Abstract
Dimethandrolone (DMA, 7α,11β-dimethyl-19-nortestosterone) has both androgenic and progestational activities, ideal properties for a male hormonal contraceptive. In vivo, dimethandrolone undecanoate (DMAU) is hydrolyzed to DMA. We showed previously that single oral doses of DMAU powder-in-capsule taken with food are well-tolerated and effective at suppressing both LH and testosterone (T), but absorption was low. We compared the pharmacokinetics and pharmacodynamics of two new formulations of DMAU, in castor oil and in SEDDS, with the previously tested powder formulation. DMAU was dosed orally in healthy adult male volunteers at two academic medical centers. For each formulation tested in this double-blind, placebo-controlled study, ten men received single, escalating, oral doses of DMAU (100 mg, 200 mg, and 400 mg) and two subjects received placebo. All doses were evaluated both fasting and with a high fat meal. All three formulations were well tolerated without clinically significant changes in vital signs, blood counts, or serum chemistries. For all formulations, DMA and DMAU showed higher maximum (p< 0.007) and average concentrations (p<0.002) at the 400mg dose, compared with the 200mg dose. The powder formulation resulted in a lower conversion of DMAU to DMA (p=0.027) compared with both castor oil and SEDDS formulations. DMAU in SEDDS given fasting resulted in higher serum DMA and DMAU concentrations compared to the other two formulations. Serum LH and sex hormone concentrations were suppressed by all formulations of 200 and 400mg DMAU when administered with food but only the SEDDS formulation was effectively suppressed serum T when given fasting. We conclude that while all three formulations of oral DMAU are effective and well-tolerated when administered with food, DMAU in oil and SEDDS increased conversion to DMA, and SEDDS may have some effectiveness when given fasting. These properties might be advantageous for the application of DMAU as a male contraceptive.
Keywords: Dimethandrolone, Male Contraception, Androgen, Pharmacokinetics, Suppression of gonadotropins
Introduction
Current methods of male contraception include condoms and vasectomy, both of which have drawbacks. While condoms are reversible and widely available, they have a high user failure rate. Vasectomies are efficacious, but invasive and not readily reversible. Therefore, efforts are underway to develop alternative male contraceptives with agents of known mechanisms of action. Male hormonal contraception uses exogenous sex steroids to suppress gonadotropin secretion and spermatogenesis, is reversible, and might provide additional health benefits for men if optimally designed (Liu, et al., 2006; Nieschlag, 2010; Page, et al., 2008; Piotrowska, et al., 2016; Wang, et al., 2016). Contraceptive efficacy studies in men with weekly intramuscular (IM) injections of testosterone enanthate or monthly injections of testosterone undecanoate have been encouraging, with high efficacy rates and few side effects (Gu, et al., 2009; World Health Organization Task Force on Methods for the Regulation of Male Fertility, 1990; World Health Organization Task Force on the Regulation of Male Fertility, 1996). However, suppression of spermatogenesis with exogenous testosterone alone is not uniform across all ethnic groups and requires supraphysiologic dosing. By combining testosterone with a progestin, suppression of spermatogenesis is enhanced (Liu, et al., 2008; Meriggiola & Bremner, 1997; Page, et al., 2008; Wang & Swerdloff, 2010). For example, the combination of testosterone implants and long-acting injections of the progestin depo-medroxyprogesterone acetate has excellent contraceptive efficacy (Turner, et al., 2003). However, injections and implants may be less desirable for some men than an oral medication such as DMAU. Oral contraceptives that are user controlled, easy to administer, and have shorter “on and off” rates, might be desirable for many couples (Glasier, 2010; Liu, et al., 2006).
Dimethandrolone (DMA) is a novel derivative of 19-nortestosterone that binds to both the androgen and the progesterone receptors, making it an attractive candidate as a single-agent male contraceptive (Attardi, et al., 2006). DMA undecanoate (DMAU), includes a long carbon chain ester at the C-17 position (Cook & Kepler, 2005). DMAU has been shown to be an effective, reversible contraceptive when dosed orally in preclinical studies. DMAU is hydrolyzed to DMA in vivo where it effectively decreases fertility in rabbits without appreciable toxicity, and is similarly non-toxic in rats and monkeys (Attardi, et al., 2011; Attardi, et al., 2011; Hild, et al., 2010). We previously reported the pharmacokinetics, safety, and tolerability of DMAU when given as a single oral dose as a powder in capsule to healthy male volunteers. However, we noted that this formulation resulted in low serum concentrations of DMA, likely due to the poor conversion of DMAU to DMA (about 3 percent) and that concomitant administration with food was required for both appreciable absorption and for conversion to DMA (Surampudi, et al., 2014). In an effort to increase the bioavailabilty of DMA, we reformulated oral DMAU into capsules in either castor oil or self-emulsifying drug delivery systems (SEDDS) and assessed their pharmacokinetics and pharmacodynamics when administered orally to healthy men. We hypothesized that utilization of these lipophilic drug delivery entities would enhance absorption and hydrolysis of DMAU, might negate the need for concomitant administration with food, and would optimize DMA serum concentrations for future evaluations of oral DMAU as a male hormonal contraceptive.
Research Participants and Methods
Research Participants
Healthy men, age 18 to 50 years, with no significant medical history or illnesses, and normal physical examination, blood count, clinical chemistries, hepatitis panel, liver function tests, prostate specific antigen (PSA) levels, electrocardiogram, and BMI less than 33 Kg/m2, were included in the study. Men were excluded if they had participated in a clinical trial involving an investigational drug within 30 days, had used hormonal therapy within the last 3 months, had a disorder of the hypothalamus/pituitary/testis, desired fertility within a year or had a pregnant partner, or had clinically significant abnormal physical or laboratory findings or an elevated PSA. Participants were recruited and enrolled at the Harbor-UCLA Medical Center/Los Angeles Biomedical Research Institute in Torrance, California and the University of Washington in Seattle, Washington. The study protocol was approved by the institutional review boards for both participating institutions. All participants provided written informed consent prior to any study procedures. The medical monitor and the investigators reviewed adverse events and safety data weekly with the provision that an external independent data safety monitoring board be notified if and when a grade 3 adverse event occurred.
Study Medications
DMAU is manufactured by Evestra Inc (San Antonio, TX). For the powder in capsule formulation, DMAU was micronized by Micron Technologies, Inc. (Malvern, PA) and encapsulated as 25 or 200 mg DMAU powder and placebo in capsules (Pharmtek Laboratories, Inc., San Diego, CA) in a cGMP environment. SRI International (Menlo Park, CA) manufactured 100 mg DMAU in castor oil/benzyl benzoate (70:30 volume: volume) and 50 mg DMAU in SEDDS capsules and corresponding placebo under Good Manufacturing Practice standards.
Study Design
All participants were assessed by a study physician to ensure that all inclusion and exclusion criteria were met. For all 3 formulations at each dose level evaluated, 10 men received active drug and 2 received placebo in a double-blind fashion. For the castor oil and SEDDS formulations, 10 participants received 100, 200, and 400 mg DMAU (for) both fasting and after a high fat meal, and two men received identical placebo capsules. One subject randomized to the DMAU in SEDDS group had no measurable DMA levels on two pharmacokinetics sampling days and his data were not included in the study (despite approval for additional procedures by the UCLA-IRB, he declined re-dosing). For the DMAU powder in capsules, participants were administered 100, 200 and 400 mg DMAU or identical placebo with a high fat meal as previously described (Surampudi, et al., 2014).
All participants were admitted to the clinical research unit within the Clinical and Translational Science Institute at each site and were observed for 24 hours with hourly vital signs monitoring following dosing. An electrocardiogram was performed 4–6 and 24 hours after drug administration. Safety laboratory tests (clinical chemistry panel, liver function tests) were measured at baseline and 24 hours after each DMAU dose. Fasting lipids and complete blood counts were quantified at baseline and approximately 7 days following each dose. Serum hormones (T, free T, estradiol, dihydrotestosterone [DHT], LH, FSH, and sex hormone binding globulin [SHBG], were measured before drug administration (time zero) and either every 4 (castor oil and SEDDS formulations) or 12 (powder formulation) hours post administration. In all cases, DMA and DMAU concentrations were quantified in blood drawn −0.5 before, 0, and 0.5, 1, 2, 4, 6, 8, 12, 18, and 24 hours after oral administration of DMAU. The participants returned to the clinic at least 7 days after the dose of DMAU for safety laboratory tests, adverse event reporting, vital signs, and hormone evaluation. Halting parameters for predefined safety criteria and adverse events were included in the protocol but these parameters were never reached.
Analytical Methods
Safety laboratory tests and lipid panels were performed at each institution’s respective clinical laboratory. All hormones were quantified by the licensed Endocrine and Metabolic Research Laboratory at Harbor-UCLA/LA Biomed using validated methods. Serum T, DHT, estradiol, DMAU and DMA were measured by liquid chromatography–tandem mass spectrometry (LC-MS/MS) (Rothman, et al., 2011; Shiraishi, et al., 2008; Surampudi, et al., 2014) and free T was calculated using a standard formula (Vermeulen, et al., 1999). Serum LH, FSH and SHBG were measured using sensitive fluoroimmunometric assays as previously described (Swerdloff, et al., 2000).
Statistical Analyses
The primary endpoints of the trial were safety and tolerability of the three formulations of DMAU. Secondary endpoints included the 24-hour PK of DMAU and DMA after oral dosing fasting and with food, as well as suppression of gonadotropins and testosterone production.
The number of participants for this Phase 1 clinical study was powered to provide at least a 0.80 probability to exclude at least 20% of participants developing Grade 3 adverse events assuming a two-sided 95% confidence interval with each dose and formulation given. The PK parameters for each full sampling day for DMAU were determined by non-compartmental methods and were primarily assessed using the area under the curve from 0–24 h (AUC0–24) of serum DMAU/DMA levels generated by the 10 blood sampling times over 24 h for each dose of DMAU and computed using the trapezoid method. Other PK parameters assessed included Cavg (average concentration over 24 h), Cmax (maximum concentration over 24 h), Cmin (minimum concentration over 24 h) and Tmax (time to reach Cmax). The elimination half-life, T1/2, was calculated assuming exponential decay when there were at least 3 measurable concentrations after Cmax.
Since a dose effect is not anticipated for zero dose, the zero dose was removed from the analysis. Also, since we anticipated a marked effect of food, we planned separate analyses under the fed and fasting conditions a priori, assuming this was verified. Mixed models incorporated repeated measurements within subjects using a compound symmetrical covariance structure were constructed to examine the effect of dose (0, 200 and 400 mg), formulation (powder, SEDDS, castor oil) and the interaction on AUC, Cavg, Cmax, Tmax and T1/2 for serum DMA, serum DMAU and the ratio of DMA to DMAU. Post-hoc Bonferroni adjusted testing was performed only when a significant main effect was detected. All analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary NC), with two-tailed p<0.05 construing statistical significance. Data are presented as mean ± SEM.
The effect of oral DMAU treatment on serum T, DHT, estradiol (E2), LH, FSH and calculated free T was analyzed by mixed model analogously for Cavg and Cmin. Models here were constructed separately using 3 levels of formulation (powder, SEDDS, castor oil) as well as 2 levels of formulation (SEDDS and castor oil) since serum concentrations for hormones were measured less frequently when the powder formulation was administered than when SEDDS and castor oil formulations were dosed. Analyses under both models yielded congruent findings, and hence we present analyses that include all 3 formulations, unless otherwise indicated.
Results
Research Participants Demographics, Disposition and Safety
There were a total of 44 participants between the two study sites, 19 for powder in capsule, 12 for castor oil, and 13 for SEDDS. A total of 8 men discontinued during the dosing periods and were replaced to ensure that for all doses 12 participants were evaluated both fasting and fed; 7 discontinued dosing during the evaluation of powder in capsule and 1 from SEDDS dosing. In all cases, discontinuation was due to scheduling or personal reasons and not due to adverse effects. Demographics for the participants in each phase of the study are shown in Table 1. Across all three groups, men had an average age of 33 years and BMI of 25.
Table 1.
Characteristics of the Participants (± standard error of the mean).
| Total (44) |
Powder (19) |
Castor Oil (12) |
SEDDS (13) |
||
|---|---|---|---|---|---|
| Race | |||||
| American Indian or Alaska Native |
2(4.5%) | 2(10.5%) | 0 | 0 | |
| Asian | 1(2.3%) | 0 | 0 | 1(7.7%) | |
| Native Hawaiian or other Pacific Islander |
1(2.3%) | 1(5.3%) | 0 | 0 | |
| Black or African American |
5(11.4%) | 2(10.5%) | 3(25%) | 0 | |
| White | 33(75%) | 12(63.2%) | 9(75%) | 12(92.3%) | |
| Other | 2(4.5%) | 2(10.5%) | 0 | 0 | |
| BMI (kg/m2) | 25.4 ± 0.5 | 25.9 ± 0.71 | 25.2 ± 1.1 | 24.9 ± 1.1 | |
| Age (years) | 33.2 ± 1.4 | 31.7 ± 2.3 | 36.4 ± 2.6 | 32.4 ± 2.4 | |
| Screening T (ng/dl) | 546 ± 29 | 546 ± 43 | 481 ± 63 | 610 ± 45 | |
All three formulations were well tolerated and there were no serious adverse events. One participant had an AST > 2-fold the upper limit of normal thought to be related to binge alcohol intake; this elevation resolved without treatment. There were no significant changes in chemistry or lipid panels and hematocrit was not significantly different between baseline and the end of the study. EKGs and QTc intervals in all participants were not significantly different from baseline and there were no clinically significant changes in vital signs in any of the participants (data not shown).
Food effects
There were marked food effects on DMAU and DMA pharmacokinetics in all three formulations. When DMAU was administered with a high fat meal (50 % calories as fat), DMAU absorption and DMA serum concentrations were increased leading to a significantly higher AUC, Cavg, and C max for all formulations compared to administration fasting (Fig. 1, P<0.001 in all cases). Hence, the data were analyzed separately for the fasting and fed conditions.
Fig. 1.
Serum DMA (upper panel) and DMAU (lower panel) concentrations after oral administration of DMAU in three formulations as a single dose 0, 100, 200 or 400 mg after fasting overnight (left panels) or a high fat meal (50% fat)(right panels). Note y -axis is log scale. (Conversion DMA 1 ng/ml =3.29 nmol/l and DMAU 1 ng/ml=2.12 nmol/l)
Pharmacokinetics of DMA and DMAU when DMAU is given with food
DMA and DMAU showed higher AUC, Cavg, and C max (P ≤ 0.001 for each) and DMA/DMAU AUC ratio (P=0.044) at the 400 mg dose, compared with the 200 mg dose (Table 2). This dose effect was true for all three formulations. Comparing the three formulations, the powder in capsule resulted in higher DMAU AUC and Cmax than dosing in castor oil (P=0.007 and P=0.029 respectively). Despite these higher serum concentrations of DMAU, administration in powder did not increase serum DMA concentrations compared to the other two formulations. In fact, powder in capsule resulted in the lowest proportion of DMAU conversion to DMA (DMA/DMAU AUC ratio) compared with either of the other two formulations (P<0.02 for powder in capsule compared to both castor oil and SEDDS, Table 2).
Table 2.
Comparison of PK parameters after Single Oral Dose of DMAU in three formulations.
| Fed | 200 mg | 400 mg | ||||
|---|---|---|---|---|---|---|
| DMAU | SEDDS (n=10) |
Castor Oil (n=10) |
Powder in Capsule (n=10) |
SEDDS (n=9) |
Castor Oil (n=10) |
Powder in Capsule (n=10) |
| AUC (ng/mL/24h) |
2233±340 | 1706±247 | 3149±470 | 3534 ±289 | 2583±400 | 6153±1143 |
| Cavg (ng/ml) | 93.0±14.1 | 71.1±10.3 | 131.2±19.6 | 147.3±12.1 | 107.6 ±16.7 | 256.4±47.6 |
| Cmax (ng/mL) | 599± 90 | 485±59 | 854±194 | 889 ±50 | 665± 109 | 1410±283 |
| Tmax (h) | 3.8 ±0.6 | 4.6±0.7 | 5.8±0.6 | 3.3±0.6 | 6.4±2.0 | 6.2±1.1 |
| HalfLife(h) | 2.0±0.63 | 1.94±0.87 | 1.72±0.57 | 2.13 ±0.71 | 2.30±0.76 | 1.83±0.61 |
| DMA | ||||||
| (n=10) | (n=10) | (n=10) | (n=9) | (n=10) | (n=10) | |
| AUC (ng/mL/24h) |
96.0±16.3 | 103.1 ±16.3 |
66.7±12.0 | 222.5 ±28.1 |
187.6 ±35.3 | 163.9± 23.2 |
| Cavg (ng/ml) | 4.0±0.7 | 4.3±0.7 | 2.8±0.5 | 9.3±1.2 | 7.8±1.5 | 6.8±1.0 |
| Cmax (ng/mL) | 19.2±4.7 | 19.2±3.3 | 12.5±2.8 | 47.9±8.1 | 36.1±8.2 | 27.7±4.3 |
| Tmax (h) | 3.8±0.8 | 4.8±0.5 | 5.8±0.5 | 3.8±0.5 | 5.6±0.9 | 6.4±1.0 |
| HalfLife(h) | 2.84±1.0 | 2.68±0.85 | 2.44±0.92 | 3.23±1.07 | 3.10±1.03 | 3.31±1.10 |
| AUC Ratio | ||||||
| DMA/DMAU AUC |
0.050 ±0.009 |
0.063 ±0.007 |
0.028 ±0.006 | 0.112±0.051 | 0.075 ±0.008 |
0.031±0.004 |
(± standard error of the mean)
There was no dose effect on the time to maximum concentration, Tmax,, for either DMAU or DMA. However, Tmax for both serum concentrations of DMA and DMAU were significantly delayed with powder in capsule compared with SEDDS (P<0.05 for both DMAU and DMA). In contrast, there was an effect of dose on the elimination half-lives of DMA and DMAU (P<0.05 for both, Table 2); importantly, however, the elimination half-lives for both DMA and DMAU were not significantly affected by formulation.
Pharmacodynamic effects of DMAU given with food
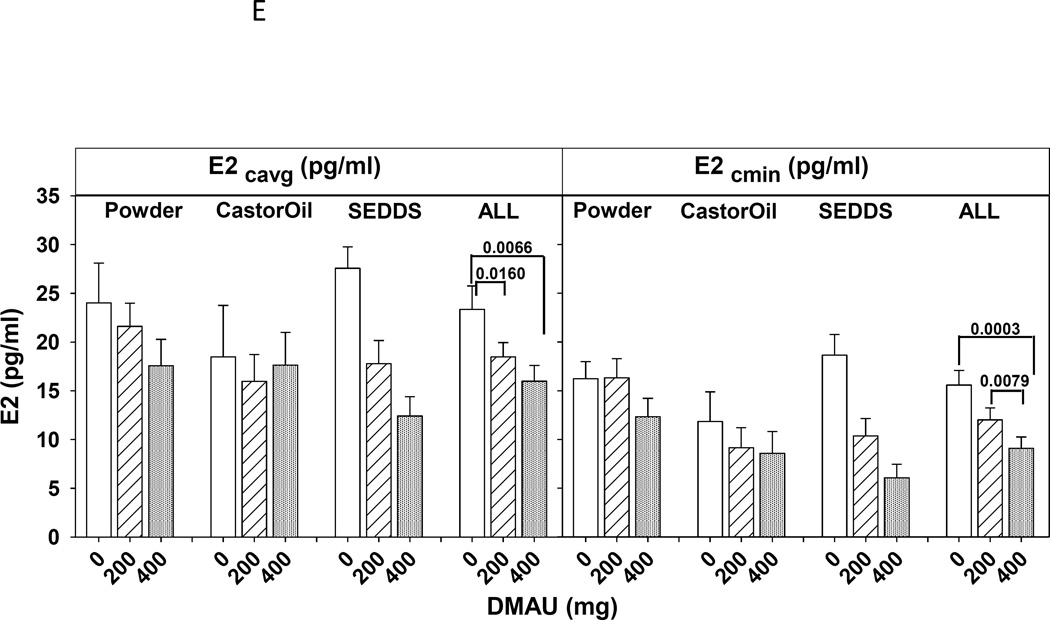
Significant dose effects were detected in Cavg and Cmin for LH, T, free T, DHT and E2 (P< 0.01 in all cases). Significant post-hoc Bonferrroni adjusted differences are illustrated in Figured 2A–E, and consistently show that 400mg was more suppressive than placebo, both in overall suppression (Cavg) and the minimum concentration achieved (Cmin) (Table 2). 400mg achieved a greater maximal suppression than 200mg for steroids T, DHT and E2: see Fig. 2C to E which shows the Cmin for each dose and formulation. There were not significant effects of the formulation on LH or any of the sex steroids examined. In contrast, there were no significant dose effects on FSH Cavg, but there was a significant dose by drug interaction for FSH Cmin (P<0.05, Fig. 2B). Post-hoc testing indicated that the 400 mg of SEDDS DMAU significantly suppressed FSH compared with SEDDS placebo (P=0.004), but no other significant differences were detected.
Fig. 2.
Serum average (Cavg) and minimum (Cmin) concentrations over 24h of LH (A), FSH (B), T(C), free T (D), estradiol (E), and DHT(F) after administration of a single dose 0 (placebo), 200, 400 mg of DMAU with a high fat meal. For simplicity the statistical differences between doses were marked by brackets only in the combined responses of gonadotropins or sex steroids of all three formulations (ALL).
Pharmacokinetics and pharmacodynamics of DMAU administered when fasting
When administered fasting, oral DMAU in the SEDDS formulation resulted in higher blood concentrations of DMAU and DMAU than the other two formulations (Fig. 1). When the 400 mg dose was compared, these differences were significant, with SEDDS DMAU resulting in higher Cavg for both DMA and DMAU than the castor oil and powder in capsule formulations (P<0.005 for each comparison for formulation by dose interaction, Bonferroni adjusted post hoc P<0.03 for each comparison).
Since only negligible concentrations of DMA were achieved when DMAU was administered in powder or castor oil, their pharmacodynamics effects were not further evaluated. We investigated whether DMAU given fasting in SEDDS decreased serum LH, FSH or T in a dose-dependent manner. When given fasting, DMAU in SEDDS resulted in a dose-dependent decrease in T Cavg (P=0.0005) and Cmin (P=0.015), and FSH Cmin (P<0.0001) without significantly impacting LH (data not shown).
Discussion
In this study we compared the pharmacokinetics of DMAU and DMA after oral administration of single, escalating doses of three formulations of DMAU. These studies are consistent with our earlier observation that administration of DMAU with food, even when formulated in oil, markedly increases absorption (Surampudi, et al., 2014), even when formulated in oil, and result in dose incremental increases in DMAU and DMA levels. Although administering DMAU in oil did not negate the significant enhancing effect of co-administration of food on DMAU absorption, oil-based formulations improved the conversion of DMAU to DMA compared to powder in capsule. Moreover, these newer formulations of DMAU dynamically suppress LH, T and the downstream metabolites of T, DHT and E2, when given with food. In contrast to the powder and castor oil formulations, under fasting conditions administration of DMAU in SEDDS provided sufficient DMA to suppress T and FSH, even after a single dose, despite resulting in markedly lower DMAU and DMA concentrations than when given with food.
This study builds upon our previous work, further demonstrating that a single oral dose of DMAU of 200 or 400 mg with food significantly suppressed serum LH, T, Free T and its metabolites E2 and DHT compared with placebo. The suppressive effect of all three formulations on sex steroids was dose dependent. The suppression of serum FSH was only evident when DMAU was given at the highest dose, 400 mg, with the SEDDS preparation, which also resulted in the highest DMA AUC (Fig 2B and Table 2). This is consistent with our prior observation that a single dose of DMAU as powder in capsule is a very effective suppressor of production of T, and suggests that oral DMAU may, when given repeatedly over time, be a potent suppressor of spermatogenesis (Surampudi, et al., 2014). The very rapid of suppression of LH and endogenous T in response to oral DMAU may be due to the dual action of DMA on both the androgen and progesterone receptors (Attardi, et al., 2006). Combinations of androgens and progestins are more effective suppressors of spermatogenesis than T alone in male contraceptive clinical trials (Liu, et al., 2008).
We were somewhat surprised that DMAU had greater oral bioavailability when given as a powder than the other two formulations when given in the fed state (Fig. 1 and Table 2), achieving greater serum DMAU Cavg than the castor oil formulation. Testosterone and its short chain esters undergo rapid first pass hepatic metabolism, limiting oral bioavailability (Tauber, et al., 1986), whereas testosterone undecanoate (TU) in castor oil has enhanced lipophilicity with absorption occurring via the intestinal lymphatics when TU is given with food (Shackleford, et al., 2003). Presumably, DMAU is also lymphatically absorbed; thus we expected that oil emulsions would enhance absorption via this route but this was not evident in this study. However, there was no effect of formulation on the AUC for DMA, the active metabolite of DMAU, due to the enhanced conversion of DMAU to DMA in vivo when given in either emulsified/oil formulation compared to powder (Table 2). How administration of DMAU in oil enhances de-esterification is not clear; however, given that only 5–10% of the DMAU is metabolized to DMA systemically, the improved DMA/DMAU ratio achieved when DMAU is given in oil is likely to be a significant advantage in multiple dose studies, allowing for markedly lower amounts of DMAU to be administered to achieve equivalent pharmacodynamic effects when DMAU is given in oil/SEDDS versus powder. This hypothesis remains to be tested in repeat dose studies as we did not observe an effect of formulation in this single dose study on the degree or extent of LH or endogenous steroid production.
Although the effects were modest, we did observe a significant effect of formulation on DMA, LH and endogenous steroid production when DMAU was administered fasting. In particular, the SEDDS formulation was superior to both powder in capsule and the castor oil formulation in achieving significant DMA and DMAU concentrations. SEDDS has also been shown to enhance the absorption of TU (Yin, et al., 2012). While the AUC for DMAU and DMA when DMAU is administered in SEDDS is still vastly lower than when given with food, by roughly an order of magnitude (Figure 1), these low levels of serum DMA achieved when DMAU is given in SEDDS may be important in longer term, real use studies. As a potential contraceptive, the levels of DMA achieved with DMAU-SEDDS occasionally dosed without concomitant food, may be sufficient for maintaining gonadotropin suppression, and perhaps inhibition of spermatogenesis, in long-term daily users.
There were very few adverse events that were ascribed to DMAU. Importantly, since the studies presented here include only single doses, and involve multiple blood draws, androgenic effects such as stimulation of erythropoiesis, suppression of sex hormone binding globulin, and potential reductions in high density lipoprotein cholesterol concentrations could not be adequately assessed in this study. Longer-term, repeat dose studies are required to further evaluate the safety of DMAU in men. To assess the effects of DMAU on spermatogenesis as well non-gonadal, androgen-sensitive organs including the prostate, bone, and muscle, longer term DMAU administration studies will be necessary. While DMAU has both androgenic and progestational activity in vitro and in preclinical rodent models, the long-term impact of LH and testosterone suppression on these hormonally sensitive tissues remains to be assessed and will be vital in developing DMAU as a male hormonal contraceptive.
In summary, single escalating doses of DMAU in three formulations: powder in capsule, castor oil, and SEDDs, up to 400 mg, were well tolerated in healthy male volunteers. When a single oral dose of DMAU was administered with a high fat meal, serum DMAU and DMA showed dose incremental increases sufficient to reversibly suppress LH and endogenous sex hormone production with all three formulations. Administration of DMAU in castor oil or SEDDS resulted in enhanced conversion of DMAU to DMA in vivo, which might be an advantage further development of these formulations over the powder in capsule. Furthermore, DMAU given in SEDDS was superior to the other two formulations when given in the fasting state, resulting in higher serum DMA concentrations sufficient for suppression of T and FSH. Further development of oral DMAU is ongoing with the goal of assessing its safety and efficacy in suppressing endogenous gonadotropins, sex steroids, and, in the long run, sperm production. These studies demonstrate that the formulation of DMAU in oil may have some advantages over powder in capsule; whether these observations hold true with multiple, repeat dosing remains to be evaluated. DMAU holds promise as a potential single agent, reversible, male hormonal contraceptive.
Acknowledgments
The Los Angeles Center was supported by the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD) Contraceptive Clinical Trial Network Contract HHSN27520130024I Task Orders HHSN 27500001, 27500002, 27500006 ; the Endocrinology, Metabolism and Nutrition training Grant (T32 DK007571), and the UCLA Clinical and Translational Science Institute (UL1TR000124) at Harbor-UCLA/LA BioMed. The Seattle Center was supported by NICHD Contraceptive Clinical Trial Network Contract HHSN275201100075U Task Orders HHSN 27500001, and 27500002, the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000423, the Center for Research in Reproduction and Contraception U54 HD 04245 (NICHD), and the National Institute of Diabetes and Digestive and Kidney Diseases training grant 5T32 DK007247-35. We thank Peter Christenson, PhD, formerly at LA Biomed for his advice in study design and data analysis plans. We thank our research coordinators Xiao-Dan Han, Elizabeth Ruiz, Kathryn Torrez-Duncan for their assistance with the study and the technical assistance of Feng Bai and Andrew Leung for DMA and DMAU assay development and validation. Finally we thank our research volunteers and the staff of the Endocrine and Metabolic Research Laboratory at Harbor-UCLA/LA Biomed and the University of Washington Center for Research in Reproduction and Contraception.
Disclosures
CW received research funding from Clarus Therapeutics, Lipocine and Besins Healthcare. She is a temporary consultant to TesoRx and Lipocine. JKA has received research funding from Clarus Therapeutics. RSS received research funding from from Clarus Therapeutics, Lipocine, Antares, and Aeterna Zentaris, Inc. He has served as a temporary consultant for Clarus Therapeutics, Novartis, TesoRex, Antares and Aeterna Zentaris, Inc.
Footnotes
Clinical Trial Number: NCT01382069
Author Contributions:
STP, RSS, JKA, DB, AC, WJB and CW designed the research study, analyzed the data, and wrote the paper. RA, JHC, AL, LH conducted the research study and analyzed the samples and reviewed and revised the paper.
References
- Attardi BJ, Engbring JA, Gropp D, Hild SA. Development of dimethandrolone 17beta-undecanoate (dmau) as an oral male hormonal contraceptive: Induction of infertility and recovery of fertility in adult male rabbits. Journal of Andrology. 2011;32:530–540. doi: 10.2164/jandrol.110.011817. [DOI] [PubMed] [Google Scholar]
- Attardi BJ, Hild SA, Reel JR. Dimethandrolone undecanoate: A new potent orally active androgen with progestational activity. Endocrinology. 2006;147:3016–3026. doi: 10.1210/en.2005-1524. [DOI] [PubMed] [Google Scholar]
- Attardi BJ, Marck BT, Matsumoto AM, Koduri S, Hild SA. Long-term effects of dimethandrolone 17beta-undecanoate and 11beta-methyl-19-nortestosterone 17beta-dodecylcarbonate on body composition, bone mineral density, serum gonadotropins, and androgenic/anabolic activity in castrated male rats. Journal of Andrology. 2011;32:183–192. doi: 10.2164/jandrol.110.010371. [DOI] [PubMed] [Google Scholar]
- Cook CE, Kepler JA. 7alpha,11beta-dimethyl-19-nortestosterone: A potent and selective androgen response modulator with prostate-sparing properties. Bioorganic and Medicinal Chemistry Letters. 2005;15:1213–1216. doi: 10.1016/j.bmcl.2004.11.076. [DOI] [PubMed] [Google Scholar]
- Glasier A. Acceptability of contraception for men: A review. Contraception. 2010;82:453–456. doi: 10.1016/j.contraception.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Gu Y, Liang X, Wu W, Liu M, Song S, Cheng L, Bo L, Xiong C, Wang X, Liu X, Peng L, Yao K. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in chinese men. Journal of Clinical Endocrinology and Metabolism. 2009;94:1910–1915. doi: 10.1210/jc.2008-1846. [DOI] [PubMed] [Google Scholar]
- Hild SA, Attardi BJ, Koduri S, Till BA, Reel JR. Effects of synthetic androgens on liver function using the rabbit as a model. Journal of Andrology. 2010;31:472–481. doi: 10.2164/jandrol.109.009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PY, Swerdloff RS, Anawalt BD, Anderson RA, Bremner WJ, Elliesen J, Gu YQ, Kersemaekers WM, McLachlan RI, Meriggiola MC, Nieschlag E, Sitruk-Ware R, Vogelsong K, Wang XH, Wu FC, Zitzmann M, Handelsman DJ, Wang C. Determinants of the rate and extent of spermatogenic suppression during hormonal male contraception: An integrated analysis. Journal of Clinical Endocrinology and Metabolism. 2008;93:1774–1783. doi: 10.1210/jc.2007-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PY, Swerdloff RS, Anawalt BD, Anderson RA, Bremner WJ, Elliesen J, Gu YQ, Kersemaekers WM, McLachlan RI, Meriggiola MC, Nieschlag E, Sitruk-Ware R, Vogelsong K, Wang XH, Wu FC, Zitzmann M, Handelsman DJ, Wang C. Determinants of the rate and extent of spermatogenic suppression during hormonal male contraception: An integrated analysis. J Clin Endocrinol Metab. 2008;93:1774–1783. doi: 10.1210/jc.2007-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PY, Swerdloff RS, Christenson PD, Handelsman DJ, Wang C Hormonal Male Contraception Summit, G. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: An integrated analysis. Lancet. 2006;367:1412–1420. doi: 10.1016/S0140-6736(06)68614-5. [DOI] [PubMed] [Google Scholar]
- Meriggiola MC, Bremner WJ. Progestin-androgen combination regimens for male contraception. Journal of Andrology. 1997;18:240–244. [PubMed] [Google Scholar]
- Nieschlag E. Clinical trials in male hormonal contraception. Contraception. 2010;82:457–470. doi: 10.1016/j.contraception.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Page ST, Amory JK, Bremner WJ. Advances in male contraception. Endocrine Reviews. 2008;29:465–493. doi: 10.1210/er.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowska K, Wang C, Swerdloff RS, Liu PY. Male hormonal contraception: Hope and promise. Lancet Diabetes Endocrinol. 2016 doi: 10.1016/S2213-8587(16)00034-6. Epub February 22, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman MS, Carlson NE, Xu M, Wang C, Swerdloff R, Lee P, Goh VH, Ridgway EC, Wierman ME. Reexamination of testosterone, dihydrotestosterone, estradiol and estrone levels across the menstrual cycle and in postmenopausal women measured by liquid chromatography-tandem mass spectrometry. Steroids. 2011;76:177–182. doi: 10.1016/j.steroids.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleford DM, Faassen WA, Houwing N, Lass H, Edwards GA, Porter CJ, Charman WN. Contribution of lymphatically transported testosterone undecanoate to the systemic exposure of testosterone after oral administration of two andriol formulations in conscious lymph duct-cannulated dogs. Journal of Pharmacology and Experimental Therapeutics. 2003;306:925–933. doi: 10.1124/jpet.103.052522. [DOI] [PubMed] [Google Scholar]
- Shiraishi S, Lee PW, Leung A, Goh VH, Swerdloff RS, Wang C. Simultaneous measurement of serum testosterone and dihydrotestosterone by liquid chromatography-tandem mass spectrometry. Clinical Chemistry. 2008;54:1855–1863. doi: 10.1373/clinchem.2008.103846. [DOI] [PubMed] [Google Scholar]
- Surampudi P, Page ST, Swerdloff RS, Nya-Ngatchou JJ, Liu PY, Amory JK, Leung A, Hull L, Blithe DL, Woo J, Bremner WJ, Wang C. Single, escalating dose pharmacokinetics, safety and food effects of a new oral androgen dimethandrolone undecanoate in man: A prototype oral male hormonal contraceptive. Andrology. 2014;2:579–587. doi: 10.1111/j.2047-2927.2014.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdloff RS, Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Longstreth J, Berman N. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. Journal of Clinical Endocrinology and Metabolism. 2000;85:4500–4510. doi: 10.1210/jcem.85.12.7045. [DOI] [PubMed] [Google Scholar]
- Tauber U, Schroder K, Dusterberg B, Matthes H. Absolute bioavailability of testosterone after oral administration of testosterone-undecanoate and testosterone. European Journal of Drug Metabolism and Pharmacokinetics. 1986;11:145. doi: 10.1007/BF03189840. [DOI] [PubMed] [Google Scholar]
- Turner L, Conway AJ, Jimenez M, Liu PY, Forbes E, McLachlan RI, Handelsman DJ. Contraceptive efficacy of a depot progestin and androgen combination in men. Journal of Clinical Endocrinology and Metabolism. 2003;88:4659–4667. doi: 10.1210/jc.2003-030107. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. Journal of Clinical Endocrinology and Metabolism. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- Wang C, Festin MP, Swerdloff RS. Male hormonal contraception: Where are we now? Curr Obstet Gynecol Rep. 2016;5:38–47. doi: 10.1007/s13669-016-0140-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Swerdloff RS. Hormonal approaches to male contraception. Curr Opin Urol. 2010;20:520–524. doi: 10.1097/MOU.0b013e32833f1b4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Task Force on Methods for the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia in normal men. World health organization task force on methods for the regulation of male fertility. Lancet. 1990;336:955–959. [PubMed] [Google Scholar]
- World Health Organization Task Force on the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertility and Sterility. 1996;65:821–829. [PubMed] [Google Scholar]
- Yin AY, Htun M, Swerdloff RS, Diaz-Arjonilla M, Dudley RE, Faulkner S, Bross R, Leung A, Baravarian S, Hull L, Longstreth JA, Kulback S, Flippo G, Wang C. Reexamination of pharmacokinetics of oral testosterone undecanoate in hypogonadal men with a new self-emulsifying formulation. J Androl. 2012;33:190–201. doi: 10.2164/jandrol.111.013169. [DOI] [PMC free article] [PubMed] [Google Scholar]