Abstract
Duchenne muscular dystrophy (DMD) leads to cardiomyopathy (CMP). The objective of this study was to estimate the association of body mass index (BMI) with CMP onset. CMP was defined as an LVEF < 55% or LVFS < 28%. Overall, 48% met criteria for CMP. We were unable to demonstrate an association between BMI Z score and age of CMP onset (HR 0.79, 95% CI 0.57 to 1.11, p=0.17) after adjusting for covariates. Duration of corticosteroid use (p=0.01), but not loss of ambulatory ability (p=0.47) was associated with age of CMP onset. We were unable to detect a significant difference in median BMI Z scores in corticosteroid treated boys compared to corticosteroid naive boys (1.11, 95% CI 0.25–1.95 vs. 1.05, 95% CI 0.01–1.86, p=0.69). No association was detected between the BMI Z scores of DMD subjects and age of CMP onset.
Keywords: Duchenne Muscular Dystrophy, Cardiomyopathy, Body mass index
11. Introduction
Duchenne Muscular Dystrophy (DMD), an X- linked recessive disorder, is caused by mutations in the dystrophin gene. DMD is common, affecting one in every 5000 boys [1,2,3]. The disease course is one of progressive muscle injury and weakness leading affected boys to experience loss of ambulation and the eventual development of respiratory and cardiac failure. Death now typically occurs in the third decade. No cure exists for DMD.
DMD cardiomyopathy (CMP) is defined by myocardial fibrosis contributing to ventricular dysfunction [4]. Most boys remain asymptomatic in the early stages of declining cardiac function, making it very difficult to apply symptom based classification systems such as the New York Heart Association (NYHA)[4]. The majority of DMD boys will have evidence of CMP, or altered ventricular function, by the age of 20 years [1,5]. A certain subset, however, has evidence of the development of altered ventricular function in the late first or early second decades. Research aimed at identifying modifiable predictors for early onset CMP is clinically relevant. Conflicting evidence exists regarding the interaction between cardiac function [6,7] and skeletal muscle function; animal and human research studies have shown influences to be both beneficial and detrimental. Adult studies have shown obesity to be a risk factor in both the progression of heart failure and to have direct myocardial effects in diabetic and hypertrophic cardiomyopathies [8,9]; however, there are limited studies on the interaction between weight and cardiovascular risk in children [10–12]. Obesity is prevalent in DMD secondary to lack of mobility and corticosteroid use, which is associated with similar metabolic derangement seen in adult studies.
Our study aimed to characterize the association of body habitus with age of CMP onset in DMD. We sought to determine if body habitus and the current medical regimen used in DMD patients is associated with the age of CMP onset. We tested the hypothesis that higher body mass index (BMI) would have negative cardiovascular associations and would be associated with earlier onset of CMP in DMD affected boys. Secondarily, we tested the association of treatment with corticosteroids and BMI and CMP onset.
2. Methods
2.1 Study Design and Recruitment
After local Institutional Review Board approval, we conducted a retrospective cohort study over a 6-year time period (2007–2013). Subjects included boys with confirmed DMD by either genetic testing or a muscle biopsy with a corresponding skeletal muscle phenotype followed in a combined Neuromuscular-Cardiology clinic at a single institution. All subjects had undergone cardiac imaging (either an echocardiogram or cardiac magnetic resonance imaging) at our institution over the study period. A total of 85 boys met inclusion criteria with complete data.
2.2 Study Protocol
After subject identification, cardiac imaging was reviewed for each subject. At our institution, cardiac imaging was routinely performed on either an annual or biannual basis. The cardiac imaging modalities reviewed for this study included echocardiography and cardiac magnetic resonance imaging (CMR). Imaging measurements collected included left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS), internal dimension of the left ventricle in diastole (LVIDd), internal dimension of the left ventricle in systole (LVIDs), and left ventricular mass. Due to the small number of subjects with complete CMR data at the time of the study (26 total over the study period), echocardiographic imaging measurements were used for the primary analysis, unless otherwise indicated. CMP was defined as either an LVEF <55% or LVFS <28%. CMP development was considered premature if a subject age 15 or younger had abnormal ventricular function by the above measurements. Due to imaging quality, when a subject lacked a measurable LVEF on the echocardiographic studies reviewed; the LVFS was used as the main outcome measure to define CMP. Each subject’s baseline study (defined as the first study performed) was documented as the initial visit; all subsequent studies and their measures were recorded until either the criteria for CMP was met or that subject’s most recent study was reached. If a subject did not have consecutive visits recorded, it was decided to include the subject in the cohort, as it was felt by the research team their imaging and body habitus information strengthened the overall study. The subject’s height and weight were recorded at the time of the imaging study with a post analysis calculated body surface area (BSA) [calculated as square root of height (cm) × weight (kg)/60] and body mass index (BMI) [calculated as weight (kg)/height2 (cm)]. BMI Z scores were also calculated based on the Center for Disease Control (CDC) BMI-for-age growth charts. Baseline characteristics collected included cardiac medication usage, and corticosteroid usage and duration. Timing of cardiac medication initiation was at the discretion of the attending physician and family, but was based on the current evidence based recommendations aimed at a proactive management approach. There was limited practice variability, as there was only one treating physician for the majority of subjects. The age of loss of ambulation for each patient, if reached during the study period, was documented longitudinally. The last imaging study prior to death was documented in those patients in whom death occurred during the study period. A subject was considered to be corticosteroid naive if they received treatment for less than 6 months.
2.3 Statistical Analysis
Descriptive techniques were reported as median (IQR) for continuous variables and frequency (percentage) for categorical variables. Linear models accounting for repeated measurements using generalized least squares were used to estimate the association of BMI Z scores over time on the percent change of LVFS or LVIDd after adjusting for covariates (duration of corticosteroid usage, and whether loss of ambulation had occurred or not). Proportional hazard regression with time dependent covariates was used to assess if BMI Z score had an association with age of CMP onset. Interval censoring was used where each subject was considered at risk from the time of the first visit until CMP onset or they were censored. Subjects were censored at their time of death or last visit. To incorporate the time varying covariate, we divided time into one-year intervals and included covariates for whether the subject was ambulatory and spline of BMI Z score during that year (BMI Z score is lagged by one year). The duration of steroid use and whether loss of ambulation had occurred or not was adjusted for in the model. Spearman correlation coefficients were calculated between duration of steroid use and BMI measurements. R statistical software (version 2013) was used for the statistical analysis. Two-sided p- values of <0.05 were considered statistically significant.
3. Results
3.1 Baseline Results
A total of 85 boys with confirmed DMD were included (Table 1). Overall, 48% (41/85) of boys met criteria for CMP by the end of the study period, with the average age of onset 15.8 years (range 9 to 29 years). Almost 73% of boys were treated with corticosteroids at some point during the disease course (62/85) with median treatment duration of 4 years. At the time of CMP development, 37% of boys (15/41) were receiving corticosteroids. A total of 8 deaths (9% rate of death) occurred in the cohort during the study period.
Table 1.A.
Baseline Demographics for Cohort *
| All DMD N=85 |
|
|---|---|
| Characteristics | N = 85 |
| Average age, y | 14.9 (3.5–37.5)a |
| Median Age at loss of ambulation, y | 10 (4–14)a |
| % of subjects still ambulatory | 32.6 |
| BSA, m2 | 1.2 [0.9,1.5]b |
| BMI, kg/m2 | 20.1 [16.5,25.9]b |
| BMI Z score | 1.1 [0.2,1.9]b |
| Fractional Shortening, % | 34 [31,37.1]b |
| CMR Ejection Fraction, % | 57 [50.0,60.9]b |
| Left ventricular mass, grams | 65.6 [51.5,82.5]b |
| LVIDd, mm | 40.0 [37,44]b |
| LVIDs, mm | 26 [24,30]b |
| Corticosteroid use, % | 52 |
Based on echocardiographic measurements unless CMR labeled
expressed as range
expressed in median [IQR]
DMD boys did not exhibit significant changes in weight over the study period. The majority of boys fell into the normal weight category [BMI for age between 5–85%, (31/85)] or the overweight/obese weight category [BMI for age greater than 85% (46/85)]. Only 9% (8/85) of boys were classified in the underweight category [a BMI for age less than 5%]. We did not detect a difference in CMP development between weight categories (p=0.81). For those that met criteria for CMP, the majority fell into either the normal weight (32%, 13/41) or overweight (42%, 17/41) categories at the time of diagnosis. The underweight category was represented in 10% (4/41) of boys. No difference was detected in the age of CMP onset between boys that changed weight categories the year prior to CMP onset (median age of 14.5 years) compared to those that did not change weight category (median age 14.25). Independent of CMP onset, in those boys with documented consecutive yearly visits, 67% (44/66) had no change in their weight category between years. In boys that shifted weight categories between any two-year period, weight loss (13/66) was more prevalent than weight gain (9/66).
3.2 Generalized linear model results
No significant difference was detected between BMI Z score and percent change of LVFS (p=0.35, 95% CI −1.90 to 0.71) or LVIDd over time (p=0.25, 95% CI −0.73 to 0.47), adjusting for duration of corticosteroid usage and whether loss of ambulation had occurred or not.
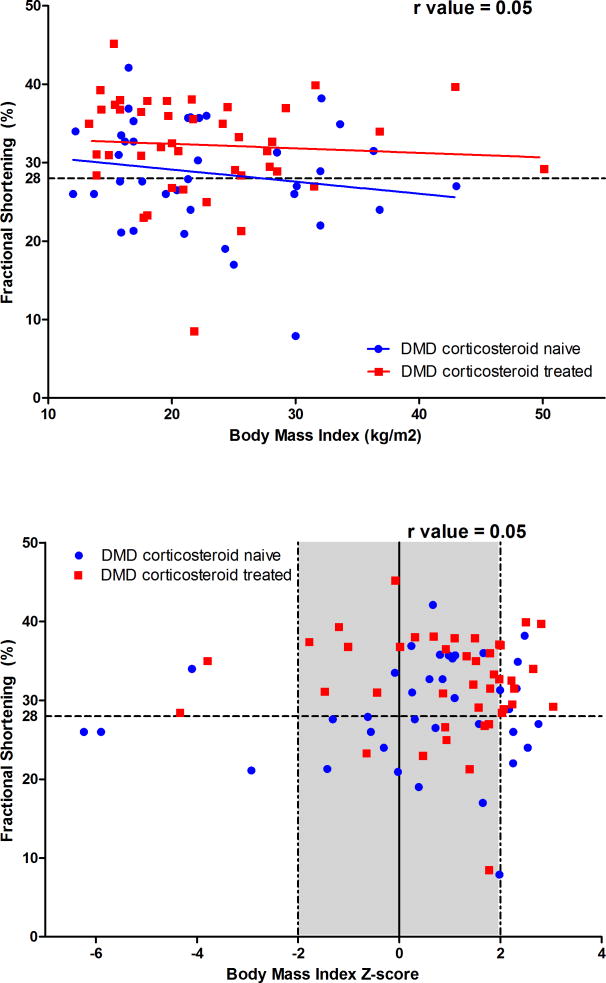
3.3 Corticosteroid Results (Figure 1)
Figure 1.
Figure 1A. Relationship between Fractional Shortening and Body Mass Index by Corticosteroid Use
Figure comparing the FS to BMI of boys treated with corticosteroids for greater than 6 months to those that did not receive corticosteroid treatment. r value for figure 1A=0.05
Figure 1B. Relationship between Fractional Shortening and Body Mass Index Z score by Corticosteroid Use
Figure comparing the FS to BMI Z score of boys treated with corticosteroids for greater than 6 months to those that did not receive corticosteroid treatment. r value for figure 1B=0.05
Affected boys that received corticosteroids for > 6 months did not differ significantly in their median BMI Z scores (1.11, IQR 0.25–1.95) compared to corticosteroid naive subjects (1.05, IQR 0.01–1.86, p=0.69). We were also unable to correlate continued corticosteroid use until the time of CMP diagnosis to median BMI Z score (1.12, IQR 0.25–1.84 vs. 1.07, IQR 0.04–1.97, p=0.67). There was a weak positive correlation between corticosteroid duration and BMI Z score (r=0.05).
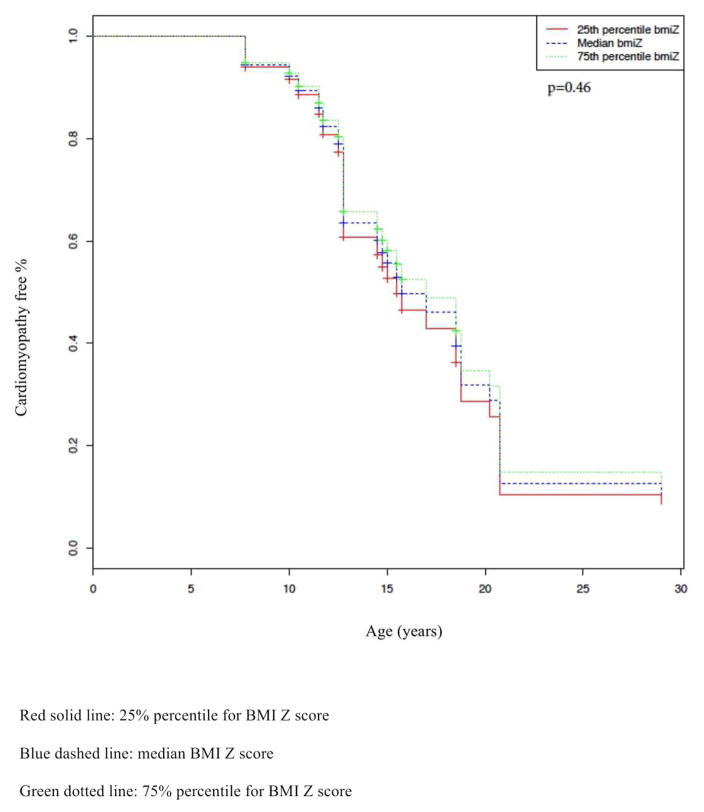
3.4 Proportional hazard regression with time dependent covariates model (Figure 2)
Figure 2.
Estimated age of being CMP free by BMI Z score
Kaplan Meir curve demonstrating the percentage (%) of boys CMP free over time by BMI Z score quartile (25%, 50%, 75%). p value for figure 2=0.46
We did not detect a significant association with body habitus (as studied using the BMI Z score) and earlier onset of CMP in our cohort. After adjusting for covariates (duration of corticosteroid usage and whether loss of ambulation had occurred or not), we did not detect a significant association between the subject’s BMI Z score and age of CMP onset (HR 0.79, when increased from 25th percentile (Z-score of 0.17) to 75th percentile (Z-score of 1.93), 95% CI 0.57 to 1.11, p=0.17). Two factors, corticosteroid usage and loss of ambulation, were evaluated for association to CMP onset in our cohort. The duration of corticosteroid usage (HR 0.4 when increased from 25th percentile (2 years) to 75th percentile (6 years), 95% CI 0.2 to 0.82, p=0.01) correlated with delayed CMP onset. Whether loss of ambulation occurred or not during the study period was not significantly associated with a change in LVFS (p=0.42) or LVIDd (p=0.28).
4. Discussion
We were unable to identify an association between the body habitus of DMD subjects and their age of CMP onset, although we cannot exclude the possibility that the size of our cohort did not provide adequate statistical power to detect a small but nonetheless meaningful association. We were also unable to confirm our secondary hypothesis, that higher BMI Z scores would be observed in those boys treated with corticosteroids for more than 6 months. We confirmed a previously known association with CMP onset and duration of steroid use; a longer duration of use is associated with a later age of development of CMP. We were unable to show a correlation between loss of ambulation and CMP onset in our cohort, however. These findings are important because they suggest that body habitus may not be associated with earlier CMP development in DMD; however, additional research is needed to corroborate these results, and to rule out the possibility of meaningful associations via more precise confidence intervals.
DMD CMP is common, with over 50% of boys exhibiting evidence of CMP by the end of their second decade [1,5]. Proactive medical therapies such as angiotensin converting enzyme inhibitors, beta-blockers, and aldosterone antagonists, can be useful in the treatment of DMD associated CMP [1,13, 14, 15, 16] and have been shown to lessen myocardial fibrosis in animal models [8]. Corticosteroids, widely used to prolong ambulation [14], have been shown to have beneficial effects on cardiac function and cardiovascular mortality [17,18]. Our study confirmed findings that previously demonstrate the beneficial effects of corticosteroids on the rate of CMP progression [18]. These results served to further validate our other findings.
We were unable to demonstrate an association between body habitus and premature onset of CMP within our patient population. We chose to use a subject’s BMI Z score as the independent variable, instead of the BSA or BMI, to more uniformly describe the standard deviation within the cohort and to allow for statistical comparisons between the group means [19]. The modality used to assess cardiac function in our study was echocardiography, as it provided the most complete data. CMR has been frequently used in this population and likely provides a more accurate and reproducible assessment of cardiac function, as well as an assessment of myocardial fibrosis. Although LVFS has adequately correlated with CMR LVEF in previous studies [20], replicating this study using CMR as the main assessment of cardiac function could be performed in a future research project.
No discernable differences were seen in the body habitus compositions between those boys that developed CMP and those with preserved cardiac function. Interestingly, boys’ weights (and BMI Z scores) remained relatively stable between years with only 28% (26/92) showing a change in their weight category during the study period. This stability may be impacted by the study duration, high usage of corticosteroids by our cohort, and degree of baseline muscle strength secondary to the juvenile average age of the cohort.
Recent studies in the mdx mouse model of DMD have raised concerns that corticosteroids could worsen myocardial fibrosis. These concerns, combined with the weight gain associated with systemic corticosteroids [21], have prompted some to question the safety of long-term use. Interestingly, we were unable to show a difference in BMI Z scores among those boys with a past history of corticosteroid use compared to those without. In addition, our results suggested that the consequence of weight gain might not be as considerable as previously thought, thus allaying some of the fears about long-term use of corticosteroids in DMD.
Certain limitations were inherent in our study. As our study population was modest, the study suffers from lack of precision for many estimates due to the sample size; including the less severe forms of muscle disease would have increased precision, but would limit the application of results and the generalizability of the findings. The lack of power could have also increased the likelihood of a type II error; but due to the rare nature of the disease, including an adequately sized cohort is not feasible without a larger scale multicenter study. In addition, the age of our cohort may have limited the analysis; however, we had a considerable proportion of affected boys that developed our primary outcome of CMP over the study period and are able to document weight patterns over multiple years for the subjects. Another limitation involved the use and predictive capacity of BMI Z scores as a surrogate for weight change in muscle wasting diseases. Other studies involving DMD patients have used weight for age ratio [22], urinary creatinine excretion [23] and MRI resting energy expenditure [24] to study weight change over time. The retrospective nature of our study did not allow the use of these alternative methods, though these approaches may prove useful in conducting future prospective studies. We are unable to comment on degree of CMP progression to clinical heart failure after the initial abnormal echocardiogram as we elected to study until CMP onset; it is possible that BMI plays a larger role in CMP progression in older subjects or subjects with already depressed LV function. The high prevalence of corticosteroid use could have led to confounding in both the age of CMP onset and weight trends in the overall cohort. Due to the retrospective nature of the study, we did not have complete data on cardiac medication usage for this cohort and removing those patients would have further reduced the study power. However, the same physician treated all patients, leading to very little practice variability.
No association was demonstrated between the BMI of DMD subjects and their age of CMP onset. The clinical significance of this result, however, warrants ongoing investigation, as this study describes novel findings. A correlation between CMP onset and corticosteroid usage was demonstrated in the cohort. Further research is needed to identify factors predictive of premature CMP onset in DMD boys, as significant advances in cardiac understanding and management are required. Future work is necessary to evaluate whether weight changes due to change in muscle mass or interventions for elevated BMI can alter the progression to CMP.
Table 1.B.
Baseline Demographics for Cohort *
| Normal weight N=31 |
Overweight/Obese N=46 |
Underweight N=8 |
P values | |
|---|---|---|---|---|
| Fractional Shortening, % | 31.9 | 31.5 | 28.4 | 0.78 |
| CMR Ejection Fraction, % | 54 | 51.9 | NA# | 0.30 |
| CMP, number | 13 | 17 | 4 | NS |
| Left ventricular mass, gramsa | 55.95 | 79.75 | 67.2 | <0.01 |
| LVIDd, mma | 41 | 41 | 39 | 0.28 |
| LVIDs, mma | 26 | 28.5 | 27.5 | 0.37 |
| Corticosteroid use, % | 45% | 57% | 50% | 0.61 |
Only 1 subject in this category had a CMR Ejection fraction.
Expressed as median
Acknowledgments
Grant Support:
Supported in part by Vanderbilt CTSA grant 1 UL1 RR024975 from NCRR/NIH
Supported by NIH T32HL 105334 “Developmental Determinants of Cardiovascular Disease”
Supported in part by the Jonah and Emory Marlin DMD Discovery Gift from the Fighting Duchenne Foundation.
This work was supported by American Heart Association Grant 13CRP14530007 (Soslow).
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K23HL123938. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Abbreviations: DMD- Duchenne Muscular Dystrophy, CMP- Cardiomyopathy, BMI- Body Mass Index, CMR- Cardiac Magnetic Resonance Imaging, LVEF- Left ventricular ejection fraction, LVFS- Left ventricular fractioning shortening, LViDd- Left ventricular internal dimension in diastole, LViDs- Left ventricular internal dimension in systole, BSA- Body Surface Area, CDC- Centers for Disease Control, IQR- Interquartile range
Author Contributions:
MM: designed the study, collected the data, assisted with data analysis, and wrote the manuscript
JHS: designed the study, collected the data, assisted with data analysis, and wrote the manuscript
MX: performed statistical analysis and assisted with manuscript preparation
BS: performed statistical analysis and assisted with manuscript preparation
JCS: performed statistical analysis and assisted with manuscript preparation
WBB: assisted with manuscript preparation
LM: designed the study, collected the data, assisted with data analysis, and wrote the manuscript
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent was not required. Study approval by Vanderbilt Institutional Review Board was obtained prior to study initiation.
Declarations of Conflicting Interests:
MM: conflicts of interest: none
JHS: conflicts of interest: none
MX: conflicts of interest: none
BS: conflicts of interest: none
JCS: conflicts of interest: none
WBB: conflicts of interest: Receives funding for clinical trials from the following: NINDS (National Institute of Neurological Disorders and Stroke), Eli Lilly and Company, Sarepta Therapeutics
LWM: conflicts of interest: none
References
- 1.Jefferies JL, Eidem BW, Belmont JW, et al. Genetic Predictors and Remodeling of Dilated Cardiomyopathy in Muscular Dystrophy. Circulation. 2005;112:2799–804. doi: 10.1161/CIRCULATIONAHA.104.528281. [DOI] [PubMed] [Google Scholar]
- 2.Mendell JR, Lloyd-Puryear M. Report of MDA muscle disease symposium on newborn screening for Duchenne muscular dystrophy. Muscle Nerve. 2013;48:21–6. doi: 10.1002/mus.23810. [DOI] [PubMed] [Google Scholar]
- 3.Prevalence of Duchenne/Becker muscular dystrophy among males aged 5–24 years - four states, 2007. MMWR Morb Mortal Wkly Rep. 2009;58:1119–22. [PubMed] [Google Scholar]
- 4.Romfh A, McNally EM. Cardiac Assessment in Duchenne and Becker Muscular Dystrophies. Curr Heart Fail Rep. 2010;7:212–8. doi: 10.1007/s11897-010-0028-2. [DOI] [PubMed] [Google Scholar]
- 5.Thomas TO, Morgan TM, Burnette WB, et al. Correlation of heart rate and cardiac dysfunction in Duchenne Muscular Dystrophy. Pediatr Cardiol. 2012;33:1175–9. doi: 10.1007/s00246-012-0281-0. [DOI] [PubMed] [Google Scholar]
- 6.Barber BJ, Andrews JG, Lu Z, et al. Oral Corticosteroids and Onset of Cardiomyopathy in Duchenne Muscular Dystrophy. J Pediatr. 2013;163(4):1080–4. doi: 10.1016/j.jpeds.2013.05.060. [DOI] [PubMed] [Google Scholar]
- 7.Townsend D, Yasuda S, Chamberlin J, et al. Cardiac Consequences to skeletal muscle-centric therapeutics for Duchenne muscular dystrophy. Trends Cardiovascul Med. 2009;19:50–5. doi: 10.1016/j.tcm.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Olivotto I, et al. Obesity and its association to phenotype and clinical course in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2013;62(5):449–457. doi: 10.1016/j.jacc.2013.03.062. [DOI] [PubMed] [Google Scholar]
- 9.Gupta PP, et al. Obesity and the obesity paradox in heart failure. Can J Cardiol. 2015;31(2):195–202. doi: 10.1016/j.cjca.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Davidson ZE, Ryan MM, Kornberg AJ, et al. Observations of Body Mass Index in Duchenne Muscular Dystrophy: A longitudinal Study. European Journal of Clinical Nutrition. 2014;68(8):892–897. doi: 10.1038/ejcn.2014.93. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Cruz M, Sanchez R, Escobar RE, et al. Evidence of Insulin Resistance and other Metabolic Alterations in Boys with Duchenne or Becker Muscular Dystrophy. Int J Endocrinol. 2015:867273. doi: 10.1155/2015/867273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayer J, Charakida M, Deanfield JE, et al. Lifetime Risk: Childhood Obesity and Cardiovascular Risk. Eur Heart J. 2015;36(22):1371–1376. doi: 10.1093/eurheartj/ehv089. [DOI] [PubMed] [Google Scholar]
- 13.Kharraz Y, Guerra J, Pessina P, et al. Understanding the Process of Fibrosis in Duchenne Muscular Dystrophy. Biomed Research International. 2014;2014:1–11. doi: 10.1155/2014/965631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duboc D, Meune C, Lerebours G, et al. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol. 2005;45(6):855–857. doi: 10.1016/j.jacc.2004.09.078. [DOI] [PubMed] [Google Scholar]
- 15.Duboc D, Meune C, Pierre B, et al. Perindopril preventive treatment on mortality in Duchenne muscular dystrophy: 10 years’ follow up. Am Heart J. 2007;154(3):596–602. doi: 10.1016/j.ahj.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 161.Rafael-Fortney JA, Chimanji NS, Schill KE, et al. Early treatment with Lisinopril and Spironolactone Preserves Cardiac and Skeletal Muscle in Duchenne Muscular Dystrophy Mice. Circulation. 2011;124:582–8. doi: 10.1161/CIRCULATIONAHA.111.031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schram G, Fournier A, Leduc H. All-Cause Mortality and Cardiovascular Outcomes with Prophylactic Steroid Therapy in Duchenne Muscular Dystrophy. J Am Coll Cardiol. 2013;61:948–54. doi: 10.1016/j.jacc.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Markham LW, Spicer RL, Khoury PR, et al. Steroid Therapy and cardiac function in Duchenne Muscular dystrophy. Pediatr Cardiol. 2005;26:768–71. doi: 10.1007/s00246-005-0909-4. [DOI] [PubMed] [Google Scholar]
- 19.Must A, Anderson SE. Body mass index in children and adolescents: considerations for population based applications. Int J Obes. 2006;30:590–4. doi: 10.1038/sj.ijo.0803300. [DOI] [PubMed] [Google Scholar]
- 20.Brunklaus A, Parish E, Muntoni F, et al. The value of cardiac MRI versus echocardiography in the preoperative assessment of patients with Duchenne muscular dystrophy. European Journal of Pediatric Neurology. 2015 doi: 10.1016/j.ejpn.2015.03.008. http://dx.doi.org/10.1016/j.ejpn.2015.03.008. [DOI] [PubMed]
- 21.DMD Care Considerations Working Group. Diagnosis and management of Duchenne Muscular Dystrophy, part I: diagnosis and pharmacological and psychosocial management. Lancet Neurol. 2010;9(1):77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 22.Martigne L, Salleron J, Mayer M, et al. Natural evolution of weight status In Duchenne Muscular Dystrophy: A retrospective audit. Br J Nutr. 2011;105(10):1486–91. doi: 10.1017/S0007114510005180. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths RD, Edwards RH. A new chart for weight control in Duchenne Muscular Dystrophy. Arch Dis Child. 1988;63(10):1256–8. doi: 10.1136/adc.63.10.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zanardi MC, Tagliabue A, Orcesi S, et al. Body Composition and Energy Expenditure in Duchenne Muscular Dystrophy. Eur J Clin Nutr. 2003;57:272–8. doi: 10.1038/sj.ejcn.1601524. [DOI] [PubMed] [Google Scholar]