A 70-year-old man with chronic lymphocytic leukemia (CLL) presented in June of 2016 with a one-month history of gradual onset, intermittent non-neutropenic fevers up to 103.4°F and night sweats, which did not improve despite treatment with broad spectrum antibiotics. Multiple blood, urine, and sputum cultures remained negative. Additionally, the patient had new onset, enlarged inguinal lymph nodes that were associated with a non-pruritic, non-tender rash on his bilateral upper and lower extremities, intermittent migratory arm swelling, and approximately 10 pounds of weight loss.
The patient was diagnosed with CLL in 2007, with unmutated IGHV and trisomy 12. He was treated first-line with idelalisib plus rituximab on a clinical trial, but was discontinued 5 years later due to intolerance. He had stable disease subsequent to discontinuation of treatment and did not receive subsequent systemic treatment for CLL. The patient developed warm antibody autoimmune hemolytic anemia in June, 2013 and was treated with various immunosuppressive regimens, undergoing splenectomy in March, 2014.
Physical examination showed a generalized erythematous, maculopapular skin rash and bulky inguinal lymphadenopathy. His initial laboratory tests demonstrated: WBC 48.1 K/uL, HGB 11.8 g/dL, PLT 371 K/uL with normal LDH and liver and renal function tests. Bone marrow (BM) examination showed CLL with trisomy 12 and NOTCH1 and BIRC3 mutations. Infectious work up was negative for all pathogens by standard testing as well as cultures of blood, urine, and sputum. Quantitative PCR for HSV 1 and 2 was negative. The patient was treated empirically with broad spectrum antimicrobial agents and one dose of intravenous immunoglobulin (IVIG) for suspected autoimmune hemolysis (Serum IgG was normal). Positron emission tomography-CT scan (PET-CT) showed generalized lymphadenopathy with F-18 fluorodeoxyglucose (FDG) uptake highest in inguinal lymph nodes, with standardized uptake value (SUV) of 9.8 (Figure 1-A). Core biopsy of an inguinal lymph node was performed to assess for Richter transformation (RT). Pathology evaluation revealed a diffuse infiltrate of intermediate-size to large cells with prolymphocytic and paraimmunoblastic morphology and interspersed, well-demarcated areas of suppurative necrosis (Figure 1-B–C). Scattered degenerated large cells with nuclear inclusions of Cowdry type A type were also observed. Immunohistochemistry (IHC) showed many lymphocytes positive for CD5 and PAX5 consistent with CLL with an increased proliferation (Ki-67) rate of 30–50%. The antibody specific for herpes simplex virus (HSV, subtypes I and II) highlighted many cells within the necrotic areas including the cells with nuclear inclusions (Figure 1-D–E). Based on these findings, the diagnosis of HSV lymphadenitis was rendered. The patient responded well to treatment with intravenous acyclovir, and was discharged in stable condition upon successful transition to valacyclovir. Infrequently we have observed, other CLL patients with suspected RT, based on PET-scan, shown by biopsy to have HSV lymphadenitis (unpublished data).
Figure 1. (A–E). Whole body PET-CT and histopathological features of a lymph node biopsy specimen involved by chronic lymphocytic leukemia (CLL) and necrosis attributable to herpes simplex virus (HSV) infection.
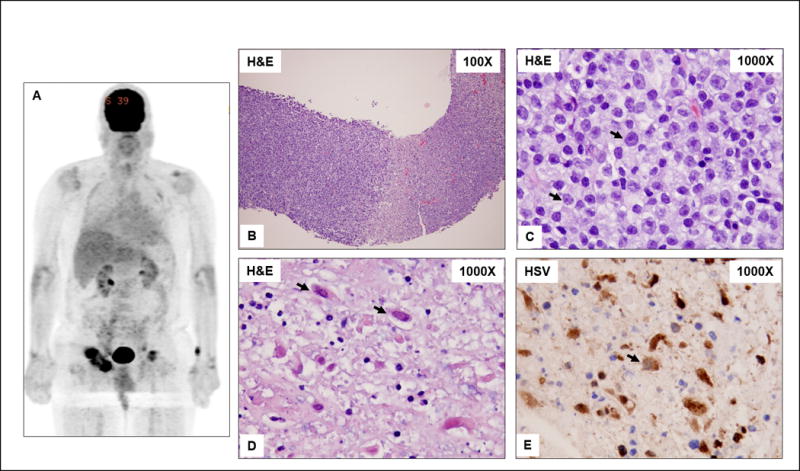
A) Enlarged, metabolically active, likely centrally necrotic, right greater and left inguinal lymph nodes. Prominent 4 × 5 cm right inguinal lymph node with SUV of 9.85 is seen. B) A core needle biopsy specimen showing replacement of lymph node by viable CLL (left of field) and necrosis (right of field). C) High magnification of CLL showing small lymphocytes and increased intermediate-size to large cells (arrows) consistent with prolymphocytes and paraimmunoblasts. D) High magnification of the necrosis showing scattered necrotic large cells with intranuclear inclusions typical of the Cowdry type A HSV inclusions (arrows). E) Immunohistochemistry for HSV (types I and II) is positive in the large cells with viral inclusions (arrows).
Progressive lymphadenopathy with high SUV on PET scan in patients with CLL can indicate disease transformation.1 However, histopathological features with biopsy/tissue analysis must be performed before finalizing the diagnosis of RT to exclude other etiologies. HSV lymphadenitis is rarely reported in patients with CLL and generally responds well to antiviral agents2,3 Furthermore, CLL lymph nodes exhibiting increased prolymphocytes or paraimmunoblasts may represent antigen activation or re-activation of latent viruses, such as HSV. In conclusion, increased prolymphocytes or paraimmunoblasts in lymph nodes from patients with CLL with PET-positive disease may not always represent accelerated CLL4 or RT; consideration of infectious etiologies as mimickers of RT5, as in this case, is warranted.
Acknowledgments
This study was supported in part by the NIH/NCI award number P30CA016672 and by NCI award number P01CA049639.
Footnotes
Contributions
NP, PJ, LJM, JLJ, NJ, JW, DPK, ZE, and WW gathered the data and evaluated the clinical presentation and pathology, and all authors have reviewed and approved the manuscript and do not have any disclosures.
References
- 1.Falchi L, Keating MJ, Marom EM, et al. Correlation between FDG/PET, histology, characteristics, and survival in 332 patients with chronic lymphoid leukemia. Blood. 2014;123:2783–90. doi: 10.1182/blood-2013-11-536169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cases M, Leduc C, Farmer PL, Richardson SE, Zoutman DE. Herpes simplex virus lymphadenitis: the elusive doppelganger in immunocompromised patients. Intern Med. 2014;53:2539–42. doi: 10.2169/internalmedicine.53.2343. [DOI] [PubMed] [Google Scholar]
- 3.Mercadal S, Martinez A, Nomdedeu B, et al. Herpes simplex and Epstein-Barr virus lymphadenitis in a patient with chronic lymphocytic leukemia treated with fludarabine. Eur J Haematol. 2006;77:442–4. doi: 10.1111/j.1600-0609.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 4.Gine E, Martinez A, Villamor N, et al. Expanded and highly active proliferation centers identify a histological subtype of chronic lymphocytic leukemia (“accelerated” chronic lymphocytic leukemia) with aggressive clinical behavior. Haematologica. 2010;95:1526–33. doi: 10.3324/haematol.2010.022277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain P, Hu S, Jabbour E, et al. Disseminated histoplasmosis as pseudo Richter’s transformation in a patient with chronic lymphocytic leukemia. Am J Hematol. 2015;90:752–3. doi: 10.1002/ajh.24029. [DOI] [PMC free article] [PubMed] [Google Scholar]


