orientations can block access of ligands to the active site [20], selective protein immobilization can retain activity while enhancing stability such as resistance to denaturation [21, 22].
Proteins are often expressed with an MBP tag to stabilize the protein during recombinant expression, including the full-length RXRα construct used in this investigation. By leaving MBP on the RXRα target protein, no time had to be expended removing the tag and then repurifying RXRα. To demonstrate the feasibility of MagMASS for natural product discovery, MBP-RXRα immobilized on NHS-magnetic beads was incubated with the known ligand LG100268 spiked into a botanical extract. The UHPLC-MS/MS chromatograms in Supplemental Figure 3 show that the botanical extract matrix did not interfere with MagMASS affinity extraction and detection of LG100268 as a ligand for immobilized RXRα. Similarly, LG100268 was detected with strong abundance using MagMASS screening of a small 20-compound library or no matrix at all (Supplemental Figure 3). Table 1 shows additional MagMASS screening data for the selective detection of LG100268 in a botanical matrix extracted from S. nigricans. In each case, the ligand LG100268 was identified with high confidence (p<0.006) compared with controls carried out using denatured protein.
Table 1.
Immobilization methods of MagMASS for all proteins investigated have an extremely high binding efficiency. Almost all of the protein for immobilization is available in the well for ligand binding.
| RXRα Constructa | Matrixb | LG100268c | 9-cis-Retinoic acidd | 13-cis-Retinoic acid |
|---|---|---|---|---|
| Enrichment ± Standard errore | ||||
| MBP-RXRα NHS | Buffer only | 17.2 ± 13.9 (N=9) p<0.01f | 6.7 ± 1.7 (N=8) p<0.01 | 1.1 ± 0.1 (N=4) p>0.2 |
| “ | Compound library | 42.4 ± 90.1 (N=8) p<0.01 | 5.6 ± 1.9 (N=8) p<0.01 | 0.9 ± 0.1 (N=5) p>0.2 |
| “ | P. palustris extract | 298.6 ± 459.7 (N=7) p<0.01 | 24.3 ± 7.2 (N=6) p<0.01 | N/A (N=4) p>0.2 |
| “ | S. nigricans extract | 72.5 ± 49.8 (N=4) p<0.01 | 12.0 ± 4.6 (N=3) p<0.05 | N/A (N=4) p>0.2 |
| MBP-RXRα amylose | Buffer only | 8.6 ± 2.0 (N=4) p<0.01 | 2.8 ± 0.9 (N=8) p=0.22 | 0.9 ± 0.1 (N=5) p>0.2 |
| “ | Compound library | 10.5 ± 4.2 (N=7) p<0.01 | 1.9 ± 0.4 (N=8) p=0.06 | 1.6 ± 0.3 (N=4) p>0.2 |
| “ | P. palustris extract | 56.8 ± 34.3 (N=9) p<0.01 | 9.5 ± 2.2 (N=9) p<0.01 | N/A (N=5) p>0.2 |
| “ | S. nigricans extract | 59.0 ± 47.0 (N=4) p<0.01 | 6.1 ± 2.2 (N=4) p<0.05 | N/A (N=5) p>0.2 |
MBP-RXRα was immobilized using either covalent attachment of amino groups via NHS on the magnetic beads or through non-covalent interaction between MPB and amylose beads.
Possible interference of ligand binding to MBP-RXRα was investigated using different matrices ranging from simple buffer to complex botanical extracts.
9-cis-Retinoic was tested as an endogenous ligand for RXRα (Kd 15.7 nM [25]), while isomeric 13-cis retinoic was used as a non-binding negative control [25].
The enrichment factor (peak area compound bound to RXRα/ peak area compound bound to denatured protein) was averaged over all replicates.
One-way paired t-test was used to evaluate the difference between results obtained using active RXRα and denatured protein.
The effects of matrix as well as substrate on MagMASS screening were investigated using not only MBP-RXRα immobilized on NHS-magnetic beads but also MBP-RXRα immobilized on amylose-magnetic beads (Table 1). Both immobilization methods produced active RXRα that efficiently bound LG100268 or the endogenous ligand 9-cis-retinoic acid in the presence of botanical extract matrix. This is indicated by the large enrichment factors in Table 1, which are calculated as the ratio of peak areas of specifically bound ligand to non-specifically bound ligand (noise). Because 9-cis-retinoic acid (Kd 15.7 nM [25]) has lower affinity for RXRα than does LB100268 (Kd 3 nM [26]), the probability of detecting 9-cis-retinoic acid was lower in the MagMASS screens (Table 1). Although 9-cis-retinoic acid could be detected with significant enrichment factors regardless of matrix when using MBP-RXRα immobilized on NHS-magnetic beads, 9-cis-retinoic acid was not always detected when assayed alone or with the 20-compound library using amylose-magnetic beads. This might have been caused by non-specific binding of 9-cis-retinoic acid to amylose that was blocked in the presence of the botanical extract. As a negative control, note that the non-ligand of RXRα, 13-cis-retinoic acid [25] (an isomer of 9-cis-retinoic acid) was not detected as a ligand of RXRα during MagMASS screening regardless of the matrix or the form of immobilized protein (Table 1).
Like other affinity selection mass spectrometric screening approaches, MagMASS may be used to rank ligands with respect to affinity for a particular receptor. Under all test conditions, the highest affinity ligand LG100268 produced the highest enrichment factor as shown in Table 1. The ligand with the next highest affinity for RXRα, 9-cis-retinoic acid, showed the next highest enrichment factors during MagMASS, and the non-ligand 13-cis-retinoic acid produced the lowest enrichment factors (Table 1). Note that enrichment factors obtained using PUF LC-MS have been used to rank human and equine estrogens based on their relative affinities for the estrogen receptors [27]. Although enrichment factors provide relative binding data that may be used to rank ligands in order of affinity, they do not provide affinity constants.
The protein construct used for magnetic bead immobilization was crucial for maintenance of protein integrity. This was particularly evident in the case of the ligand binding domain (LBD) of RXRα. Although active in solution during PUF-MS screening assays, RXRα-LBD completely lost the ability to bind ligands such as LG100268 after immobilization on NHS-magnetic beads but not when immobilized on amylose beads (Supplemental Figure 4). We hypothesize that truncated proteins such as RXRα-LBD have fewer sites available for NHS tethering to occur, and this increases the likelihood of covalent immobilization through amino acids side chains at or near the active site. This might block the active site or cause tertiary structural changes of the protein at the active site, either of which might lower the affinity of the receptor for ligands.
As a solution to the problem of maintaining activity upon immobilization of RXRα, we retained MBP on RXRα (which had been used during protein expression and purification) and immobilized the protein on amylose magnetic beads. Because MBP binds amylose, amylose-functionalized beads may be used to immobilize RXRα-MBP without affecting the conformation or sterically hindering ligand access to the active site (Supplemental Figure 4). As illustrated by these examples, optimization of protein construct and type of functionalized magnetic bead need to be experimentally determined for each protein target during the development of new MagMASS assays.
Protein consumption during MagMASS screening is minimal, requiring only 100 pmol per well. RXRα-MBP was stable for at least 24 hours after immobilization with NHS beads. Immobilization on NHS beads requires approximately 4 h, but this step may be eliminated when using amylose magnetic beads. MagMASS is a fast, automatable assay requiring 1.5 hours to prepare each 96-well plate. UHPLC separation is the rate limiting step. In comparison, PUF-MS has not yet been automated and requires 9 h to process each 96 well plate.
Conclusions
Natural products compound diversity in botanical and microbial extracts is currently not being leveraged adequately by the pharmaceutical industry largely because these mixtures are slower to screen than discreet compounds in combinatorial libraries. To address this issue, MagMASS offers a new approach for high-throughput natural product screening that is fast, automatable, requires minimal protein and extract, and yields reproducible screening results. Compared with our previous PUF-MS approach, MagMASS is over 100-fold faster due to a combination of using UHPLC in place of HPLC, faster sample processing using magnetic beads in place of ultrafiltration, and using 96-well plates. Nevertheless, PUF-MS still has advantages such as the convenience of skipping the immobilization process and higher ligand recovery.
MagMASS is unique in comparison to other high throughput screening techniques in that it provides information about the binding activity directly using mass spectrometry, it is inexpensive, and the magnetic beads add versatility to its application. The versatility of MagMASS extends beyond screening for ligands of the active sites of receptors and enzymes. For example, functional assays may be carried out using enzymes immobilized using our MagMASS approach in which the incubation mixture is analyzed for a reaction product. MagMASS does not require displacing or binding to a particular active site, as all sites are available for binding ligands. Consequently, it is important to note that MagMASS may be used to identify allosteric ligands in addition to those binding to the active site of the immobilized protein. Binding of ligands to allosteric sites may be differentiated from binding at the active site by demonstrating binding or displacement in the presence of a high affinity active site ligand.
Supplementary Material
Acknowledgments
The authors thank Matthew Redinbo for providing the RXRα plasmid. This work was supported by the grants R01 AT007659 and T32 AT007533 from the NIH National Center for Complementary and Integrative Health. The authors acknowledge the Chicago Botanic Garden for donating all plant material used during the development of this assay. All plant extractions were performed with support from NIH grants P50AT000155-13S1 and P50AT000155 from the Office of Dietary Supplements and the National Center for Complementary and Integrative Health.
References
- 1.Newman DJ, Cragg GM. Natural Products As Sources of New Drugs over the 30 Years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eldridge GR, Vervoort HC, Lee CM, Cremin PA, Williams CT, Hart SM, Goering MG, O’Neil-Johnson M, Zeng L. High-throughput method for the production and analysis of large natural product libraries for drug discovery. Anal Chem. 2002;74:3963–71. doi: 10.1021/ac025534s. [DOI] [PubMed] [Google Scholar]
- 3.Beutler JA. Current Protocols in Pharmacology. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2009. Natural Products as a Foundation for Drug Discovery. [DOI] [PubMed] [Google Scholar]
- 4.Boehm MF, Zhang L, Zhi L, McClurg MR, Berger E, Wagoner M, Mais DE, Suto CM, Davies JA, Heyman RA. Design and synthesis of potent retinoid X receptor selective ligands that induce apoptosis in leukemia cells. J Med Chem. 1995;38:3146–55. doi: 10.1021/jm00016a018. [DOI] [PubMed] [Google Scholar]
- 5.Wagner CE, Jurutka PW, Marshall PA, Groy TL, van der Vaart A, Ziller JW, Furmick JK, Graeber ME, Matro E, Miguel BV, Tran IT, Kwon J, Tedeschi JN, Moosavi S, Danishyar A, Philp JS, Khamees RO, Jackson JN, Grupe DK, Badshah SL, Hart JW. Modeling, synthesis and biological evaluation of potential retinoid X receptor (RXR) selective agonists: novel analogues of 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)ethynyl]benzoic acid (bexarotene) J Med Chem. 2009;52:5950–66. doi: 10.1021/jm900496b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heyman Ra, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- 7.Mascrez B, Ghyselinck NB, Chambon P, Mark M. A transcriptionally silent RXR supports early embryonic morphogenesis and heart development. Proc Natl Acad Sci. 2009;106:4272–4277. doi: 10.1073/pnas.0813143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J, Wang H, Tang X. Rexinoid inhibits Nrf2-mediated transcription through retinoid X receptor alpha. Biochem Biophys Res Commun. 2014;452:554–9. doi: 10.1016/j.bbrc.2014.08.111. [DOI] [PubMed] [Google Scholar]
- 9.Duvic M, Martin AG, Kim Y, Olsen E, Wood GS, Crowley CA, Yocum RC. Worldwide Bexarotene Study Group: Phase 2 and 3 clinical trial of oral bexarotene (Targretin capsules) for the treatment of refractory or persistent early-stage cutaneous T-cell lymphoma. Arch Dermatol. 2001;137:581–93. [PubMed] [Google Scholar]
- 10.Dong D, Noy N. Heterodimer formation by retinoid X receptor: Regulation by ligands and by the receptor’s self-association properties. Biochemistry. 1998;37:10691–10700. doi: 10.1021/bi980561r. [DOI] [PubMed] [Google Scholar]
- 11.Xia G, Boerma LJ, Cox BD, Qiu C, Kang S, Smith CD, Renfrow MB, Muccio DD. Structure, energetics, and dynamics of binding coactivator peptide to the human retinoid X receptor a ligand binding domain complex with 9-cis-retinoic acid. Biochemistry. 2011;50:93–105. doi: 10.1021/bi101288y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conda-Sheridan M, Park E-J, Beck DE, Reddy PVN, Nguyen TX, Hu B, Chen L, White JJ, van Breemen RB, Pezzuto JM, Cushman M. Design, synthesis, and biological evaluation of indenoisoquinoline rexinoids with chemopreventive potential. J Med Chem. 2013;56:2581–605. doi: 10.1021/jm400026k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson-Rechavi M, Carpentier AS, Duffraisse M, Laudet V. How many nuclear hormone receptors are there in the human genome? Trends Genet. 2001;17:554–6. doi: 10.1016/s0168-9525(01)02417-9. [DOI] [PubMed] [Google Scholar]
- 14.Moras D, Gronemeyer H. The nuclear receptor ligand-binding domain: structure and function. Curr Opin Cell Biol. 1998;10:384–91. doi: 10.1016/s0955-0674(98)80015-x. [DOI] [PubMed] [Google Scholar]
- 15.Bourguet W, Vivat V, Wurtz JM, Chambon P, Gronemeyer H, Moras D. Crystal structure of a heterodimeric complex of RAR and RXR ligand-binding domains. Mol Cell. 2000;5:289–98. doi: 10.1016/s1097-2765(00)80424-4. [DOI] [PubMed] [Google Scholar]
- 16.van Breemen RB, Huang CR, Nikolic D, Woodbury CP, Zhao YZ, Venton DL. Pulsed ultrafiltration mass spectrometry: A new method for screening combinatorial libraries. Anal Chem. 1997;69:2159–2164. doi: 10.1021/ac970132j. [DOI] [PubMed] [Google Scholar]
- 17.Nikolic D, van Breemen RB. Screening for inhibitors of dihydrofolate reductase using pulsed ultrafiltration mass spectrometry. Comb Chem High Throughput Screen. 1998;1:47–55. [PubMed] [Google Scholar]
- 18.Choi Y, van Breemen RB. Development of a screening assay for ligands to the estrogen receptor based on magnetic microparticles and LC-MS. Comb Chem High Throughput Screen. 2008;11:1–6. doi: 10.2174/138620708783398340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sucholeiki I, Toledo-Sherman LM, Hosfield CM, Boutilier K, DeSouza LV, Stover DR. Novel magnetic supports for small molecule affinity capture of proteins for use in proteomics. Mol Divers. 2004;8:9–19. doi: 10.1023/b:modi.0000006780.36844.64. [DOI] [PubMed] [Google Scholar]
- 20.McFadden JJ, Junop MS, Brennan JD. Magnetic “fishing” assay to screen small-molecule mixtures for modulators of protein-protein interactions. Anal Chem. 2010;82:9850–57. doi: 10.1021/ac102164d. [DOI] [PubMed] [Google Scholar]
- 21.Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, Rastinejad F. Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature. 2008;456:350–6. doi: 10.1038/nature07413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang D-H, Yuwen L-X, Peng L-J. Parameters Affecting the Performance of Immobilized Enzyme. J Chem. 2013 http://dx.doi.org/10.1155/2013/946248.
- 23.Singh RK, Tiwari MK, Singh R, Lee J-K. From protein engineering to immobilization: promising strategies for the upgrade of industrial enzymes. Int J Mol Sci. 2013;14:1232–77. doi: 10.3390/ijms14011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Homaei AA, Sariri R, Fabio Vianello F, Stevanato R. Enzyme immobilization: an update. J Chem Biol. 2013;6:185–205. doi: 10.1007/s12154-013-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allenby G, Bocquel MT, Saunders M, Kazmer S, Speck J, Rosenberger M, Lovey A, Kastner P, Grippo JF, Chambon P. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc Natl Acad Sci USA. 1993;90:30–4. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boehm MF, Zhang L, Zhi L, McClurg MR, Berger E, Wagoner M, Mais DE, Suto CM, Davies JA, Heyman RA, Nadzan AM. Design and synthesis of potent retinoid X receptor selective ligands that induce apoptosis in leukemia cells. J Med Chem. 1995;38:3146–55. doi: 10.1021/jm00016a018. [DOI] [PubMed] [Google Scholar]
- 27.Sun Y, Gu C, Liu X, Liang W, Yao P, Bolton JL, van Breemen RB. Ultrafiltration tandem mass spectrometry of estrogens for characterization of structure and affinity for human estrogen receptors. J Am Soc Mass Spectrom. 2005;16:271–79. doi: 10.1016/j.jasms.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


