Abstract
Background
Medicare beneficiaries with multiple chronic conditions (MCCs) are typically seen by multiple providers, particularly specialists. Clinically appropriate referrals to multiple specialists may compromise the continuity of care for MCC beneficiaries and create care plans that patients may find challenging to reconcile, which may impact patient outcomes.
Objective
To examine whether glycemic control or lipid control was associated with the number of prescribers of cardiometabolic medications.
Research Design, Subjects and Measures
A retrospective cross-sectional cohort analysis of 51,879 elderly Medicare FFS beneficiaries with diabetes and 129,762 beneficiaries with dyslipidemia living in 10 east coast states. Glycemic control was defined as having an HbA1c<7.5. Lipid control was defined as an LDL<100 for beneficiaries with heart disease or diabetes or an LDL<130 for all other beneficiaries. We examined the association between the number of prescribers of cardiometabolic medications and disease or lipid control in 2011 via logistic regression, controlling for age, gender, race, Medicaid enrollment, 17 chronic conditions and state fixed effects.
Results
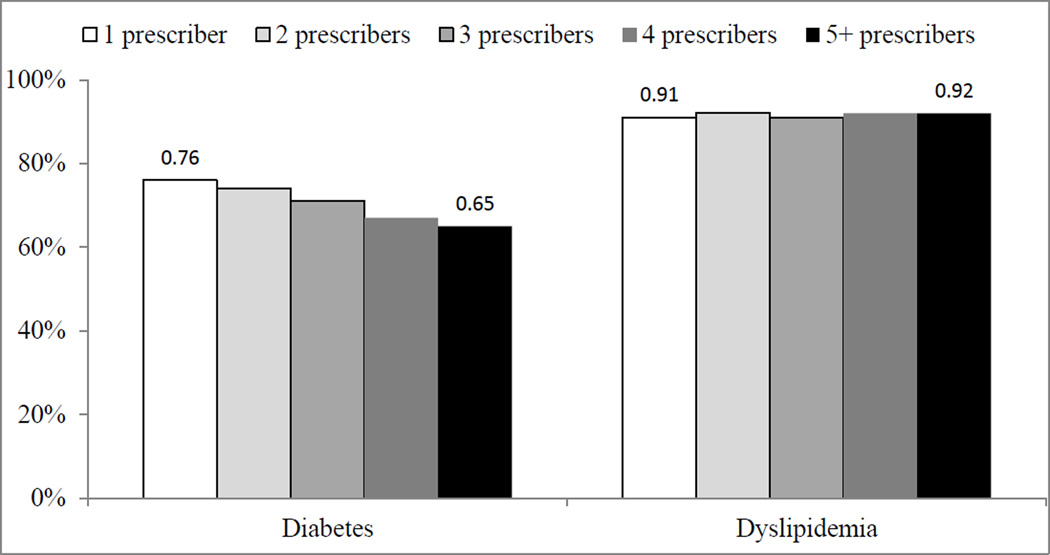
Among beneficiaries with diabetes, 76% with one prescriber had well controlled diabetes in 2011, which decreased to 65% for beneficiaries with 5+ prescribers. In adjusted analyses, Medicare beneficiaries with three or more prescribers were less likely to have glycemic control than beneficiaries with a single prescriber. Among those with dyslipidemia, nearly all (91–92%) beneficiaries had lipid control. After adjustment for demographics and comorbidity burden, beneficiaries with three prescribers were less likely to have lipid control than beneficiaries with a single prescriber.
Conclusions
Multiple prescribers were associated with worse disease control, possibly because patients with more severe diabetes or dyslipidemia have multiple prescribers or because care fragmentation is associated with worse disease control.
Keywords: Medicare, beneficiary, glycemic control, lipid control, prescribers, chronic conditions, diabetes, dyslipidemia, medication
Introduction
Medicare beneficiaries are typically seen by multiple providers for acute and chronic care,1 particularly specialists who provide time-limited consultation on newly diagnosed conditions or acute exacerbations of existing conditions.2 Primary care physicians may also engage specialists to co-manage the complex care of beneficiaries on an ongoing basis, because a majority of beneficiaries have multiple chronic conditions (MCC). Clinically appropriate referrals to multiple specialists may compromise the continuity of care for beneficiaries and create care plans that patients may find challenging to reconcile3,4, unless specialists proactively coordinate care or repatriate patients back to the primary care provider who initiated the referral.5
In numerous studies, more continuous care (measured as a higher concentration of outpatient visits with fewer providers) has been associated with lower hospital admission rates and lower health expenditures.6–10 Having fewer prescribers (as opposed to providers) has also been associated with lower rates of hospital admission and emergency department visits in a cohort of Veterans11 and with lower rates of medication utilization.12–17 In a study of 1,400 adults aged 17 and older from the Third National Health and Nutrition Examination Survey collected in 1988–1991, patients who had a usual provider of care or usual site of care had better glycemic control than patients with no usual source.18 A second study of 256 community health center patients in Texas found that better continuity of care was associated with better diabetes control.19 Results from these studies would suggest that continuous care improves disease control in non-Medicare cohorts and reduces emergent care needs, which together may reduce patients’ need to seek emergency department and inpatient care.
No prior study has examined the association between continuity of care and disease control in Medicare FFS beneficiaries, because lab values have not previously been available and the prior studies examining care continuity and disease control were on non-Medicare community-based samples. The measure of continuity examined here – number of prescribers of cardiometabolic medications – is different from continuity measures considered in prior studies in three ways. First, it is based on the subset of providers who prescribe medications instead of providers seen during outpatient visits regardless of having prescribed medications. Second, this prescriber measure is disease-specific by only considering prescribers of cardiometabolic medications instead of all-cause outpatient visits for any reason. Third, the number of prescribers is examined as a simple count instead of an index as most care continuity measures are constructed. This continuity measure has been associated with an important predictor of disease control (medication refill adherence) in prior studies of Veterans and Medicaid patients.16,17
Methods
Data and Participants
In this retrospective cohort study, we used 2010–2011 Beneficiary Summary Files (BSF) and Medicare Part D files for all Medicare FFS beneficiaries living in 10 east coast states (NY, NJ, MD, DE, VA, NC, SC, GA, FL, AL). Demographic characteristics were identified from the BSF and comorbid conditions were obtained from the BSF’s Chronic Condition Warehouse (CCW) segment. The CCW segment identifies 27 chronic conditions as far back as 1999, based on standardized algorithms that draw from inpatient and outpatient Medicare claims.20 The 2010–2011 Part D Prescription Drug Event (PDE) files were used to identify which beneficiaries were taking oral medications to manage diabetes or dyslipidemia (see Appendix A for list of medications), and the 2010 Prescriber Characteristics File (PCF) was used to identify the count and specialty of prescribers of oral cardiometabolic medications. The disease control outcomes were obtained from 2011 laboratory results data from a large national vendor with significant market share in the 10 states under investigation.21
MCC beneficiaries in this study were 65–80 years old on January 1, 2011, enrolled in all of 2010 and 2011 in Parts A, B and D, alive on December 31, 2011, and had a diagnosis for diabetes or dyslipidemia on or before December 30, 2010 based on end-of-year chronic condition indicators (see Appendix B and C for definitions and codes). Finally, beneficiaries included for analysis had glycemic or lipid testing in 2011 with matching lab results data and had more than 90 days of cardiometabolic medications in 2010 from prescribers whose specialty could be determined from the Part D Prescriber Characteristics File. Lab results were matched to Medicare data on individual beneficiaries based on a patient identifier that was available in both datasets. The requirement to have matching lab results data resulted in a 48% sample reduction in the diabetes cohort and a 47% reduction in the dyslipidemia cohort. Beneficiaries were excluded either because they did not have a lab test in 2011 or they had a lab test that was processed by different laboratory vendor. We excluded beneficiaries with end-stage renal disease due to differences in patterns of MCC care management and beneficiaries with hypertension because we lacked blood pressure control data.
The final analytic cohort included 51,879 beneficiaries with diagnosed diabetes and 129,762 beneficiaries with diagnosed dyslipidemia, which were the fifth most prevalent (28%) and second most prevalent (45%) conditions in 2010, respectively.22 Beneficiaries with diabetes but no lab data were significantly different in most characteristics from beneficiaries with lab data, including being slightly older, taking more medications and having a higher prevalence of most chronic conditions (Appendix D). Similarly, beneficiaries with dyslipidemia but no lab data were significantly different from beneficiaries with lab data.
Outcome and Explanatory Variable of Interest
In the diabetes cohort, the binary outcome of glycemic control was constructed from the last available glycated hemoglobin (HbA1c) in 2011 laboratory results data. Glycemic control was defined as having an HbA1c<7.5 based on clinical guidelines for this age group.23 In the dyslipidemia cohort, the binary outcome of lipid control was constructed from the same laboratory results data. Lipid control was defined as an LDL<100 for beneficiaries with heart disease or diabetes or an LDL<130 for all other beneficiaries.23 We examined diabetes control because poor glycemic control puts beneficiaries with type 1 diabetes at risk for developing retinopathy, nephropathy, neuropathy and heart disease24, and puts beneficiaries with type 2 diabetes at risk for retinopathy and nephropathy.25 We examined lipid control because poor lipid control puts beneficiaries with dyslipidemia at risk for heart disease, myocardial infarction and stroke.26
The explanatory variables of interest were the total number of all prescribers of medications to manage diabetes, hypertension or dyslipidemia, which was constructed by linking the 2010 PCF to the 2010 PDE data via CCW Prescriber identifiers. In contrast to prior studies that used continuity indices to reflect concentration of visits among providers, 6,7 we constructed a prescriber count based on the total number of unique prescribers who wrote a prescription for a filled (or refilled) cardiometabolic medication without regard for prescriber specialty. This prescriber continuity measure has face validity, includes prescribers involved in the clinic management of these conditions of interest, and does not require exclusion of beneficiaries with too few visits to calculate the index.7 These 2010 prescriber variables were lagged by one year from the 2011 disease control outcome to reduce simultaneity bias.
Data Analysis
We generated means and standard deviations for continuous variables, proportions of binary variables, and bivariate associations between control of glycemia or lipids and the number of prescribers. For each cohort, logistic regression of well controlled disease in 2011 was estimated as a function of a categorical non-specific prescriber variable (2 prescribers, 3 prescribers, 4 prescribers, 5+ prescribers) in 2010 with one prescriber serving as the reference group and other covariates. The prescriber variable was lagged one year from the disease control outcome to reduce the reverse causality that could arise by modeling these variables and outcomes in the same year.
All regressions adjusted for age, gender, race, Medicaid enrollment, 17 chronic conditions available from the BSF’s Chronic Condition segment, the number of all medications filled in 2010 and state fixed effects. The chronic conditions included hypothyroidism, atrial fibrillation, anemia, asthma, benign prostatic hyperplasia, cancer (as a combination of indicators for breast, colorectal, prostate, lung, and endometrial cancer), chronic kidney disease, COPD, dementia/Alzheimer’s disease/related conditions, depression, heart failure, ischemic heart disease, osteoporosis, rheumatoid arthritis/osteoarthritis, and stroke. This study was approved by the institutional review board of the Duke University Health System.
Results
Patient Characteristics and Diabetes Control by Number of Prescribers
In the diabetes cohort, the average age was 72.5, a majority (56%) was female, 74% were white race, 17.7% were dually enrolled in Medicaid and 80% had comorbid dyslipidemia. The average beneficiary took 11.7 medications for all conditions and had 1.8 prescribers of cardiometabolic medications (Table 1). The proportion of Medicare beneficiaries with well controlled diabetes in 2011 was 76% for beneficiaries with one prescriber, which decreased to 65% for beneficiaries with 5+ prescribers (Figure 1).
Table 1.
Baseline (2010) characteristics of the diabetes and dyslipidemia cohorts
| Variables | Diabetes (N=51,879) |
Dyslipidemia (N=129,762) |
|---|---|---|
| Age (Mean, SD) | 72.5 (4.4) | 72.5 (4.4) |
| Female (%) | 55.8% | 58.2% |
| Caucasian race (%) | 73.9% | 83.7% |
| African-American race (%) | 15.8% | 9.4% |
| Other race (%) | 10.2% | 6.8% |
| Medicaid status (%) | 17.7% | 11.8% |
| Diabetes mellitus (%) | 100% | 42.2% |
| Hypertension (%) | 86.6% | 82.8% |
| Hyperlipidemia (%) | 80.2% | 100% |
| Heart failure (%) | 16.8% | 13.5% |
| Atrial fibrillation (%) | 8.1% | 8.3% |
| Dementia/Alzheimer’s/Related (%) | 5.9% | 4.9% |
| Anemia (%) | 29.3% | 25.1% |
| Asthma (%) | 5.0% | 4.8% |
| Cancer (%) | 8.9% | 9.4% |
| Chronic kidney disease (%) | 20.3% | 15.3% |
| COPD (%) | 10.8% | 10.9% |
| Depression (%) | 10.1% | 10.6% |
| Benign prostatic hyperplasia (%) | 7.3% | 7.9% |
| Acquired hypothyroidism (%) | 7.1% | 9.1% |
| Ischemic heart disease (%) | 42.6% | 43.7% |
| Osteoporosis (%) | 4.7% | 8.0% |
| Rheumatoid Arthritis/Osteoarthritis (%) | 31.5% | 32.7% |
| Stroke (%) | 4.2% | 4.2% |
| Number of all medications(Mean, SD) | 11.7 (5.8) | 10.1 (5.5) |
| # of prescribers of cardiometabolic medications with known specialty, 2010 (Mean, SD) |
1.8 (1.0) | 1.6 (0.9) |
Note: COPD = Chronic obstructive pulmonary disease, SD = standard deviation
Figure 1.
Unadjusted Proportion of Medicare FFS Beneficiaries with Diabetes or Dyslipidemia who have Well Controlled Disease, by Number of Unique Prescribers
In adjusted analyses (Table 2), beneficiaries with diabetes and multiple prescribers had lower odds of having glycemic control than beneficiaries with a single prescriber (3 prescribers: odds ratio (OR)=0.86; 95% confidence interval (CI)=0.81–0.92; 4 prescribers: OR=0.77; 95% CI=0.70–0.85; ≥5 prescribers: OR=0.78; 95% CI=0.68–0.88). Beneficiaries also had lower odds of having glycemic control if they were African-American (OR=0.76, 95% CI=0.72, 0.81), enrolled in Medicaid (OR=0.92, 95% CI=0.87, 0.98) or had comorbid congestive heart failure (OR=0.82, 95% CI=0.78, 0.87), chronic kidney disease (OR=0.87, 95% CI=0.82, 0.91), or ischemic heart disease (OR=0.93, 95% CI=0.89, 0.97). Older beneficiaries had higher odds of having glycemic control than beneficiaries aged 65–69 (age 70–74: OR=1.08, 95% CI=1.03, 1.13; age 75+: OR=1.34, 95% CI=1.28, 1.41). Beneficiaries also had higher odds of having glycemic control if they had hypertension (OR=1.11, 95% CI=1.04, 1.18), dyslipidemia (OR=1.19, 95% CI=1.13, 1.25), anemia (OR=1.10, 95% CI=1.05, 1.15), asthma (OR=1.13, 95% CI=1.03, 1.24), cancer (OR=1.13, 95% CI=1.05, 1.22), COPD (OR=1.14, 95% CI=1.07, 1.23), depression (OR=1.19, 95% CI=1.10, 1.27), benign prostatic hyperplasia (OR=1.32, 95% CI=1.21, 1.43), hypothyroidism (OR=1.15, 95% CI=1.06, 1.25), osteoporosis (OR=1.38, 95% CI=1.24, 1.53) or rheumatoid arthritis/osteoarthritis (OR=1.23, 95% CI=1.17, 1.28).
Table 2.
Logistic Regression Results of 2011 Disease Control
| Diabetes Cohort | Dyslipidemia Cohort | |
|---|---|---|
| 1 Prescriber in 2010 | Reference | Reference |
| 2 Prescribers in 2010 | 0.95 (0.91, 1.00) | 0.99 (0.95, 1.04) |
| 3 Prescribers in 2010 | 0.86 (0.81, 0.92) | 0.92 (0.86, 0.99) |
| 4 Prescribers | 0.77 (0.70, 0.85) | 0.96 (0.85, 1.09) |
| 5+ Prescribers | 0.78 (0.68, 0.88) | 0.99 (0.83, 1.20) |
| Age(65–69) | Reference | Reference |
| Age(70–74) | 1.08 (1.03, 1.13) | 1.08 (1.03, 1.13) |
| Age(75+) | 1.34 (1.28, 1.41) | 1.24 (1.18, 1.30) |
| White | Reference | Reference |
| African-American | 0.76 (0.72, 0.81) | 0.65 (0.61, 0.69) |
| Other race | 0.80 (0.75, 0.86) | 0.76 (0.70, 0.82) |
| Male | Reference | Reference |
| Female | 1.10 (1.06, 1.15) | 0.54 (0.52, 0.57) |
| Medicaid enrolled | 0.92 (0.87, 0.98) | 0.84 (0.79, 0.89) |
| Diabetes | -- | 1.51 (1.44, 1.57) |
| Hypertension | 1.11 (1.04, 1.18) | 1.20 (1.14, 1.26) |
| Dyslipidemia | 1.19 (1.13, 1.25) | -- |
| Congestive heart failure | 0.82 (0.78, 0.87) | 0.90 (0.84, 0.96) |
| Atrial fibrillation | 1.03 (0.96, 1.11) | 1.37 (1.25, 1.49) |
| Dementia/Alzheimer's/Related | 1.04 (0.95, 1.13) | 0.87 (0.80, 0.95) |
| Anemia | 1.10 (1.05, 1.15) | 1.02 (0.97, 1.07) |
| Asthma | 1.13 (1.03, 1.24) | 0.99 (0.90, 1.08) |
| Cancer | 1.13 (1.05, 1.22) | 0.99 (0.92, 1.06) |
| Chronic kidney disease | 0.87 (0.82, 0.91) | 1.02 (0.96, 1.08) |
| COPD | 1.14 (1.07, 1.23) | 1.00 (0.94, 1.07) |
| Depression | 1.19 (1.10, 1.27) | 0.75 (0.71, 0.80) |
| Benign prostatic hyperplasia | 1.32 (1.21, 1.43) | 1.08 (0.98, 1.19) |
| Acquired hypothyroidism | 1.15 (1.06, 1.25) | 0.99 (0.93, 1.06) |
| Ischemic heart disease | 0.93 (0.89, 0.97) | 1.21 (1.16, 1.27) |
| Osteoporosis | 1.38 (1.24, 1.53) | 1.10 (1.03, 1.18) |
| Rheumatoid Arthritis/ Osteoarthritis | 1.23 (1.17, 1.28) | 0.95 (0.91, 0.99) |
| Stroke | 0.97 (0.88, 1.08) | 0.97 (0.88, 1.07) |
| Number of medications (all) | 0.97 (0.96, 0.97) | 0.99 (0.98, 0.99) |
| c-statistic Sample Size |
0.60 51,879 |
0.63 129,762 |
Note: adherence = 1 if continuous PDC > 80%; =0 otherwise; fixed effects for the 10 states were also included with Florida as the reference state. Diabetes control = 1 if HbA1c<7.5. Lipid control = 1 if LDL<100 for beneficiaries with heart disease or diabetes or an LDL<130 for all other beneficiaries.
Patient Characteristics and Lipid Control by Number of Prescribers
In the dyslipidemia cohort, the average age was 72.5, a majority (58%) was female, 84% were white race, 12% were dually enrolled in Medicaid and 42% had comorbid diabetes. The average beneficiary took 10.1 medications for all conditions and had 1.6 prescribers of cardiometabolic medications. The unadjusted proportion of Medicare beneficiaries with well controlled dyslipidemia in 2011 was similar (91–92%) across the number of prescribers (Figure 1). In adjusted analysis (Table 2), beneficiaries with dyslipidemia and three prescribers had lower odds of having lipid control than beneficiaries with a single prescriber (OR=0.92; 95% confidence interval (CI)=0.86–0.99).
Beneficiaries had lower odds of having glycemic control if they had comorbid congestive heart failure (OR=0.90, 95% CI=0.84, 0.96), dementia, Alzheimer’s or related disorders (OR=0.87, 95% CI=0.80, 0.95), depression (OR=0.75, 95% CI=0.71, 0.80) or rheumatoid arthritis/osteoarthritis (OR=0.95, 95% CI=0.91, 0.99). Older beneficiaries had higher odds of glycemic control than beneficiaries aged 65–69 (age 70–74: OR=1.08, 95% CI=1.03, 1.13; age 75+: OR=1.24, 95% CI=1.18, 1.30). Beneficiaries also had higher odds of glycemic control if they had diabetes, (OR=1.51, 95% CI=1.44, 1.57), hypertension (OR=1.20, 95% CI=1.14, 1.26), atrial fibrillation (OR=1.37, 95% CI=1.25, 1.49), ischemic heart disease (OR=1.21, 95% CI=1.16, 1.27) or osteoporosis (OR=1.10, 95% CI=1.03, 1.18).
Discussion
The impact of care continuity on health care utilization and expenditures of Medicare beneficiaries has been examined in prior work,6 but no prior studies have examined the impact of the number of prescribers on disease control in this population. To our knowledge, this is the first study to examine the association between the number of prescribers and disease control for Medicare FFS beneficiaries with diabetes or dyslipidemia. The role of care continuity in control of chronic conditions is important to understand, since poorly controlled disease puts MCC beneficiaries at risk for adverse events that lead to emergency department visits and hospitalizations.27 Care discontinuity experienced by MCC beneficiaries may partly explain why they have more hospital visits, more physician office visits and higher Medicare expenditures than beneficiaries with 0–1 conditions.28,29 MCC beneficiaries may also be at greater risk of being prescribed drugs with harmful interactions than other beneficiaries due to their complex medication regimens.30,31
In the cohort of beneficiaries with diabetes, multiple prescribers were associated with worse glycemic control. The significant results are consistent with two prior studies in which better continuity of care was associated with better glycemic control, which were based on much smaller, non-Medicare samples and data that was collected 15–20 years prior to the 2011 data used in this study.18,19 Beneficiaries with dyslipidemia and three prescribers had modestly worse lipid control than beneficiaries with single prescriber. Having two prescribers or four or more prescribers was not associated with lipid control. It is possible that the number of prescribers was associated with glycemic control but not lipid control, because diabetes regimens often involve polypharmacy, while lipid-lowering regimens are more straightforward by often requiring only a single statin. The diabetes cohort results were farther from the null than the lipid cohort results, but future research that accounts for the extent of patients’ polypharmacy is needed to re-examine these associations.
There are at least two explanations for the diabetes findings. First, seeing multiple prescribers could adversely impact disease control via non-adherence to medications or lifestyle change recommendations. In separate analyses of this cohort, we found a null association between availability of oral diabetes medications and number of prescribers,32 which suggests that the medication non-adherence pathway to disease control may not be an important factor. It is possible that patients with multiple prescribers have worse disease control due to non-adherence to lifestyle recommendations. However, we did not have data on provider recommendations or patient behavior to be able to evaluate this lifestyle pathway. The second possible explanation is unobserved confounding of disease severity drives these results, such that beneficiaries with more severe diabetes are simply referred to more providers, some of whom prescribe medications. We did not have data to control for disease severity at baseline, unobserved confounding is plausible and is a limitation of this study. Future work that captures these unobservable factors would elucidate the underlying mechanisms to inform improvements to providers’ management of MCC patients. There are additional limitations that must be acknowledged. These results may not generalize beyond the Medicare FFS beneficiaries in these 10 states in 2010–2011, and may not generalize to beneficiaries who were excluded due to the lack of laboratory values. Older adults receiving care from integrated health systems with more information continuity due to connected electronic health records may have different results from those reported here. Further, clinical significance (especially for lipid results) is not implied by statistical significance. Finally, claims data lack information on geriatric conditions (e.g., urinary incontinence, falls, dizziness, visual impairment) that commonly co-occur with the medical conditions measured.33,34
We unexpectedly found that female sex was positively associated with diabetes control but negatively associated with lipid control, the latter of which is consistent with prior gender comparisons in Medicare Advantage enrollees in 200435 and gender comparisons in the Multi-Ethnic Study of Atherosclerosis.36 The positive association between female sex and diabetes control finding was inconsistent with results from these prior two studies,35,36 so merits re-examination in future research.
Over the course of their enrollment in the traditional fee-for-service (FFS) program, the care of many MCC beneficiaries becomes fragmented as they are referred to specialists with complementary expertise to address newly diagnosed conditions or acute exacerbations of existing conditions. Some specialists will repatriate a beneficiary back to the primary care provider5 after addressing the issues that prompted referral, while other providers will decide to co-manage a beneficiary with the primary care provider.
This study builds on prior care continuity studies on older adults because we examine continuity of medication management and its association with disease control, which may improve understanding of the association between continuity of care and health care utilization. This analysis was made possible by the linkage of laboratory results data to Medicare FFS claims,21 which creates the opportunity to conduct patient safety and quality analyses that were not previously possible.
Supplementary Material
Acknowledgments
This project was supported by support from the Agency for Healthcare Research and Quality (AHRQ) R01HS023085. Dr. Maciejewski is also supported by a Research Career Scientist award from the Department of Veterans Affairs (RCS 10-391). Dr. Voils was also supported by a Research Career Scientist award from the Department of Veterans Affairs (RCS 14-443). This work was also supported by the Office of Research and Development, Health Services Research and Development Service, Department of Veterans Affairs. This work was presented at the 2015 research meetings of AcademyHealth, the International Health Economics Association and AHRQ. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veteran Affairs or Duke University.
Dr. Maciejewski reported receiving institutional grants from the Department of Veterans of Affairs, the Agency for Healthcare Research and Quality and an institutional contract from the National Commission on Quality Assurance; and ownership of Amgen stock due to his spouse’s employment. Dr. Voils reported receiving institutional grants from the Department of Veterans of Affairs. Dr. Curtis reported receiving institutional grants from GlaxoSmithKline, Boston Scientific, and Gilead. Dr. Wang reported receiving institutional grants from the Department of Veterans of Affairs and the National Institute of Diabetes and Digestive Disorders.
Footnotes
Conflict of Interest Disclosures: All other authors have no conflicts of interest to disclose.
References
- 1.Pham HH, Schrag D, O'Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for performance. N Engl J Med. 2007 Mar 15;356(11):1130–1139. doi: 10.1056/NEJMsa063979. [DOI] [PubMed] [Google Scholar]
- 2.Forrest CB. A typology of specialists' clinical roles. Arch Intern Med. 2009 Jun 8;169(11):1062–1068. doi: 10.1001/archinternmed.2009.114. [DOI] [PubMed] [Google Scholar]
- 3.Voils CI, Sleath B, Maciejewski ML. Patient perspectives on having multiple versus single prescribers of chronic disease medications: results of a qualitative study in a veteran population. BMC Health Serv Res. 2014 Oct 25;14(1):490. doi: 10.1186/s12913-014-0490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zulman DM, Jenchura EC, Cohen DM, Lewis ET, Houston TK, Asch SM. How Can eHealth Technology Address Challenges Related to Multimorbidity? Perspectives from Patients with Multiple Chronic Conditions. J Gen Intern Med. 2015 Aug;30(8):1063–1070. doi: 10.1007/s11606-015-3222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackerman SL, Gleason N, Monacelli J, et al. When to repatriate? Clinicians' perspectives on the transfer of patient management from specialty to primary care. J Gen Intern Med. 2014 Oct;29(10):1355–1361. doi: 10.1007/s11606-014-2920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussey PS, Schneider EC, Rudin RS, Fox DS, Lai J, Pollack CE. Continuity and the costs of care for chronic disease. JAMA Intern Med. 2014 May;174(5):742–748. doi: 10.1001/jamainternmed.2014.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyweide DJ, Anthony DL, Bynum JP, et al. Continuity of care and the risk of preventable hospitalization in older adults. JAMA Intern Med. 2013 Nov 11;173(20):1879–1885. doi: 10.1001/jamainternmed.2013.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romaire MA, Haber SG, Wensky SG, McCall N. Primary care and specialty providers: an assessment of continuity of care, utilization, and expenditures. Med Care. 2014 Dec;52(12):1042–1049. doi: 10.1097/MLR.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 9.Mainous AG, 3rd, Gill JM. The importance of continuity of care in the likelihood of future hospitalization: is site of care equivalent to a primary clinician? Am J Public Health. 1998 Oct;88(10):1539–1541. doi: 10.2105/ajph.88.10.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill JM, Mainous AG, 3rd, Nsereko M. The effect of continuity of care on emergency department use. Arch Fam Med. 2000 Apr;9(4):333–338. doi: 10.1001/archfami.9.4.333. [DOI] [PubMed] [Google Scholar]
- 11.Maciejewski ML, Powers BJ, Sanders LL, et al. The intersection of patient complexity, prescriber continuity and acute care utilization. J Gen Intern Med. 2014 Apr;29(4):594–601. doi: 10.1007/s11606-013-2746-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamblyn RM, McLeod PJ, Abrahamowicz M, Laprise R. Do too many cooks spoil the broth? Multiple physician involvement in medical management of elderly patients and potentially inappropriate drug combinations. CMAJ. 1996 Apr 15;154(8):1177–1184. [PMC free article] [PubMed] [Google Scholar]
- 13.Col N, Fanale JE, Kronholm P. The role of medication noncompliance and adverse drug reactions in hospitalizations of the elderly. Arch Intern Med. 1990 Apr;150(4):841–845. [PubMed] [Google Scholar]
- 14.Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc. 2005 Sep;53(9):1518–1523. doi: 10.1111/j.1532-5415.2005.53523.x. [DOI] [PubMed] [Google Scholar]
- 15.Green JL, Hawley JN, Rask KJ. Is the number of prescribing physicians an independent risk factor for adverse drug events in an elderly outpatient population? Am J Geriatr Pharmacother. 2007 Mar;5(1):31–39. doi: 10.1016/j.amjopharm.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Farley JF, Wang CC, Hansen RA, Voils CI, Maciejewski ML. Continuity of antipsychotic medication management for Medicaid patients with schizophrenia. Psychiatr Serv. 2011 Jul;62(7):747–752. doi: 10.1176/ps.62.7.pss6207_0747. [DOI] [PubMed] [Google Scholar]
- 17.Hansen RA, Voils CI, Farley JF, et al. Prescriber continuity and medication adherence for complex patients. Ann Pharmacother. 2015 Mar;49(3):293–302. doi: 10.1177/1060028014563266. [DOI] [PubMed] [Google Scholar]
- 18.Mainous AG, 3rd, Koopman RJ, Gill JM, Baker R, Pearson WS. Relationship between continuity of care and diabetes control: evidence from the Third National Health and Nutrition Examination Survey. Am J Public Health. 2004 Jan;94(1):66–70. doi: 10.2105/ajph.94.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parchman ML, Pugh JA, Noel PH, Larme AC. Continuity of care, self-management behaviors, and glucose control in patients with type 2 diabetes. Med Care. 2002 Feb;40(2):137–144. doi: 10.1097/00005650-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Gorina Y, Kramarow EA. Identifying chronic conditions in Medicare claims data: evaluating the Chronic Condition Data Warehouse algorithm. Health Serv Res. 2011 Oct;46(5):1610–1627. doi: 10.1111/j.1475-6773.2011.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammill BG, Curtis LH, Qualls LG, Hastings SN, Wang V, Maciejewski ML. Linkage of Laboratory Results to Medicare Fee-for-Service Claims. Med Care. 2015 Nov;53(11):974–979. doi: 10.1097/MLR.0000000000000420. [DOI] [PubMed] [Google Scholar]
- 22.Lochner KA, Cox CS. Prevalence of multiple chronic conditions among Medicare beneficiaries, United States, 2010. Prev Chronic Dis. 2013;10:E61. doi: 10.5888/pcd10.120137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Expert Panel on Detection EToHBCi, Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001 May 16;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 24.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005 Dec 22;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008 Oct 9;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 26.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD004816. doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geller AI, Shehab N, Lovegrove MC, et al. National estimates of insulin-related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern Med. 2014 May;174(5):678–686. doi: 10.1001/jamainternmed.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson G. Chronic Conditions: Making the Case for Ongoing Care. Princeton, NJ: 2010. [Google Scholar]
- 29.Services CfMaM. Chronic Conditions among Medicare Beneficiaries, Chartbook, 2012 Edition. Baltimore, MD: Centers for Medicare and Medicaid Services; 2012. [Google Scholar]
- 30.Holmes HM, Luo R, Kuo YF, Baillargeon J, Goodwin JS. Association of potentially inappropriate medication use with patient and prescriber characteristics in Medicare Part D. Pharmacoepidemiol Drug Saf. 2013 Jul;22(7):728–734. doi: 10.1002/pds.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinman MA, Miao Y, Boscardin WJ, Komaiko KD, Schwartz JB. Prescribing quality in older veterans: a multifocal approach. J Gen Intern Med. 2014 Oct;29(10):1379–1386. doi: 10.1007/s11606-014-2924-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maciejewski ML, Hammill BG, Voils CI, et al. Prescriber Continuity and Medication Use in Older Adults with Multiple Chronic Conditions. under review. [Google Scholar]
- 33.Cigolle CT, Langa KM, Kabeto MU, Tian Z, Blaum CS. Geriatric conditions and disability: the Health and Retirement Study. Ann Intern Med. 2007 Aug 7;147(3):156–164. doi: 10.7326/0003-4819-147-3-200708070-00004. [DOI] [PubMed] [Google Scholar]
- 34.Lee PG, Cigolle C, Blaum C. The co-occurrence of chronic diseases and geriatric syndromes: the health and retirement study. J Am Geriatr Soc. 2009 Mar;57(3):511–516. doi: 10.1111/j.1532-5415.2008.02150.x. [DOI] [PubMed] [Google Scholar]
- 35.Chou AF, Brown AF, Jensen RE, Shih S, Pawlson G, Scholle SH. Gender and racial disparities in the management of diabetes mellitus among Medicare patients. Women's health issues : official publication of the Jacobs Institute of Women's Health. 2007 May-Jun;17(3):150–161. doi: 10.1016/j.whi.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Winston GJ, Barr RG, Carrasquillo O, Bertoni AG, Shea S. Sex and racial/ethnic differences in cardiovascular disease risk factor treatment and control among individuals with diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2009 Aug;32(8):1467–1469. doi: 10.2337/dc09-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.