Abstract
FT-based high performance mass analyzers yield increased resolving power and mass measurement accuracy, yet require increased duration of signal acquisition that can limit many applications. The implementation of stronger magnetic fields, multiple detection electrodes for harmonic signal detection and an array of multiple mass analyzers arranged along the magnetic field axis have been used to decrease required acquisition time. The results presented here show that multiple ICR mass analyzers can also be implemented orthogonal to the central magnetic field axis. The orthogonal ICR cell system presented here consisting of two cells (master and slave cells) was constructed with printed circuit boards and installed within a single superconducting magnet and vacuum system. A master cell was positioned as is normally done with ICR cells, on the central magnetic field axis and a slave cell was located off this central axis, but directly adjacent and alongside the master cell. To achieve ion transfer between cells, ions that were initially trapped in the master cell were drifted across the magnetic field into the slave cell with application of a small DC field applied perpendicularly to the magnetic field axis. A subsequent population of ions was injected and accumulated in the master cell. Simultaneous excitation of cyclotron motion of ions in both cells was carried out, ICR signals from each cell were independently amplified and recorded in parallel. Presented here are the initial results of successful parallel spectral acquisition with this orthogonal dual ICR cell array.
Graphical Abstract
Introduction
FT-ICR MS offers unique advantages in many research fields including proteomics[1–3], metabolomics[3, 4], and others[3], due to its high resolving power and mass accuracy for improved structural analysis. However, one limitation of the technique stems from the relatively longer signal acquisition time required to obtain higher resolving power and mass accuracy. The resolving power can be estimated by simple approximation where R is resolving power, ω+ is measured cyclotron frequency and t is the required signal acquisition duration.[5, 6] Therefore, one approach to decrease required signal analysis time for high resolution mass spectral acquisition is to use stronger magnetic fields,[7, 8] and to use multiple detection electrodes for the analysis of harmonic signals of the fundamental frequency. Increased magnetic field and increased number of multiple detection electrodes result in increased detected frequencies and therefore, increase the achieved resolving power for signal acquisition of the same duration. In recent years, FTICR-MS instruments equipped with 21 T were developed at National Labs [9] and [10] and ICR cell designs with multiple electrodes have been demonstrated to enhance harmonic signals and improve acquisition rates.[7, 11–14] In addition, the use of multiple high resolution mass analyzers that can be operated in parallel was recently introduced as a complementary approach to help address this limitation.[15] In that work, multiple mass analyzers were aligned with the central magnetic field axis and trapped ion populations were sequentially filled in each cell in the array (up to five cells were demonstrated). Once the array was fully populated, all trapped ions were excited and detected at the same time so that five spectra could be acquired during the time required for only a single acquisition event. This approach enabled simultaneously high resolution analysis of biomolecules across a wide mass range with improved analysis speed directly proportional to the number of analyzers.
In multisection ICR cells, ions are able to be drifted from cell to cell along [16–19] or perpendicularly [20–23] to the magnetic field lines. The drift ICR cells were generally used for the study of ion-molecule reaction. For example, ions in an ICR cell move freely along the magnetic field axis and are trapped in 3 dimensions by combined electric and magnetic fields.[24, 25] The Finnigan model 2001 FTMS and the Nicolet model 2000 FTMS spectrometers,[26] employed dual ICR cells and were based on the concept of ion-drift along the magnetic field lines. In those systems, the dual ICR cell was aligned with the magnetic field, differentially pumped, and ions were generated in the high-pressure “source” cell and then transferred to the low-pressure “analyzer” cell for detection. These systems were not designed for parallel acquisition and were intended to benefit from a higher pressure cell for improved ion-molecule reactions and a lower pressure cell for improved ICR signal detection that could be implemented within a single superconducting magnet. In a similar concept, but based on the capability of cross-field drift of ions, Wronka and co-workers developed a drift ICR cell whereby ions could be generated in a higher pressure cell and drifted to a lower pressure ICR cell within a single electromagnet-based system. The Ridge group drift cell consisted of ion source, analyzer, and ion collector regions [20]. Recently, Nagornov et al. also demonstrated the ability to trap ions in ICR cells and vary the position of the ion cloud prior to ion excitation. Small DC voltages to were applied to detection and excitation electrodes to move ions off the central axis and decrease the contribution of magnetron motion to detected signals.[27]
While linear ICR cell arrays offer potential to expand the number of detectors to 5 or even more if detector size is further decreased, the possibility to expand further along the magnetic field axis is limited by available high field region within current magnet designs. However, the possibility to drift ions across the magnetic field axis offers the potential to develop non-linear ICR cell arrays and thus, the number of detectors in an array can potentially be greatly increased. The study presented here illustrates our efforts to investigate cross-magnetic field drift to evaluate its potential use in parallel ICR signal acquisition. These results were achieved with a novel dual cell design with cells arranged relative to one another in a direction orthogonal to the central magnetic field axis. Cross-field drift was found to be useful for efficient transfer of ions from a cell located on the field axis after their accumulation to a neighboring cell off the central axis. Filling both cells and use of a single excitation and detection event showed successful parallel spectral acquisition within this orthogonal dual cell array.
Experimental Section
LTQ FT-ICR MS
A hybrid linear ion trap Fourier transform ion cyclotron resonance mass spectrometry (LTQ FT-ICR MS; Thermo Scientific, Bremen, Germany) equipped with a 7 T actively shielded superconducting magnet (Oxford Nanoscience, Oxon, Uk) was used to acquire all experimental data. The LTQ FT-ICR MS was modified with an orthogonal ICR cell array, a preamplifier array, a custom multi-pin feedthrough flange on the source side of the vacuum system and wiring after the removal of a cylindrical Ultra ICR cell that was originally equipped with the system. After installation, the system was pumped and baked out overnight. For ion formation, electrospray ionization (ESI) was used as an ion source with syringe pump infusion of samples at a rate of 3.0 µL/min. A spray voltage of 4.5 kV was applied to a sample solution through a metal union for ionization. The ions were injected into the LTQ for initial accumulation and were then transferred into the ICR cell array through the original equipment octapole ion guide. Ion populations inside the LTQ were accumulated with automatic gain control (AGC) on and set to 1.0 × 105. The pressure indicated on the ion gauge in the cell region during all experiments was approximately 0.4×10−10 Torr.
ICR cell array design
The initial design of an orthogonal dual ICR cell array used here had two independent cells in which one cell (master cell) was positioned on the central magnetic field axis and another (slave cell) beside it as illustrated in Figure 1. Except for detection electrodes which were constructed with copper wires,[28] all electrodes in this ICR cell were constructed with 6 identical printed circuit boards (PCBs) using gold-coated copper including two excitation plates, two drift plates, and front/back lens plates as shown in Figure 2. The excitation plates shown in Figs. 2a and b were segmented into two sections; one was used for the master cell and another was used for the slave cell. Each section had 5 segments. The middle segments were used as excitation electrodes for master and slave cells. The length and width of the excitation electrodes were 1.8″ and 0.5″, respectively. The segments next to the excitation electrodes were 0.1″ in width and were used as trapping electrodes. The segments next to the trapping electrodes were 0.25″ in width and were held at a ground potential in all experiments. Figs. 2c and d show the drift plates and the 5 segments used on each plate. The middle segment in each drift plate was used as a drift electrode to transfer ions from a master cell to a slave cell by applying DC voltages to these electrodes. The segments next to the drift electrodes were used as trapping electrodes. The segments next to the trapping electrodes were held at ground potential in all experiments. The front/back lens plates were segmented into two electrodes; entrance/exit lens electrodes for a master cell and front/back lens electrodes for a slave cell, as shown in Fig. 2e. The entrance/exit lens electrodes had a 0.2″ diameter hole at the center of the electrode through which the transferred ions from LTQ came into the master cell. To form the orthogonal ICR cell array shown in Fig. 2f, excitation and drift plates were soldered to front and back lens plates. After that, 24 AWG copper wires inserted into the holes for copper wire detection electrodes shown in Fig. 2e were soldered to the cell array. Fig. 2g shows the orthogonal ICR cell array with the removal of the drift plates to reveal the copper wires used as detection electrodes which were placed above the excitation electrodes (0.1″ away from the electrodes). The trapping electrode segments were electrically coupled together by soldering 22 AWG copper wires on the pads on a cell outer surface to make trapping electrodes. The solder used for the ICR cell was 99.3/0.7 Sn-Cu lead-free solder alloy. The entire length of the orthogonal ICR cell array was 2.54 inch. All individual PCB components for preparing an orthogonal ICR cell array were designed using a circuit board layout program EAGLE ver. 7.3.0 (CadSoft Computer, Pembroke Pines, FL) and manufactured by OSH Park (Advanced Circuits, Aurora, CO).
Figure 1.
An orthogonal ICR cell array (A). A master cell was positioned on the central magnetic field axis and a slave cell beside it. To obtain parallel mass spectra, ions from LTQ were trapped in the master cell (B), and then the trapped ions were transferred to the slave cell by applying drift voltages to drift electrodes for cross-field drift of ions (C). The next transferred ions from LTQ were injected to the recently vacated master cell (D). After filling the two cells, the trapped ions were excited and detected in parallel.
Figure 2.
Individual PCB components and assembled drift ICR cell array. Excitation plate outer surface (A) showing pads and inner surface (B) showing excitation and trapping electrodes printed on board. Drift plate outer surface (C) and inner surface (D). Front and back lenses plates (E). Drift ICR cell array assembly outside (F) and inside (G) showing copper wires used as detection electrodes.
Figure 3.
DC voltage profiles produced by MIPS to independently control DC voltage to trap ions in each cell. A) inject and trap ions in a master cell, B) drift ions from the master cell to a slave cell, C) Trap the drifted ions in the slave cell until detection. D) inject and trap ions in the vacated master cell, E) ICR signals detection, and F) quench.
The assembled ICR cell array was placed at the end of the octapole ion guide in the place of the ThermoFisher Ultra ICR cell. The detection electrode pairs from each cell were connected to a custom vacuum-compatible preamplifier array (GAA Custom Engineering), which was mounted at 0.6″ away from the front lens plate of the orthogonal ICR cell array, by using Kapton-coated wires (22 AWG, Accu-Glass Products, Inc. Valencia, CA) to allow parallel ICR signal amplification. The preamp array consisted of 3 single preamplifiers[15] although for this work only 2 preamplifiers were used.
To populate both cells in the orthogonal cell array, trapping and drift voltages applied to each electrode were independently controlled with a multi-channel programmable DC power supply (Modular Intelligent Power Source (MIPS), GAA Custom Engineering, Benton City, WA, USA).[15] Figure 3 shows the DC voltage profiles used to trap and drift ions for the orthogonal dual cell array. In this graph, the black color trace indicates the DC voltage changing with time for a LTQ back lens electrode. The purple color traces indicate the DC voltages changing with time for the master’s front/back trapping electrodes. The red color traces indicate the time-dependent DC voltages applied to the drift electrodes. The green color traces indicate the time-dependent DC voltages applied to the slave’s front/back trapping electrodes. To trap ions in both cells, the first population of ions transferred from the LTQ was injected into a master cell by applying a negative voltage to the master’s front trapping electrode and a positive voltage to the master’s back trapping electrode. To trap the ions in the master cell, the negative voltage applied to the master’s front trapping electrode was switched to a positive voltage, but the positive voltage applied to the master’s back trapping electrode remained constant (A). The trapped ions were then drifted to the slave cell by applying small positive and negative voltages to two drift electrodes for a few milliseconds (B). The drifted ions were trapped in the slave cell by applying a positive voltage to the slave’s front/back trapping electrodes that were kept until detection (C). Finally, the second transferred ions from LTQ were injected to the vacated master cell by applying a negative voltage to the master’s front trapping electrode and a positive voltage to the master’s back trapping electrode. To trap the ions in the master cell, the applied positive voltage to the back trapping electrode remained constant, and the negative voltage to the front trapping electrode was switched to a positive voltage (D). The entrance/exit and front/back lens electrodes were simultaneously controlled with each front/back trapping electrodes to inject and trap ions in the cells in all experiments. After filling both cells, cyclotron motion of trapped ions in both cells was excited simultaneously with the waveform amplitude of 0.1 (ThermoFisher Excalibur software) applied to both cells, and then ICR signals were detected with the gradually decreased trapping voltages (E). Parallel ICR signals from both cells were simultaneously amplified using the independent preamplifier array that was connected to the detection electrodes of each cell. Each amplifier output signal was connected to a single vacuum feedthrough pin with kapton-coated wire and then to a Saleae digitizer (Logic 8, Saleae. South San Francisco, CA, USA). The number of samples and sample rate were set to 131072 and 781250, respectively. DC power (±2.5V) to operate the in-vacuum preamplifier was supplied by an external power supply. The digitized time domain signals were transferred to a computer through a USB interface and processed with ICR-2LS (http://omics.pnl.gov/software/icr-2ls).
Sample Preparation
All peptides and Ultramark 1621 (a mixture of fluorinated phosphazenes) were purchased from Sigma (St. Louis, MO, USA). HPLC grade methanol and acetic acid were obtained from Fisher Scientific (Pittsburgh, PA, USA). 10 µM standard peptide solutions were prepared by dissolving them in 1:1 (v/v) water/methanol solvent mixtures containing 0.1% (v/v) of acetic acid. An Ultramark 1621 stock solution was prepared by dissolving 10 µL of Ultramark 1621 in 10 mL of acetonitrile. A 10 mL solution of Ultramark 1621 was prepared by dissolving 100 µL of the stock solution of Ultramark 1621 in a solution of 1% acetic acid in 50:50 methanol:water.
Result and discussion
Characterization of Drift Voltages and Transfer Event Duration
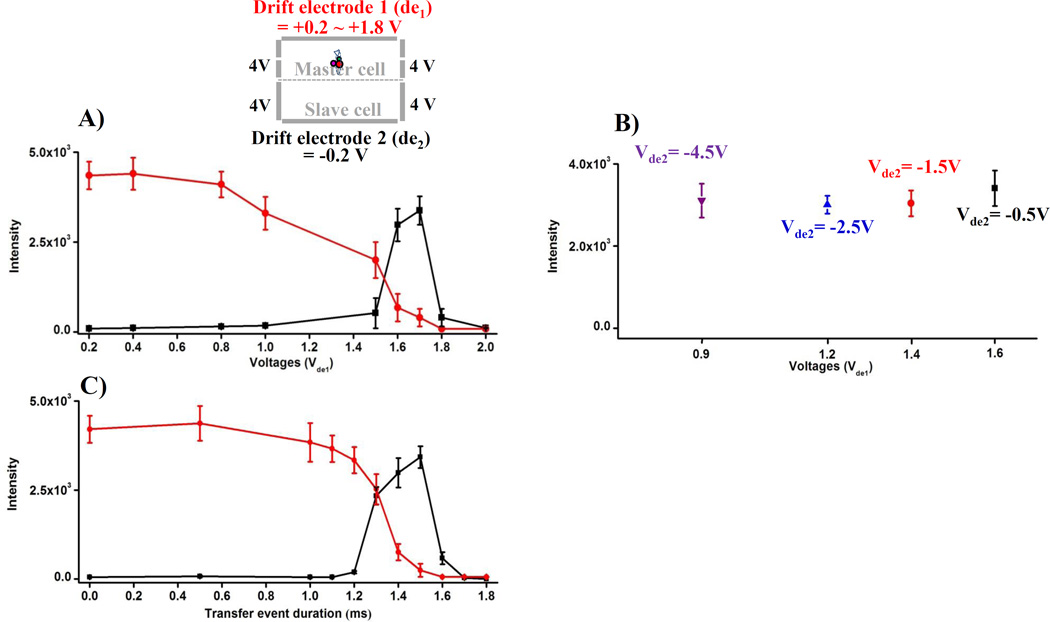
The unique orthogonal dual ICR cell array developed in this work allows characterization of the cross-magnetic field drift process by measurement of ions in either cell as a function of the parameters, drift time and drift electrostatic field. Both parameters were investigated to identify optimal values used for subsequent studies using a 10 µM solution of Ultramark 1621. For this experiment, optimized trapping potentials of −6V and +4V were applied to the master cell front and back trapping electrodes, respectively, during injection of ions. After ion injection, the applied voltages were switched to +4 V on both electrodes to trap ions in the master cell. The trapped ions were drifted by applying drift voltages and trapped in a slave cell by applying an optimized trapping potential (+4 V) to the slave cell simultaneous with the drift voltages. After drift of ions, cyclotron motion of trapped ions was excited simultaneously with the waveform amplitude of 0.1, and then ICR signals were detected with a decreased trapping voltage (+ 3V). To identify the optimal drift voltages and drift time, the peak intensities of the base peak observed in both cells were measured during drift voltage and time variations. For characterization of drift voltages, the voltage applied to the master cell drift electrode (Vde1) was varied over the range from 0 to +2.0 V for fixed Vde2 = −0.2 V. The duration of drift potential applications was 1.5ms. From each experimental condition, mass spectra from both cells were obtained 4 times and the standard deviation of measured peak intensities was calculated. Fig 4a shows that increasing the difference between the applied drift potentials increases the measured peak intensity obtained from the slave cell until +1.7 V, and decreases the peak intensity obtained from the master cell from +0.8 V. Further increase of the difference in drift potential difference (ΔVde1–de2) by increasing Vde1 beyond 1.7 V resulted in decreased slave cell peak intensities. To further investigate drift voltage-dependencies, the applied drift voltages (Vde1) were varied in the range from 0 to +2.0 V for different fixed Vde2 = −0.5 V, −1.5 V, −2.5 V and −4.5 V. Fig 4b shows that as Vde2 is made more negative, a lower drift voltage (Vde1) was observed to be required for optimum slave cell peak intensity. Moreover, the observed optimum signals appear much more sensitive to Vde1 changes as compared to Vde2 changes because the ion cloud trapped in the master cell is initially closer to Vde1 than Vde2. The effective potential experienced by the ions should decrease by roughly the square of the distance between the ions and the electrode[29].
Figure 4.
Characterization of drift voltages and transfer event duration. A) Vde1 was varied in the range from +0.2 to +1.8 V for a fixed Vde2 = −0.2 V, and B) Derived optimum Vde1 for different fixed Vde2 = −4.5V, −2.5V, −1.5V and −0.5V to obtain the most abundant peak intensity in the slave cell (transfer time = 1.5 ms). C) The duration of the drift event was investigated for the drift voltages (Vde1 = +1.7V and Vde2 = −0.2V) applied at 3.5 ms after a +4 V trapping voltage was applied to a master cell to trap ions. Applied drift voltages remained constant for transfer event duration as shown.
To optimize transfer event duration, drift voltages (Vde1 = +1.7 V and Vde1 = −0.2 V) were applied to the drift electrodes at 3.5 ms after a +4 V trapping voltage was applied to the master cell front trapping electrode to trap ions. During ion drift, applied drift voltages remained constant for various time durations (0 to 1.8ms). Fig 4c shows the observed variation of measured base peak intensity as a function of transfer event duration. Increasing the transfer event duration increased the number of drifted ions to a slave cell, but decreased the number of ions in the master cell. With transfer duration of 1.5 ms or greater, decreased the slave cell base peak intensity was observed.
Parallel Mass Spectra With and Without Drift Voltages
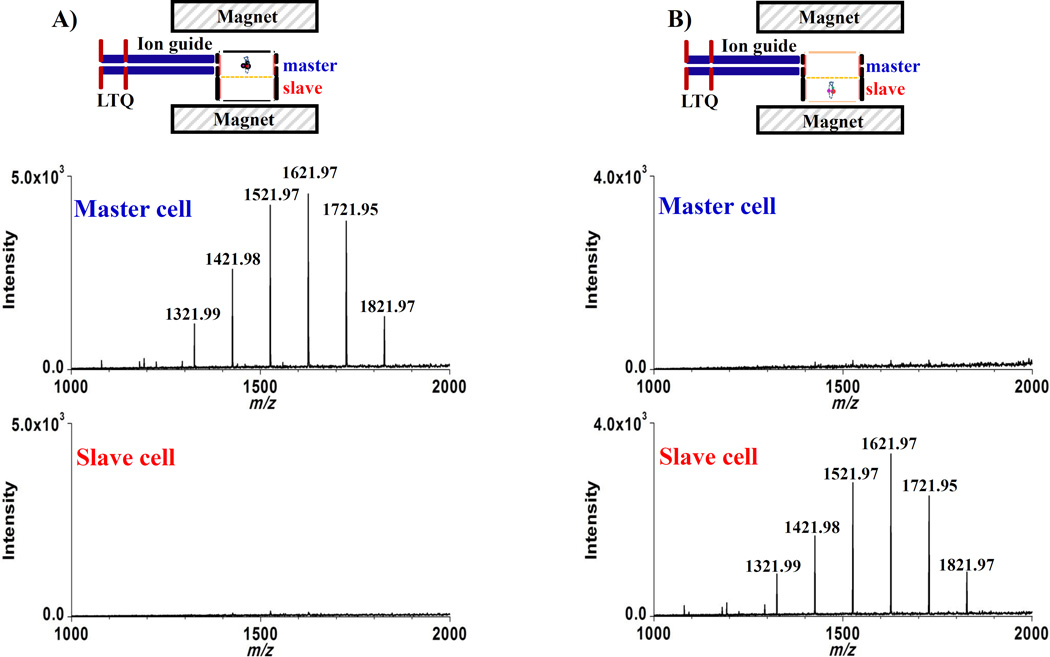
To confirm independent function of slave and master cells, ICR signals were obtained from both cells at the same time with and without drifting a trapped ion population from master to slave cells. To test master cell performance, the ions from LTQ were trapped in the master cell by applying the trapping voltage (+4 V) to the master cell front/back trapping electrodes. During these steps, the slave cell front/back trapping electrodes, and drift electrodes were kept at 0 V. After the ions were trapped in the master cell, RF voltage was applied to the excitation electrodes for both cells to excite ion cyclotron motion and ICR signals from each cell were acquired. Figure 5 shows the resulting mass spectra. As shown in Fig. 5a, expected abundant ion signals from Ultramark 1621 were observed in the master cell, but no peaks were detected in the slave cell. To further test independent function of the master cell, a +4 V trapping voltage was simultaneously applied to the slave cell with the trapping voltage applied to trap ions in the master cell, and the applied trapping voltages to both cells were decreased to +3V for detection. Although the trapping voltage (+4 V) was applied to the slave cell during trapping ions in the master cell, no peaks were detected in the slave cell without application of the ion drift event.
Figure 5.
Parallel mass spectra obtained from both cells at the same time before (A) and after (B) drift of ions by applying dc voltages to drift electrodes to transfer ions from a master cell to a slave cell. Prior to trapping ions to investigate independent function of the slave cell, the drift ICR cell was quenched to remove any residual ions from the previous measurement.
Prior to trapping ions to investigate independent function of the slave cell, the ICR cell was quenched to remove any residual ions from the previous measurement and then ions from LTQ were trapped in the master cell by applying a +4 V trapping voltage, as above. The trapped ions were then drifted to the slave cell by application of optimized drift voltages (Vde1 = +1.7 V and Vde2 = −0.2 V for 1.5ms) and simultaneously, a +4 V trapping voltage was applied to the slave cell front and back trapping electrodes before cyclotron excitation. After the ion drift event, 0 V was applied to the master cell front and back trapping electrodes. After ion excitation, the applied slave cell trapping voltages were decreased to +3V and ICR signals from both cells were acquired in parallel. The resulting mass spectra (Fig. 5b) acquired with 0 V applied to the master cell after drift of ions show reversed results; no ion signals observed in the master cell, but, ion signals were detected in the slave cell. Additionally, experiments performed where +4 V trapping voltages were maintained on master cell trapping electrodes after the ion drift event produced similar results: ions signals in the slave cell, no ion signals in the master cell.
Parallel Detection of Equivalent Ion Populations
To demonstrate parallel detection of equivalent ion populations, the first accumulated population of ions in the LTQ was trapped in the master cell by applying a +4 V trapping voltage. Trapped ions were drifted to a slave cell by applying drift voltages (Vde1 = +1.7 V and Vde2 = −0.2 V for 1.5ms) and then trapped in the slave cell by applying +4 V to its trapping electrodes. After drifting trapped ions to the slave cell, a second population of ions was accumulated in the LTQ, injected and trapped in the vacated master cell by applying a trapping voltage (+ 4V). After ion populations were trapped in both cells, a single cyclotron excitation waveform was applied to excitation electrodes in both cells simultaneously. After excitation, the trapping voltages applied to both cells were decreased to +3 V and the ICR signals from both cells were acquired in parallel. The resulting mass spectra and corresponding time domain signals (transients) are shown in Figure 6 and supporting information (Figure S-1). After drift of ions, no obvious discrimination in S/N and intensity variations observed between master and slave cells resultant from cross-magnetic field drift used for ion transfer. The transmission efficiency for ions between m/z = 1000 to 2000 estimated by (the peak intensity from the slave cell/the peak intensity from the master cell) × 100 is 75% or higher.
Figure 6.
Parallel detection of equivalent ion populations using calibration. Master (A) and slave (B) cells were filled with equivalent ions. All ions were simultaneously excited, ion signals were amplified, recorded and processed to yield parallel spectral acquisition in a drift ICR cell array.
Calibration and mass accuracy
To perform mass calibration for each cell with external calibration, equivalent populations of Ultramark ions were trapped in both cells and parallel ICR signals were acquired for 0.3s. The trapping potentials applied to both cells for cyclotron excitation were +4V and +3 V for signal acquisition. Applied ion drift voltages were Vde1 = +1.7 V and Vde2 = −0.2 V for 1.5ms as described above. Calibration for both cells was accomplished with a two-parameter calibration equation, m/z = A/f + B/f2.[5, 30] In this equation, the A term is related to magnetic field strength and the B term related to magnetron motion and the electric field generated primarily from trapping potentials, and f is the measured cyclotron frequency. To calculate the appropriate mass calibration parameters, the equation was re-written as f × m/z = A + B/f and the observed peaks were mapped on f × m/z versus 1/f plots as shown in Supporting Information (Figure S-2). The plots show that the points fit linear equations, from which A and B parameters of the calibration equation for each cell are obtained; A = 108,199,088.25 and B = − 902,890,884.58 for the master cell, and A = 108,199,142.79 and B = − 906,722,854.42 for the slave cell. After calibrating the observed ion peaks in each cell by the calibration equation, the mass measurement accuracies were calculated for the Ultramark 1621 ion peaks and are shown in Table 1 and mass measurement error values on the order of 1 ppm were obtained from the both cells in the orthogonal dual ICR cell array.
Table 1.
Calculated mass error for each peak observed from both cells
| Master cell | |||||
|---|---|---|---|---|---|
| Theoretical m/z |
Theoretical frequency (Hz) |
Measured m/z |
Measured frequency (Hz) |
Calibrated m/z |
ppm |
| 1321.9843 | 81837.6282 | 1322.9674 | 81776.8713 | 1321.9863 | 1.6 |
| 1421.9779 | 76082.2120 | 1423.0419 | 76025.3061 | 1421.9798 | 1.4 |
| 1521.9715 | 71083.0576 | 1523.1511 | 71027.9544 | 1521.9744 | 1.2 |
| 1621.9651 | 66700.2961 | 1623.2642 | 66646.8706 | 1621.9670 | 1.2 |
| 1721.9587 | 62826.5458 | 1723.4366 | 62772.6537 | 1721.9520 | −1.6 |
| Slave cell | |||||
| 1321.9843 | 81837.6340 | 1323.0446 | 81772.1048 | 1321.9864 | 1.9 |
| 1421.9779 | 76082.2149 | 1423.1392 | 76020.1098 | 1421.9806 | 1.7 |
| 1521.9715 | 71083.0580 | 1523.2313 | 71024.2162 | 1521.9691 | −1.6 |
| 1621.9651 | 66700.2943 | 1623.3892 | 66641.7309 | 1621.9673 | 1.4 |
| 1721.9587 | 62826.5420 | 1723.5304 | 62769.2346 | 1721.9554 | −1.9 |
Revolving power
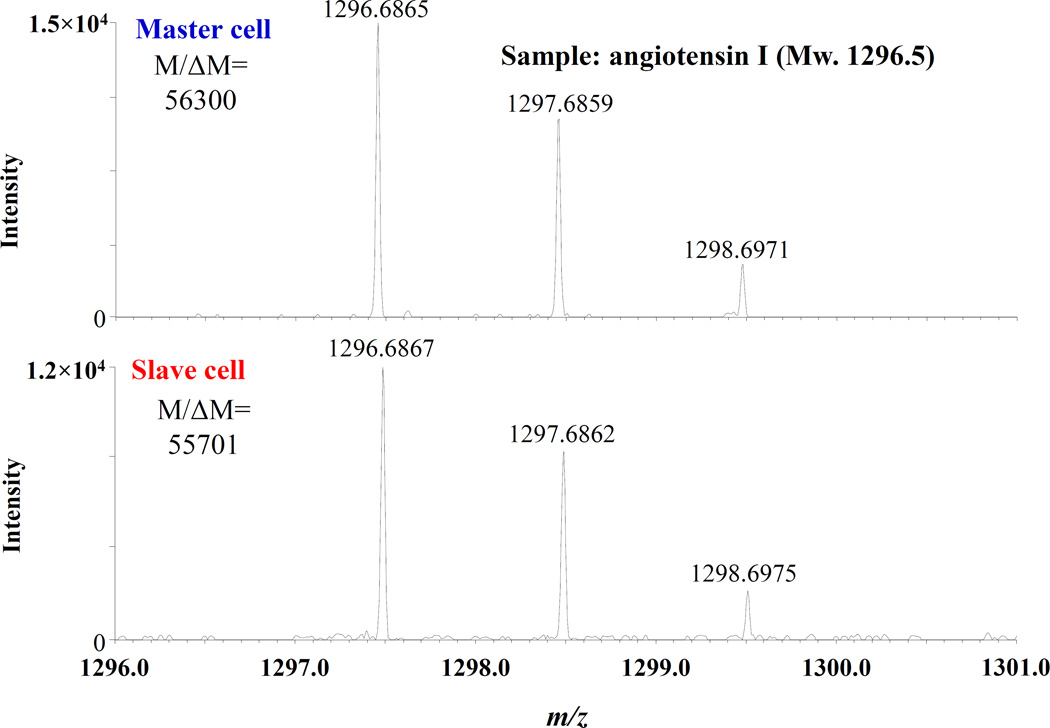
One question regarding the use of multiple cells positioned orthogonal to the magnetic field axis relates to whether parallel, stable ICR signals can be generated in both cells where the ICR orbit center in the slave cell is removed from the magnetic field central axis. If positioning the ions in the slave cell is not compatible with stable, coherent cyclotron motion, high resolution spectral acquisition would be precluded. To investigate resolving power of this orthogonal cell configuration, mass spectra from both cells were evaluated with the analysis of the peptide angiotensin I (MW=1296.5). The trapping voltages applied to both cells were +4 V, and the applied ion drift conditions used to transfer ions to the slave cell were Vde1 = +1.7 V and Vde2 = −0.2 V for 1.5ms. All voltages used to inject, trap, drift and eject ions were the same as described above. Figure 7 shows resulting mass spectra acquired by accumulation and injection of equivalent angiotensin ion populations for excitation and detection in either master or slave cells. Thus, to at least the level of moderately high resolving power that would be acquired during on line liquid chromatography separation of proteome samples, the performance of orthogonal cells can help further increase the number of detectors available in a mass spectrometer array. Future efforts will investigate additional orthogonal cells and further refined cell geometries to provide additional benefits.
Figure 7.
High resolution spectra of the peptide angiotensin I showing resolving power R(FWHM) of nearly 60,000 in both cells.
Multiple injections of different ion populations
One benefit demonstrated previously for a cell array[15] is that each cell can be used to detect different ion populations. To test this capability with the orthogonal dual cell array, two different ion populations were accumulated in each cell in the array (Figure 8). For these experiments, ions from different mass ranges or different ion types were selected or produced in the LTQ, transferred to selected cells and parallel ICR signals were acquired. AGC target values for the FT analyzer were set to 5.0 × 106, 6.0 × 105 and 8.0 × 105 for the full MS target, the SIM target and MSn target, respectively. The spectra in Fig 8a show results of multiple injections of differing ion populations in each cell. For this experiment, full MS analysis was performed in the master cell while the slave cell was used to trap and detect only selected ions at m/z = 1522. As shown in Fig 8a a distribution of Ultramark ions and single peak were observed in the master cell and the slave cell, respectively. Fig. 8b shows simultaneous acquisition of MS and MS/MS spectra of Ultramark ions. In this case, the full distribution of Ultramark ions was transferred and detected in the slave cell, while fragment ions from the Ultramark species near m/z = 1622 were transferred to and detected in the master cell. These results illustrate that orthogonal cells can be used to provide parallel detection of independent mass ranges or independent stages of mass analysis. These capabilities can further increase the number and type of measurements that can be performed during chromatographic separations.
Figure 8.
Multiple injections of different ion populations. (A) All ions (m/z 1000–2000) accumulated in the LTQ were injected into the master cell and ion at m/z 1522 was selected and injected into slave cell. (B) The fragmented ions for the selected ion at m/z 1622 in LTQ were transferred to a master cell and the accumulated ions in the LTQ were transferred to a slave cell for full MS analysis.
CONCLUSIONS
FT-ICR MS is a powerful instrument for the study of complex biological samples due to its ability to acquire high performance measurements; however, this typically requires longer signal acquisition times to achieve high resolution. One approach to decrease required signal analysis time for high resolution mass spectral acquisition is to use multiple high resolution mass analyzers.[15] In this study, we developed a new multiple mass analyzer called an orthogonal dual ICR cell array that consists of master and slave cells. The master cell was positioned on the central magnetic field axis with the slave cell positioned immediately adjacent to the master cell. To obtain parallel ICR signals, ions were trapped in both cells by independently controlling the applied voltages to trapping and drift electrodes. After filling both cells, a common RF waveform was applied to the excitation electrodes of both cells and ICR signals were acquired in parallel. With the current orthogonal ICR cell array, no obvious discrimination in S/N and intensity variations were observed between master and slave cells as a result of cross-magnetic field drift used for ion transfer. The observed transmission efficiency between cells for all ions was 75 % or higher. High resolution spectra (60,000 FWHM) were observed to be achievable with either the master or slave cell. This study shows that the capability of cross-field drift of ions can be used to transfer ions with non-linear multiple mass analyzers and potentially extend the number of ICR cells beyond those possible with linear arrays. Taken together with our previously reported linear 5 cell array technology,[15] the present results suggest that parallel acquisition of 10 or more spectra is possible with a two or three dimensional ICR cell array.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health through grant 5R01GM097112.
References
- 1.Bantscheff M, Lemeer S, Savitski M, Kuster B. Quantitative mass spectrometry in proteomics: critical review update from 2007 to the present. Anal. Bioanal. Chem. 2012;404:939–965. doi: 10.1007/s00216-012-6203-4. [DOI] [PubMed] [Google Scholar]
- 2.Yates JR, Ruse CI, Nakorchevsky A. Proteomics by Mass Spectrometry: Approaches, Advances, and Applications. Annu. Rev Biomed Eng. 2009;11:49–79. doi: 10.1146/annurev-bioeng-061008-124934. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths WJ, Wang Y. Mass spectrometry: from proteomics to metabolomics and lipidomics. Chem. Soc Rev. 2009;38:1882–1896. doi: 10.1039/b618553n. [DOI] [PubMed] [Google Scholar]
- 4.Courant F, Antignac J-P, Dervilly-Pinel G, Le Bizec B. Basics of mass spectrometry based metabolomics. Proteomics. 2014;14:2369–2388. doi: 10.1002/pmic.201400255. [DOI] [PubMed] [Google Scholar]
- 5.Marshall AG, Hendrickson CL, Jackson GS. Fourier transform ion cyclotron resonance mass spectrometry: A primer. Mass Spectrom. Rev. 1998;17:1–35. doi: 10.1002/(SICI)1098-2787(1998)17:1<1::AID-MAS1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 6.Amster IJ. Fourier Transform Mass Spectrometry. J. Mass Spectrom. 1996;31:1325–1337. [Google Scholar]
- 7.Nagornov KO, Gorshkov MV, Kozhinov AN, Tsybin YO. High-Resolution Fourier Transform Ion Cyclotron Resonance Mass Spectrometry with Increased Throughput for Biomolecular Analysis. Anal. Chem. 2014;86:9020–9028. doi: 10.1021/ac501579h. [DOI] [PubMed] [Google Scholar]
- 8.Scigelova M, Hornshaw M, Giannakopulos A, Makarov A. Fourier Transform Mass Spectrometry. Mol. Cell. Proteomics. 2011;10 doi: 10.1074/mcp.M111.009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendrickson CL, Quinn JP, Kaiser NK, Smith DF, Blakney GT, Chen T, et al. 21 Tesla Fourier Transform Ion Cyclotron Resonance Mass Spectrometer: A National Resource for Ultrahigh Resolution Mass Analysis. J. Am. Soc. Mass Spectrom. 2015;26:1626–1632. doi: 10.1007/s13361-015-1182-2. [DOI] [PubMed] [Google Scholar]
- 10.Shaw JB, Lin T-Y, Leach FE, Tolmachev AV, Tolić N, Robinson EW, et al. 21 Tesla Fourier Transform Ion Cyclotron Resonance Mass Spectrometer Greatly Expands Mass Spectrometry Toolbox. J. Am. Soc. Mass Spectrom. 2016:1–8. doi: 10.1007/s13361-016-1507-9. [DOI] [PubMed] [Google Scholar]
- 11.Misharin AS, Zubarev RA. Coaxial multi-electrode cell (‘O-trap’) for high-sensitivity detection at a multiple frequency in Fourier transform ion cyclotron resonance mass spectrometry: main design and modeling results. Rapid Commun. Mass Spectrom. 2006;20:3223–3228. doi: 10.1002/rcm.2724. [DOI] [PubMed] [Google Scholar]
- 12.Misharin AS, Zubarev RA, Doroshenko VM. Fourier transform ion cyclotron resonance mass spectrometer with coaxial multi-electrode cell (‘O-trap’): first experimental demonstration. Rapid Commun. Mass Spectrom. 2010;24:1931–1940. doi: 10.1002/rcm.4593. [DOI] [PubMed] [Google Scholar]
- 13.Nikolaev EN, Rakov VS, Futrell JH. Analysis of harmonics for an elongated FTMS cell with multiple electrode detection. Int. J. Mass Spectrom. Ion Processes. 1996;157–158:215–232. [Google Scholar]
- 14.Vorobyev A, Gorshkov MV, Tsybin YO. Towards data acquisition throughput increase in Fourier transform mass spectrometry of proteins using double frequency measurements. Int. J. Mass spectrom. 2011;306:227–231. [Google Scholar]
- 15.Park S-G, Anderson GA, Navare AT, Bruce JE. Parallel Spectral Acquisition with an Ion Cyclotron Resonance Cell Array. Anal. Chem. 2016;88:1162–1168. doi: 10.1021/acs.analchem.5b02987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wobschall D. Ion Cyclotron Resonance Spectrometer. Rev. Sci. Instrum. 1965;36:466–475. [Google Scholar]
- 17.Miasek PG, Beauchamp JL. A novel trapped-ion mass spectrometer for the study of ion-molecule reactions. International Journal of Mass Spectrometry and Ion Physics. 1974;15:49–66. [Google Scholar]
- 18.Hunt DF, Shabanowitz J, McIver RT, Hunter RL, Syka JEP. Ionization and mass analysis of nonvolatile compounds by particle bombardment-quadrupole-Fourier transform mass spectrometry. Anal. Chem. 1985;57:765–768. doi: 10.1021/ac00280a043. [DOI] [PubMed] [Google Scholar]
- 19.Alford JM, Williams PE, Trevor DJ, Smalley RE. Metal cluster ion cyclotron resonance. Combining supersonic metal cluster beam technology with FT-ICR. Int. J. Mass Spectrom. Ion Processes. 1986;72:33–51. [Google Scholar]
- 20.Wronka J, Strobel F, Ridge DP. Adaptation of an ICR drift cell for tandem ICR. Int. J. Mass Spectrom. Ion Processes. 1986;71:303–307. [Google Scholar]
- 21.Heninger M, Fenistein S, Durup-Ferguson M, Ferguson EE, Marx R, Mauclaire G. Radiative lifetime for v = 1 and v = 2 ground state NO+ ions. Chem. Phys. Lett. 1986;131:439–443. [Google Scholar]
- 22.Mauclaire G, Heninger M, Fenistein S, Wronka J, Marx R. Radiative relaxation of vibrationally excited ions. Int. J. Mass Spectrom. Ion Processes. 1987;80:99–113. [Google Scholar]
- 23.Mauclaire G, Derai R, Fenistein S, Marx R. Energy disposal in thermal-energy charge transfer reactions determined by kinetic energy measurements of product ions: Ne++O2→O++O+Ne and Ar++O2→O2++Ar. The Journal of Chemical Physics. 1979;70:4017–4022. [Google Scholar]
- 24.Mauclaire G, Sourisseau C. An improved balanced drift voltage control for ion cyclotron resonance drift cells. Int. J. Mass Spectrom. Ion Processes. 1988;83:215–221. [Google Scholar]
- 25.Beauchamp JL. Ion Cyclotron Resonance Spectroscopy. Annu. Rev. Phys. Chem. 1971;22:527–561. [Google Scholar]
- 26.Shea RC, Petzold CJ, Liu J-a, Kenttämaa HI. Experimental Investigations of the Internal Energy of Molecules Evaporated via Laser-induced Acoustic Desorption into a Fourier-transform Ion Cyclotron Resonance Mass Spectrometer (LIAD/FT-ICR) Anal. Chem. 2007;79:1825–1832. doi: 10.1021/ac061596x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozhinov AN, Tsybin OY, Tsybin YO. Ion Trap with Narrow Aperture Detection Electrodes for Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2015;26:741–751. doi: 10.1007/s13361-015-1089-y. [DOI] [PubMed] [Google Scholar]
- 28.Hanson CD, Castro ME, Kerley EL, Russell DH. Field-corrected ion cell for ion cyclotron resonance. Anal. Chem. 1990;62:520–526. [Google Scholar]
- 29.Child CD. Discharge From Hot CaO. Physical Review (Series I) 1911;32:492–511. [Google Scholar]
- 30.Ledford EB, Rempel DL, Gross ML. Space charge effects in Fourier transform mass spectrometry. II. Mass calibration. Anal. Chem. 1984;56:2744–2748. doi: 10.1021/ac00278a027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.