Abstract
Background
Use of chronic blood transfusions as a treatment modality in patients with blood disorders places them at risk for iron overload. Since patients with beta thalassemia major (TM) are transfusion-dependent, most studies on iron overload and chelation have been conducted in this population. While available data suggest that compared to TM, patients with sickle cell disease (SCD) have a lower risk of extra-hepatic iron overload, significant iron overload can develop. Further, previous studies have demonstrated a direct relationship between iron overload and morbidity and mortality rates in SCD. However, reports describing the outcome for patients with SCD and cardiac iron overload are rare.
Study Design and Methods
We performed a retrospective analysis and identified two SCD patients with cardiac iron overload. We provide detailed descriptions of both cases and their outcomes.
Results
Serum ferritin levels ranged between 17,000 – 19,000 μg/L. Both had liver iron concentrations (LIC) in excess of 35 mg of iron per gram of dried tissue (mg Fe/g dry wt) as well as evidence of cardiac iron deposition on magnetic resonance imaging (MRI). One patient died of an arrhythmia and had evidence of severe multi-organ iron overload via autopsy. On the other hand, following appropriate therapy, a second patient had improvement in cardiac function.
Conclusion
Improper treatment of iron overload in SCD can lead to a fatal outcome. Alternatively, iron overload may potentially be prevented or reversed with judicious use of blood transfusions and early use of chelation therapy, respectively.
Keywords: sickle cell disease, cardiac iron overload, blood transfusion, iron chelation
Introduction
Despite the approval of hydroxyurea by the US Food and Drug Administration (FDA) for the treatment of adults with sickle cell disease (SCD), red blood cell (RBC) transfusion therapy remains an integral part of SCD management. Blood transfusion is not without risks; among them iron overload and alloimmunization.1 The role of blood transfusions has been well-defined in the prophylaxis and treatment of stroke, as well as therapy for acute chest syndrome, aplastic crisis, and acute splenic sequestration with a significant drop from baseline hemoglobin.2 However, SCD patients are often transfused during uncomplicated painful episodes despite evidence that blood transfusions have no proven benefit in such a setting.3 More importantly, in cases where sporadic blood transfusions are clinically indicated, iron levels are not frequently monitored, and chelation therapy not initiated early enough, or at all.4
Much of the data available on the cardiac implications of iron overload and the benefit of chelation stem from studies conducted in patients with beta thalassemia major (TM).5 Large studies on the long-term effects of transfusion and iron overload in SCD are lacking; on the other hand, many studies report that an increased serum ferritin level is associated with earlier death and a more severe phenotype.3,6 Recently, Meloni and colleagues (2014) described the largest group of SCD patients with clinically significant cardiac iron overload (n=6). They further show that cardiac iron and ejection fraction can improve with appropriate iron chelation therapy. Despite a comparatively lower risk, SCD patients are therefore not entirely protected from cardiac iron toxicity.7 We report clinical findings of two chronically transfused SCD patients with diffuse iron overload, including significant cardiac loading. Both patients had symptomatic, radiologic and serologic evidence of cardiac iron toxicity, including depressed T2* values. Severe cardiac iron overload led to the death of one patient while the symptoms and clinical markers improved following aggressive chelation therapy in the other patient, suggesting that severe iron overload-associated end organ damage may be reversible and should be avoided in patients with SCD.
In this work, we performed a retrospective chart review. Cardiac magnetic resonance imaging (MRI) using a 1.5 Tesla scanner was ordered for 66 patients enrolled on our Natural History protocol (Clinical Trials.gov identifier NCT00081523) and our transplant protocols (NCT02105766, NCT00061568, and NCT00977691) between December 7, 2005 and September 6, 2016 at the NIH Clinical Center or Suburban Hospital, and 39 patients underwent liver biopsies with reportable LICs. All patients provided informed consent, and the protocols were approved by the National Heart, Lung, and Blood Institute Institutional Review Board. Two adults with SCD and cardiac iron overload were identified and their medical records reviewed. Iron overload was quantified using laboratory reports of serum ferritin levels, transferrin saturation, and liver iron concentration (LIC). The MRIs measured T2* of the liver and heart.
Case Reports
Case 1
A 42-year-old African-American man with SCD (HbSS) presented for evaluation for a nonmyeloablative peripheral blood stem cell transplant protocol, on which he was subsequently enrolled. His clinical course included multiple vaso-occlusive episodes requiring hospitalizations, supraventricular tachycardia, and congestive heart failure. Medications at the time of evaluation included digoxin and metoprolol. For five years prior, he had taken 500 mg/day of hydroxyurea (6.6 mg/kg/day), without any dose adjustments. Serum ferritin was 17,268 μg/L, direct bilirubin 1.6 mg/dL (reference 0.0 - 0.2 mg/dL), creatinine 0.95 mg/dL (reference 0.6 - 1.1 mg/dL); left ventricular ejection fraction (LVEF) was as low as 42% (normal ≥ 55%), and tricuspid regurgitant velocity (TRV) 2.8 m/s (normal <2.5 m/s). On transjugular parenchymal liver biopsy, LIC was 49.7 mg Fe/g dry weight (normal <2 mg Fe/g) with marked fibrosis (Ishak score 5). MRI revealed severe myocardial and hepatic iron overload with a cardiac T2* of 10.9 ms (<20 ms is diagnostic) and hepatic T2* < 2.5 ms (normal 25-30 ms).
The patient had been transfused more than 200 RBC units in his lifetime, but had not received iron chelation therapy. He was started on deferasirox at 20 mg/kg/day with the plan to transition him to deferoxamine. Shortly thereafter, the patient collapsed at home and died the weekend before his planned admission to start transplant conditioning. Autopsy revealed diffuse systemic siderosis, with iron loading in his thyroid gland, liver, testes, kidneys, adrenals, pancreas and heart as shown in Figure 1. His heart weighed 760 g (reference 270 – 280 g) and liver 3,700 g (reference 1440 - 1680 g). Sections from the posterior left ventricle showed cardiac myocytes containing contraction bands, but no inflammatory infiltrate or necrosis. Liver histology showed evidence of nodular regenerative hyperplasia and bridging fibrosis between portal areas, consistent with cirrhosis. An atheromatous plaque was found within the right coronary artery occupying about 75% of the lumen; the left coronary artery was clear. The cause of death was determined to be most likely arrhythmia.
Figure 1.
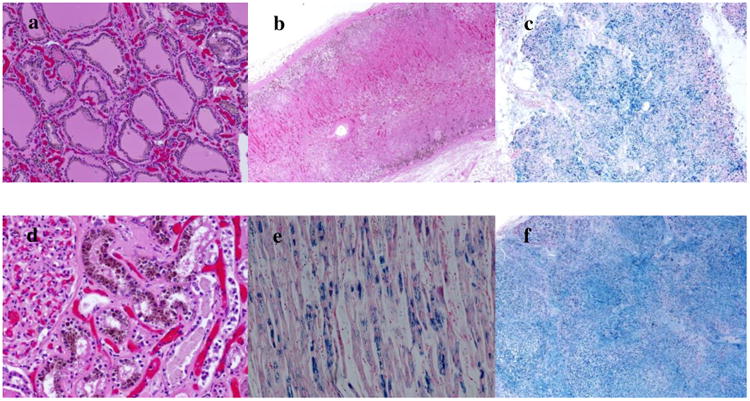
Autopsy findings in Patient 1 which revealed diffuse siderosis in the a) Thyroid, b) Adrenals, c) Pancreas d) Kidney, e) Heart, and f) Liver.
Case 2
A 35-year-old African-American woman with SCD (HbSS) was evaluated under our screening protocol with a diagnosis of decompensated congestive heart failure, acute kidney injury, and vaso-occlusive crisis. She presented with chest pain, lower extremity edema, and abdominal distention. Prior treatment regimens included carvedilol, digoxin, hydralazine, and isosorbide dinitrate. Serum ferritin was 19,147 μg/L, LIC 35.7 mg Fe/g dry weight, direct bilirubin 8.4 mg/dL, and creatinine 6.2 mg/dL. LVEF determined by MRI and echocardiogram was 38% and TRV 3.1 m/s. On telemetry, she had supraventricular tachycardia with heart rate ranging from 170 - 190 beats per minute and 5-beat run ventricular tachycardia. MRI revealed evidence of iron deposition in subcutaneous fat, and reduced signal in the liver, spleen, and pancreas. Cardiac and hepatic T2* were 12 ms and < 2 ms, respectively.
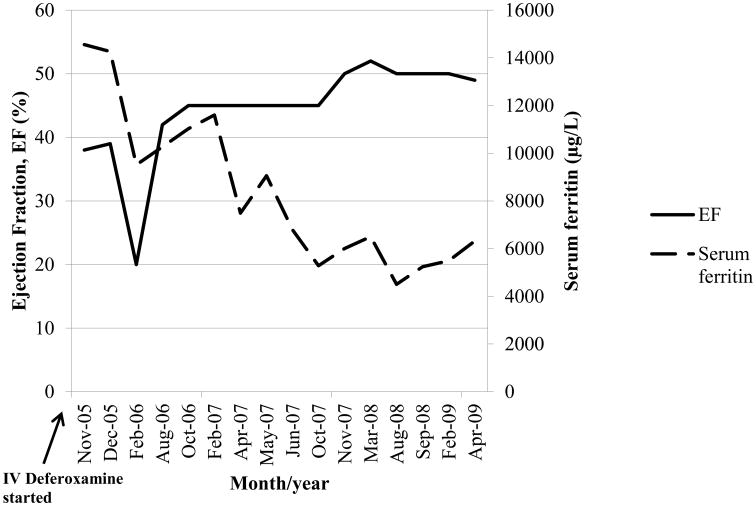
The patient had received more than 200 RBC units in her lifetime. She was previously diagnosed with iron overload and was intermittently treated with deferoxamine, but not at the time of admission. Hydroxyurea was initiated at 27 mg/kg/day and ascorbic acid at 500 mg daily with intravenous deferoxamine at 70 mg/kg/day daily for 8 to 12 hours for the initial 4 months, then 58 mg/kg/day via continuous infusion for 8 months, followed by 32 mg/kg/day via continuous infusion. An implantable cardioverter defibrillator was placed following sustained ventricular tachycardia. Medical therapy was continued over the course of four years. The patient experienced an acute decrease in her LVEF while hospitalized for a vaso-occlusive episode and sepsis. However, her LVEF improved from a baseline of 38 to 52% and the lowest serum ferritin level recorded following chelation was 4502 μg/L, see Figure 2. Subsequent interrogation of her defibrillator revealed no recurrent ventricular tachycardia and short episodes of supraventricular tachycardia which were medically controlled.
Figure 2.
Left ventricular ejection fraction and serum ferritin following chelation with intravenous deferoxamine.
Discussion
Iron-mediated organ injury is not only a function of absolute iron levels, but is modulated by differences in the pathophysiology of SCD and TM, as well as duration of transfusion.8 Unlike SCD patients, TM patients may develop iron overload even without transfusion because of abnormal hepcidin regulation and increased gut absorption. Additionally, the chronic inflammatory physiology and increase in markers of oxidative stress associated with SCD contribute to these differences.8-10 Lastly, because TM patients begin transfusions earlier in life, they are exposed to transfusion-associated iron overload longer than their SCD counterparts.
A recent study reported 32 children with SCD undergoing chronic transfusion therapy who did not develop evidence of significant cardiac iron overload11. However, in the two adult patients that we present, we show that ongoing transfusions can lead to significant cardiac damage. We also report in 1 patient (Case 1) atherosclerosis which is rare in patients with SCD. Therefore, the iron overload may have contributed to the development of the atheromatous plaque.12 It is imperative to monitor iron levels in SCD patients with a long-standing history of chronic and /or intermittent transfusions therapy.
Although a threshold LIC at which patients should be evaluated for cardiac overload has not been established, Fung et al found a significant association between the degree of liver iron loading and myocardial siderosis on autopsy in the Multi-Center Study of Iron Overload.2 Additionally, LIC values over 15 mg/g dry weight indicate risk of cardiac iron toxicity and premature death in TM.9 The LIC values reported in our study were closer to those reported by patients with SCD who had cardiac iron overload (22-53 mg/g)7 as compared to those patients who did not have cardiac iron overload (median 8, range 2-23 mg/g).11 Interestingly, for our 37 patients without cardiac iron overload who underwent liver biopsy, median LIC was 6 (range 1-44 mg/g). Six subjects had an LIC >20 mg/g. Therefore, patients with SCD and LIC >20 mg/g may be more likely to have cardiac iron overload as compared to patients with LIC <20 mg/g; however, LIC >20 mg/g is not diagnostic.
Serum ferritin is the most common biomarker used to estimate total body iron and has been shown to have a strong positive correlation with LIC in SCD and TM.9,13 In SCD, its accuracy is limited by fluctuations due to inflammation, liver dysfunction, ascorbate deficiency, hemolysis, and hepatitis C.4,14 Levels are increased during vaso-occlusive crises, and do not represent steady state when measured at such a time.3 With this in mind, it may still serve as a useful tool in assessing iron overload and response to chelation therapy when levels are measured at steady state. The autopsy findings of our patient (Case 1) support cardiac T2* as an accurate measure of elevated cardiac iron stores. Cardiac T2* is inversely related to the probability of reduced LVEF15 and T2* < 20 ms has been found to correlate with decreased LVEF in both SCD and TM,9,13 as was the case in both of our patients. We suggest that SCD patients with cardiac T2 * of <20 ms should be treated aggressively with intravenous chelation, which was successful in our patient (Case 2).
We demonstrated for the first time significant improvement in ferritin levels and LVEF collected serially over time, as well as alleviation of edema and ascites in Case 2 following chelation and other supportive treatment. Unfortunately, we were not able to follow Case 1 long enough to observe significant changes. Ventricular dysfunction resulting from cardiac iron accumulation has been shown to be improved after early chelation therapy.5,16 Indeed, we have shown that aggressive chelation was also beneficial in a patient who developed severe cardiac iron overload and systolic dysfunction.
Our study was limited by its retrospective nature, small sample size, and assessment of patients in a tertiary facility, which may over-represent the findings in the general population. Further, because of the anemia as well as vascular occlusion of large and small vessels that occur with SCD, it is unclear how much of the cardiac pathology was due to the cardiac iron overload as opposed to the underlying SCD. Larger, multi-center prospective studies are warranted to further assess cardiac iron overload in patients with SCD.
Cardiac iron overload studies in TM patients should be applied with caution to the SCD population due to the differences in disease pathophysiology. More independent studies on the role of iron overload in morbidity and mortality in SCD patients are indicated. Both of our patients received more than 200 RBC units before appropriate iron chelation therapy was initiated, and consequently developed severe cardiac iron overload. These findings underscore the need to closely monitor the number of RBC units transfused, and to initiate chelation therapy early in the course of transfusion therapy. Iron chelation should be considered after the first 10-20 transfusions, when the LIC as determined by liver biopsy or more commonly MRI is >7 mg Fe/g dry weight, or when the baseline serum ferritin is >1,000 μg/L.8 In summary, it is imperative for clinicians to understand the role of iron overload in SCD, to prescribe transfusions judiciously, to routinely monitor ferritin levels in transfused patients with SCD, and to have a low threshold to initiate iron chelation therapy.
Acknowledgments
This research was funded by the intramural programs of the Molecular and Clinical Hematology Branch, NHLBI and NIDDK, NIH and the Hematology Branch, NHLBI, NIH. The authors would like to thank Anna Conrey, CRNP and Mary Jackson, RN for assistance in ordering and retrieving information regarding cardiac MRIs, Theo Heller MD and the Hepatology group for performing liver biopsies, and Andrew Arai MD and his team for assistance with cardiac MRIs performed at Suburban Hospital.
Footnotes
Conflict of Interest: The authors have disclosed no conflicts of interest.
References
- 1.Vichinsky E, Butensky E, Fung E, et al. Comparison of organ dysfunction in transfused patients with SCD or beta thalassemia. Am J Hematol. 2005;80(1):70–74. doi: 10.1002/ajh.20402. [DOI] [PubMed] [Google Scholar]
- 2.Fung EB, Harmatz P, Milet M, et al. Morbidity and mortality in chronically transfused subjects with thalassemia and sickle cell disease: A report from the multi-center study of iron overload. Am J Hematol. 2007;82(4):255–265. doi: 10.1002/ajh.20809. [DOI] [PubMed] [Google Scholar]
- 3.Ballas SK. Iron overload is a determinant of morbidity and mortality in adult patients with sickle cell disease. Semin Hematol. 2001;38(1 Suppl 1):30–36. doi: 10.1016/s0037-1963(01)90058-7. [DOI] [PubMed] [Google Scholar]
- 4.Lambing A, Kachalsky E, Mueller ML. The dangers of iron overload: bring in the iron police. J Am Acad Nurse Pract. 2012;24(4):175–183. doi: 10.1111/j.1745-7599.2011.00680.x. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim el SH, Rana FN, Johnson KR, White RD. Assessment of cardiac iron deposition in sickle cell disease using 3.0 Tesla cardiovascular magnetic resonance. Hemoglobin. 2012;36(4):343–361. doi: 10.3109/03630269.2012.679376. [DOI] [PubMed] [Google Scholar]
- 6.Feld JJ, Kato GJ, Koh C, et al. Liver injury is associated with mortality in sickle cell disease. Aliment Pharmacol Ther. 2015;42(7):912–921. doi: 10.1111/apt.13347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meloni A, Puliyel M, Pepe A, Berdoukas V, Coates TD, Wood JC. Cardiac iron overload in sickle-cell disease. Am J Hematol. 2014;89(7):678–683. doi: 10.1002/ajh.23721. [DOI] [PubMed] [Google Scholar]
- 8.Porter J, Garbowski M. Consequences and management of iron overload in sickle cell disease. Hematology Am Soc Hematol Educ Program. 2013;2013:447–456. doi: 10.1182/asheducation-2013.1.447. [DOI] [PubMed] [Google Scholar]
- 9.Thuret I. Post-transfusional iron overload in the haemoglobinopathies. C R Biol. 2013;336(3):164–172. doi: 10.1016/j.crvi.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Porter JB. Pathophysiology of transfusional iron overload: contrasting patterns in thalassemia major and sickle cell disease. Hemoglobin. 2009;33(1):S37–45. doi: 10.3109/03630260903346627. [DOI] [PubMed] [Google Scholar]
- 11.Badawy SM, Liem RI, Rigsby CK, Labotka RJ, DeFreitas RA, Thompson AA. Assessing cardiac and liver iron overload in chronically transfused patients with sickle cell disease. Br J Haematol. 2016 doi: 10.1111/bjh.14277. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Real JM, Manco M. Effects of iron overload on chronic metabolic diseases. Lancet Diabetes Endocrinol. 2014;2(6):513–526. doi: 10.1016/S2213-8587(13)70174-8. [DOI] [PubMed] [Google Scholar]
- 13.Voskaridou E, Douskou M, Terpos E, et al. Magnetic resonance imaging in the evaluation of iron overload in patients with beta thalassaemia and sickle cell disease. Br J Haematol. 2004;126(5):736–742. doi: 10.1111/j.1365-2141.2004.05104.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoffbrand AV, Taher A, Cappellini MD. How I treat transfusional iron overload. Blood. 2012;120(18):3657–3669. doi: 10.1182/blood-2012-05-370098. [DOI] [PubMed] [Google Scholar]
- 15.Pennell DJ, Udelson JE, Arai AE, et al. Cardiovascular function and treatment in beta-thalassemia major: a consensus statement from the American Heart Association. Circulation. 2013;128(3):281–308. doi: 10.1161/CIR.0b013e31829b2be6. [DOI] [PubMed] [Google Scholar]
- 16.Westwood MA, Shah F, Anderson LJ, et al. Myocardial tissue characterization and the role of chronic anemia in sickle cell cardiomyopathy. J Magn Reson Imaging. 2007;26(3):564–568. doi: 10.1002/jmri.21018. [DOI] [PubMed] [Google Scholar]