Abstract
Background and objective
Idiopathic pulmonary fibrosis (IPF) is a progressive disease with poor prognosis and variable clinical course. Though MMP-7 is emerging as an important IPF biomarker, reproducibility across studies is unclear. We aimed to determine whether a previously reported prognostic threshold for MMP-7 was predictive of mortality in an independent cohort of IPF patients.
Methods
MMP7 concentrations obtained from heparinised plasma samples were determined by ELISA in 97 patients with IPF and 41 healthy controls. The association of the previously published heparin plasma MMP-7 threshold of 12.1ng/ml with all-cause-mortality or transplant-free-survival (TFS) was determined, both as an independent biomarker or as part of the modified personal clinical and molecular mortality index (m-PCMI).
Results
MMP-7 plasma concentrations were significantly higher in IPF patients compared to healthy controls (14.40±6.55 vs. 6.03±2.51ng/ml, p<0.001). The plasma MMP-7 threshold of 12.1 ng/ml was significantly associated with both all-cause-mortality and TFS (unadjusted Cox proportional hazard ratio (HR) =25.85 and 15.49, 95% confidence interval (CI): 10.91-61.23 and 5.41-44.34, respectively, p<0.001). MMP-7 concentrations, split by 12.1 ng/ml, were significantly (p<0.05) predictive of mortality and TFS after adjusting for age, gender, smoking and baseline pulmonary function parameters, in a multivariate Cox proportional hazards model. MMP-7 concentrations were negatively correlated with DLCO (r=−0.21, p=0.02), and positively with a mortality risk scoring system (GAP) that combines age, gender, FVC and DLCO (r=0.32, p=0.001).
Conclusion
Our study confirms that MMP-7 concentrations could be used to accurately predict outcomes across cohorts and centers, when similar collection protocols are applied.
Keywords: MMP7, prognostic biomarker, IPF, heparin plasma
Introduction
Idiopathic Pulmonary Fibrosis (IPF) is a chronic lung disease that is often fatal within 3-5 years after initial diagnosis 1. Despite extensive research efforts, its etiology in humans still remains largely unknown, and no curative drug therapies are available 2-4. The clinical course and rate of disease progression are heterogeneous and relatively unpredictable 5-7. Previous studies aimed to identify clinical and physiologic parameters that predict mortality and defined multidimensional indices to estimate the risk of death in patients with IPF 3, 8-10. However, none of these clinical markers predicts disease behavior accurately enough to guide patient management decisions such as priority for lung transplantation, timely referral to palliative care or other therapeutic decisions 11.
Although fibrosis in IPF is limited to the lung, evidence suggests that proteins and genes in peripheral blood differentiate IPF patients from healthy controls and may predict mortality 12. Of these, matrix metalloproteinase (MMP)-7 has emerged as an important biomarker, reproducibly indicative of disease presence, severity and prognosis 13-15. Plasma MMP-7 plasma concentrations in IPF are linked with two single-nucleotide polymorphisms in the gene's promoter region, suggesting potential genetic basis for MMP-7 upregulation 16. We have demonstrated that plasma MMP-7 concentration predicted mortality in two cohorts of patients with IPF (n=241) and developed a personal clinical and molecular mortality index, (PCMI) that accurately distinguished high from low risk mortality groups 13. More recently, plasma MMP-7 concentration was reported as an independent predictor of survival in a prediction model containing clinical indices and MUC5B genotype, in two cohorts with 586 IPF patients 17. These findings were replicated by Song et al in a moderately sized cohort of patients with IPF (n=118) 15. Finally, plasma MMP-7 has been proposed as a univariate predictor of early interstitial lung disease abnormalities in first degree relatives of patients with familial interstitial pneumonia 18. Despite these impressive data, the implementation of MMP-7 in clinical decision making has significantly lagged 19. The most important reason for this is lack of standardization. Sample collection protocols and matrices differed across studies, as did individual study derived prognostic cut-off points, thus justifiably preventing generalization of results.
In this study we aimed to determine whether a prognostic threshold identified in a previous study would be significantly predictive of outcome in a completely independent cohort. To this end, we decided not to derive MMP-7 cut-off thresholds based on our own cohort. Instead, given similarities in sample management procedures and collection matrices, we chose to test whether the plasma MMP-7 threshold identified by Song et al 15, was an accurate prognostic marker in our cohort.
Methods
Patients and Plasma Samples
Source data included samples and clinical information from 97 patients with IPF from the Yale Center of Excellence for Interstitial Lung Disease and 41 age and sex matched healthy donors recruited from the local New Haven Community. IPF diagnosis was based on guidelines of the American Thoracic Society and European Respiratory Society 2. All patients and healthy donors provided written informed consent and a protocol incorporating biomarker-studies was approved by the Institutional Review Board (IRB), Yale School of Medicine (HIC#0706002766).
MMP-7 Quantification
This was a prospective observational cohort study. Yale patient samples were obtained at routine visits from treatment naïve subjects that were not currently enrolled in clinical studies. Blood was collected in heparin tubes using a routine procedure and was immediately (within 10 minutes after blood collection) centrifuged at 4°C, 1200×g, for 10 min). Plasma was aliquoted and frozen at −80°C until analysis by enzyme-linked immunosorbent assay (ELISA) for MMP-7. The ELISA kit chosen (R&D Systems, Inc.) has been widely used 14, 20, 21 and has a reported sensitivity of: 0.094 ng/ml, an assay range:0.2-10 ng/ml and minimal cross-reactivity (0.5%) (R&D Systems, Inc.). The resultant trend line produced an r2 of at least 0.99 and coefficient variability (CV) for intra-assay variations was 4.79% indicating significant reproducibility among duplicates. Heparin plasma samples were diluted 1:5. All assays were performed in duplicate, and the mean values were reported.
Statistical Analysis
Data were statistically analyzed using Med. Calc. version 14. Comparisons of plasma MMP-7 concentrations in IPF patients and healthy donors were performed using Mann Whitney U test or unpaired two-tailed t-test. For baseline characteristics comparisons between IPF patients and controls or between IPF subgroups (high and low plasma MMP-7 concentrations) we used the Fisher's exact test. For age, pulmonary function tests, GAP and m-PCMI indices we used unpaired, two tailed, Student t-test. Spearman correlations were used to assess associations between MMP-7 and functional parameters. In analyses of all-cause-mortality and transplant-free-survival (TFS), patients were followed from enrollment to death or censored at either the end of follow-up or time of lung transplantation or ≥10% drop in FVC%predicted. For mortality patients were censored at transplant and deaths were considered events. For TFS, transplants and deaths were both counted as events. Derivation of a modified version of personal clinical and molecular mortality (m-PCMI) index, a risk prediction rule published by us 13, was based on the following formula: PCMI: 114 * I (Male) + 2 * (100% - FVC%Predicted) + 3* (100% – DLCO%Predicted) + 111 * I (MMP-7≥ 12.1 ng/ml) where the indicator function I( ) is unity (one) if and only if the condition inside the parentheses is true. Associations of the previously published plasma MMP-7 threshold of 12.1ng/ml 15 with all-cause-mortality, TFS or disease progression as measured by drop of ≥10% in FVC%predicted, were evaluated using Kaplan Meier approach. The multivariate Cox regression Hazard ratio models were used to investigate the associations between MMP-7 cut-off point of 12.1 ng/ml and mortality or TFS or drop of ≥10% in FVC%predicted. The covariates considered included age, gender, smoking, forced vital capacity (FVC %predicted) and diffusing lung capacity of carbon monoxide (DLco %predicted) 9.
RESULTS
Patients and healthy controls demographic data
Baseline characteristics of patients and healthy controls are in Table 1. Our study population included 97 patients with IPF and 41 healthy controls matched for age and gender. Patients with IPF were predominantly white (87.6%), male (78.4%) and former smokers (65.9%). Baseline mean FVC was 75.9 %predicted and DLCO 51 %predicted. Diagnosis was based on surgical lung biopsy in 23.7% of our patients. The median follow-up time was 563 days and 7% of our patients received a lung transplant. The rate of death in the first and second year of follow up was 11% and 14%, respectively. As expected, healthy controls had a significantly higher percentage of never smokers, and lower percentage of ex-smokers compared to patients with IPF (85.4 vs.27.8% and 14.6 vs 65.9%, p<0.001)
Table 1. Baseline characteristics of the study population.
P-values were calculated using the Fisher's exact test except for age, pulmonary function tests and GAP index where an unpaired, two tailed, Student t-test was used. DLCO%: carbon monoxide diffusing capacity, percent predicted, FVC%: forced vital capacity, percent predicted
| IPF | Controls | p-value | |
|---|---|---|---|
| n | 97 | 41 | |
| Age (yrs) | |||
| Mean ± SD | 70.0 ± 8.0 | 68.0±14.3 | 0.42 |
| Gender, n (%) | |||
| Males | 76 (78.4°%) | 28 (68.3%) | 0.28 |
| Females | 21 (21.6%) | 13 (31.7%) | |
| Ethnic origin, n (%) | |||
| White | 85 (87.6%) | 37 (90.2) | 0.78 |
| Others | 12 (12.4%) | 4 (9.8%) | |
| Smoking Status, n (%) | |||
| Current Smokers | 6 (6.2%) | 0 (0%) | 0.18 |
| Ex-Smokers | 64 (65.9%) | 6 (14.6%) | <0.001 |
| Never Smokers | 27 (27.8%) | 35 (85.4%) | <0.001 |
| Surgical Lung Biopsy, n (%) | 23 (23.7%) | ||
| FVC (% predicted) | 75.9 ± 19.3 | ||
| DLCO (% predicted) | 51.0 ± 17.2 | ||
| Supplement oxygen, n (%) | 49 (50.5%) | ||
| GAP score (mean±SD) | 4.08 ± 1.50 | ||
| Stage I, n (%) | 29 (29.9%) | ||
| Stage II, n (%) | 51 (52.6%) | ||
| Stage III, n (%) | 17 (17.5%) | ||
Plasma MMP-7 concentrations are higher in patients with IPF compared to Healthy Controls
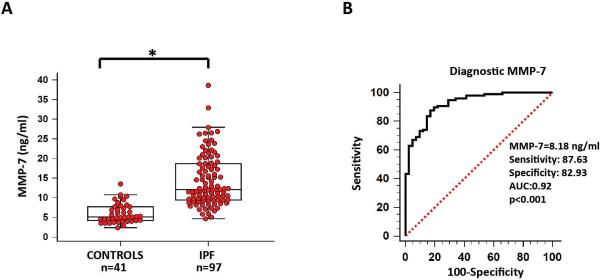
Mean plasma MMP-7 concentrations were significantly elevated in patients with IPF compared to controls (14.40 ± 6.55 vs. 6.03 ± 2.51, p<0.001) (Table 2 and Figure 1A). Receiver operating characteristic (ROC) curve analysis (Figure 1B) revealed that the cut-off value of 8.18 ng/ml could distinguish patients with IPF from controls with a sensitivity of 87.63% and specificity of 82.93% (AUC: 0.92, p<0.001).
Table 2. Plasma MMP-7 in patients with IPF and healthy controls.
P-values were calculated using the unpaired, two-tailed Student t-test or Mann Whitney U test.
| CONTROLS | IPF | p-value | |
|---|---|---|---|
| n | 41 | 97 | |
| Mean ± SD (ng/ml) | 6.03 ± 2.51 | 14.40 ± 6.55 | <0.001 |
| Median (range) (ng/ml) | 5.12 | 12.07 | <0.001 |
| 95% CI for the median | 4.63 – 6.45 | 11.32 – 14.58 |
* Lower limit of detection (0.16 ng/mL).
Figure 1. Increased plasma MMP-7 concentrations differentiate patients with IPF from controls.
A) Whisker Box-plots showing that plasma MMP-7 concentrations were significantly higher in patients with IPF compared to control subjects (12.07 vs 5.12 ng/ml, 95% CI: 11.32 – 14.58 vs. 4.63 – 6.45, *p<0.001), B) Diagnostic accuracy of plasma MMP-7 for IPF is presented by receiver operating characteristic (ROC) curve analysis. ROC curve analysis identified the optimal cut-off concentrations of plasma MMP-7 (8.18 ng/ml) that could differentiate patients with IPF from controls with the highest sensitivity (87.63) and specificity (82.93). The area under the curve (AUC) between patients with IPF and control subjects was 0.92.
Plasma MMP-7 threshold of 12.1 ng/ml and m-PCMI predict mortality
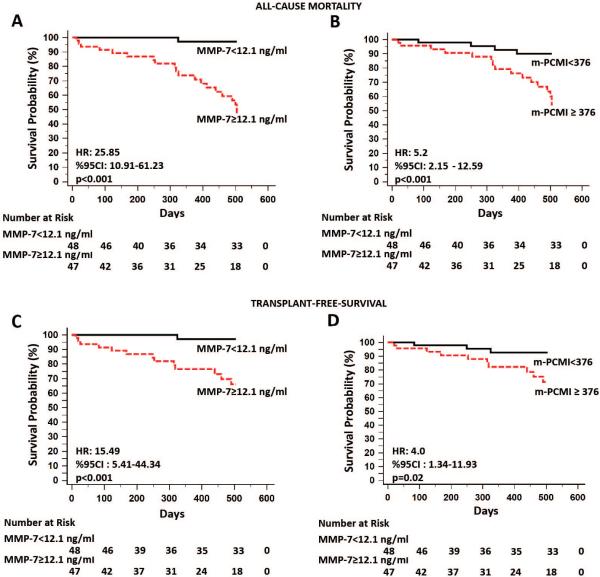
Previously published 15 plasma MMP-7 threshold of 12.1 ng/ml was also incorporated into a multidimensional prediction rule, denominated PCMI. Given that the cut-off value of plasma MMP-7 in the current cohort was different (12.1 vs. 4.3 ng/ml) than the one published by Richards et al 13 , a new version of PCMI was generated and called modified- (m)-PCMI. Plasma MMP-7 cut-off levels of 12.1 ng/ml and median m-PCMI index of 376 were used as dichotomous variables and significantly associated with both all-cause-mortality (unadjusted HR =25.85 and, 95%CI: 10.91 to 61.23, p<0.001 and HR:5.2, 95%CI: 2.15 to 12.59, respectively, p<0.001) and TFS (unadjusted HR=15.49, 95%CI: 5.41 to 44.34, p<0.001 and HR: 4.0, 95%CI:1.34 to 11.93, p=0.02, respectively) as shown in Kaplan-Meier curves in Figure 2. ROC curve analysis revealed that plasma MMP-7 threshold of 12.1ng/ml exhibited a sensitivity of 95.2%, 92.9% and specificity of 63.2%, 57.8% for predicting all-cause-mortality and TFS, respectively.
Figure 2. Increased plasma MMP-7 concentrations and personal clinical and molecular mortality index (PCMI) are highly predictive of all-cause-mortality and transplant-free-survival in patients with IPF.
(A, C) The cut-off threshold of 12.1 ng/ml of plasma MMP-7 was used as a dichotomous variable and clearly differentiated high from low risk mortality groups as assessed by significant associations with both all-cause-mortality and transplant-free-survival. The red curves indicate the Kaplan-Meier plot for all-cause-mortality or transplant-free survival by samples with MMP-7 concentrations in the highest MMP7 quantile (≥12.1 ng/ml). The black curves indicate the same plot for the lowest quantile (<12.1 ng/ml). Risk tables below indicate the number of objects at risk for each group at each time point. (B, D) The same cut-off point of 12.1 ng/ml was incorporated into a multidimensional prediction rule, denominated modified (m)-PCMI. m-PCMI concentrations split by the median (376) showed strong correlations with both all-cause-mortality and transplant-free-survival. The red curves indicate the Kaplan-Meier plot for all-cause-mortality or transplant-free survival by samples with m-PCMI concentrations in the highest m-PCMI quantile (≥ 376). The black curves indicate the same plot for the highest quantile (<376). Risk tables below indicate the number of objects at risk for each group at each time point.
Following adjustment for age, gender, smoking and functional parameters of disease severity MMP-7 threshold of 12.1 ng/ml still remained a strong predictor of all-cause-mortality and TFS (HR: 41.87, 95%CI: 4.30 to 407.92, p=0.0013 and HR: 48.21, 95%CI: 3.49 to 690.19, p=0.0039, respectively) (Supplementary Tables S1 and S2). The associations between DLco and all-cause-mortality as well as TFS were significant in the multivariate model (p=0.006 and p=0.03, respectively). With regard to GAP score, only when used as a univariate predictor, the association with all-cause-mortality and TFS was significant (p=0.01 and 0.02). IPF patients with higher plasma MMP-7 levels (≥12.1ng/ml) exhibited more severe disease as assessed by almost significant reductions in DLCO (44.19 ± 14.35 vs. 50.58 ± 18.65, p=0.06), as well as subsequent increases in GAP (median: 5 vs. 4, p=0.02) and m-PCMI (444.19 ± 75.20 vs. 285.44 ± 93.86, p<0.001) indices, compared to patients with lower plasma MMP-7 levels (<12.1ng/ml). All-cause-mortality was 41% (20 deaths) in the high MMP-7 group versus 2% (1 death) in the low MMP-7 group (Table 3).
Table 3. Baseline clinical and functional characteristics between High and Low plasma MMP-7 patients.
P-values were calculated using the Fisher's exact test except for age, pulmonary function tests, GAP and m-PCMI index where an unpaired, two tailed, Student t-test was used. DLCO%: carbon monoxide diffusing capacity, percent predicted, FVC%: forced vital capacity, percent predicted, m-PCMI: modified personal clinical and molecular mortality index, NA: Not Applicable.
| Variable | High MMP-7 (≥12.1 ng/ml) | Low MMP-7 (<12.1 ng/ml) | p-value |
|---|---|---|---|
| n | 49 | 48 | |
| Age (yrs) | |||
| Mean ± SD | 69.43 ± 6.97 | 70.58 ± 8.89 | 0.48 |
| Gender, n (%) | 0.04 | ||
| Males | 43 (91.4%) | 34 (70.8%) | |
| Females | 6 (8.6%) | 14 (29.2%) | |
| Smoking status, n (%) | 0.25 | ||
| Current Smokers | 5 (10.2%) | 1 (2.1%) | |
| Ex-Smokers | 31 (63.2%) | 33 (68.7%) | |
| Never Smokers | 13 (26.6%) | 14 (29.2%) | |
| Supplemental Oxygen, n (%) | 19 (46.3%) | 26 (55.3%) | 0.11 |
| Survival (days) | |||
| Median (IQ range) | 412 (192 – 504) | NA | NA |
| DLCO (%predicted)* | 44.19 ± 14.35 | 50.58 ± 18.65 | 0.06 |
| FVC (%predicted)* | 67.55 ± 16.35 | 72.83 ± 16.35 | 0.30 |
| GAP* (Median-IQ range) | 5 (4-5) | 4 (3-5) | 0.02 |
| m-PCMI* | 444.19 ± 75.20 | 285.44 ± 93.86 | <0.001 |
2 patients were excluded from analysis due to missing functional parameters.
Plasma MMP-7 concentration correlates with functional and clinical predictors of mortality
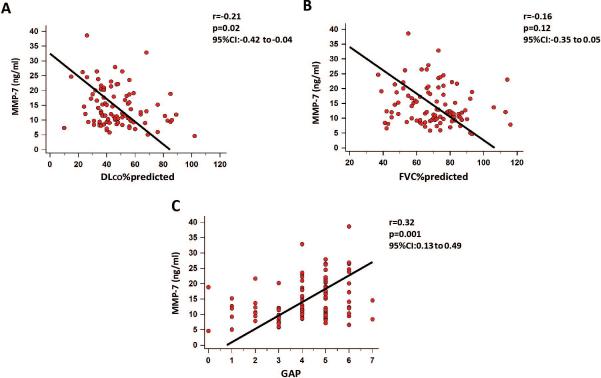
Plasma MMP-7 concentration, used as a continuous variable, correlated with disease severity across multiple measures of patient functional status. Baseline plasma MMP-7 concentration was negatively correlated with DLCO (r=−0.21, 95%CI: −0.42 to −0.04, p=0.02) (Figure 3A) but not with FVC %predicted (r=−0.16, 95%CI: −0.35 to 0.05, p=0.12) (Figure 3B). Plasma MMP-7 concentrations also showed a strong positive association with the GAP score (r=0.32, 95%CI: 0.13 to 0.49, p=0.001) (Figure 3C). Baseline plasma MMP-7 concentration (split by the cut-off of 12.1 ng/ml) did not correlate with disease progression as measured by absolute or relative% drop in FVC both at univariate (unadjusted HR:1.8, 95%CI: 0.8 to 4.2, p=0.2 and HR:1.3, %95CI: 0.6 to 2.6, p=0.5) and multivariate cox regression hazard ratio analyses (HR: 2.2, %95CI:0.8 to 5.5, p=0.1 and HR: 1.2, %95CI:0.6 to 2.5, p=0.6) adjusted for age, gender, smoking and DLCO, respectively.
Figure 3. Plasma MMP-7 concentrations correlate with clinical and functional indicators of disease severity in patients with IPF.
Spearman correlations showed weak, yet significant correlation between MMP-7 and measures of patient functional status including (A) DLCO %predicted. (B) No correlations with FVC %predicted were noted. MMP-7 was also strongly correlated with (C) GAP score.
DISCUSSION
In this study we validated the prognostic accuracy of a previously reported threshold (12.1ng/ml) 15 of plasma MMP-7 concentrations in a moderately sized cohort of patients with IPF (n=97). We first showed that plasma MMP-7 concentrations are significantly elevated in patients with IPF compared to controls. We then used the 12.1 ng/ml-threshold of plasma MMP-7 as a dichotomous variable and confirmed previously published associations13, 14 with all-cause-mortality and TFS. In addition, we incorporated the MMP-7 cut-off value of 12.1 ng/ml into a multidimensional risk prediction rule and generated a modified version of PCMI that displayed sufficient prognostic power. Finally, plasma MMP-7 concentrations exhibited strong correlations with functional and clinical predictors of mortality.
IPF presents a major challenge in clinical research due to its unpredictable clinical course and the lack of easily reproducible surrogate markers for patient relevant outcomes 22. In recent years, there has been significant progress in identifying proteins 13, 23-25, gene variants 17, 26, and changes in blood gene expression 12 that distinguish IPF patients with distinct clinical outcomes. Despite these efforts, currently available molecular biomarkers present with appreciable limitations that hamper their widespread clinical applicability 19, 27.
MMP-7, a WNT/β-catenin target gene, is a matrix metalloprotease over-expressed in hyperplastic epithelial cells in IPF 28, 29. MMP-7 deficient mice are relatively protected from bleomycin-induced lung fibrosis 28, 30. Overexpression of MMP-7 by epithelial cells may be pro-fibrotic through several mechanisms including cleavage and activation of numerous pro-fibrotic substrates, such as osteopontin 31 and tumor necrosis factor-alpha 30, 32. An association of elevated concentrations of MMP7 with increased mortality in patients with IPF and other chronic lung diseases has been found repeatedly 33-36. However, the potential implementation of MMP-7 as a prognostic biomarker in clinical practice has been delayed by the lack of reproducible and uniform cut-off thresholds across multiple studies. Different collection matrices 37, 38 may account for these highly variable and conflicting thresholds. The studies by Richards and Rosas et al 13, 14 and Peljito et al 17, used EDTA-containing tubes whereas Song et al 15 used heparin plasma samples. None of the studies tried to validate previously derived thresholds. Thus, we decided to test the ability of a threshold derived in a Korean cohort of patients with IPF to segregate patients with significantly different outcomes in our cohort. The choice of the MMP-7 threshold was based on similar collection matrices (heparin plasma), collection procedures (single center), and methods of analysis (ELISA) as well as study design. Our cohort was slightly older (62.8 vs 70.0) and had more advanced disease as assessed by lower baseline mean DLCO concentrations (65 vs. 51) than the Ulsan University cohort. Consistent with that, the mean plasma MMP-7 concentrations of the Ulsan University cohort 15 were slightly lower than ours (13.7 vs 14.4 ng/ml). Impressively the threshold derived at Ulsan University was significantly associated both with all-cause-mortality and TFS (unadjusted HR: 25.85 and 15.49, respectively) and exhibited higher sensitivity and specificity in predicting all-cause-mortality compared to those reported by Song et al (95% vs. 71% and 63.2% vs.54%, respectively). These findings confirmed the original observations that IPF patients can be segregated based on their blood MMP-7 concentrations to those with low mortality and those with high mortality. Importantly our results established, for the first time in IPF, that a threshold derived in one center, can accurately predict survival in a completely separate cohort.
In a previous study we derived a multidimensional risk prediction rule (PCMI) that combined demographic, functional and molecular components and was highly predictive of mortality in two independent cohorts of patients with IPF 13. m-PCMI, generated by inserting the University of Ulsan derived MMP-7 cut-off threshold of 12.1 ng/ml to the original PCMI equation, was significantly predictive of mortality. These results replicate and validate the predictive value of the PCMI as a combined clinical and molecular predictor of mortality in IPF.
Our study had several limitations. First, this was a moderately sized and relatively underpowered study. Therefore, the high HR reported for this small population is likely to overestimate the risk associated with elevated MMP-7 concentrations in the general IPF population. Second, the follow-up period was relatively short (approximately 1.5 years) compared to those reported in other similar studies 13; however, was sufficient to reproduce the prognostic significance of heparin plasma MMP-7 in IPF patients. Last, while we observed significantly increased plasma MMP-7 concentrations in IPF patients, our study was not designed to test whether plasma MMP-7 concentrations could be used to reliably differentiate patients with IPF from controls or other diseases. Several other studies 14, 33, 36, 39, 40 have suggested that MMP-7, most commonly in combination with other molecular biomarkers may distinguish IPF from other chronic lung diseases but they remain limited in size and need further validation.
In summary, to our knowledge this the first study that reproduces prognostic accuracy of a previously published threshold of an IPF peripheral blood biomarker in an entirely different cohort of patients. Our results demonstrate that, when similar sample management procedures and collection matrices (heparin plasma) are applied, baseline plasma MMP-7 concentrations exhibit remarkable and highly reproducible prognostic value across different cohorts. To better determine the specific role of MMP-7 in the management of patients with IPF, additional studies that include assessment of changes in plasma MMP-7 concentration over time and disease progression, as well as response to therapy will be required.
Supplementary Material
Summary at a Glance.
The generalizability of the prognostic value of MMP-7 in IPF has been questioned. We reproduced prognostic accuracy of a previously published threshold of MMP-7 in an independent cohort of patients with IPF based on similarities in collection matrices. MMP-7 predictive value is robust when heparin plasma is used as collection matrix.
Acknowledgements
The authors gratefully acknowledge the IPF patients who consented to participation in this study and the numerous clinical researchers involved in the collection of samples and clinical data. This work was funded by the National Institutes of Health (UO1 HL108642 and R01 HL127349 to N.K. and R01 HL109033 E.L.H).
Abbreviations
- BALF
Broncho-alveolar Lavage Fluid
- DLCO
Diffusing Lung capacity of Carbon monoxide
- FVC
Forced Vital Capacity
- IPF
Idiopathic Pulmonary Fibrosis
- MMP-7
Matrix Mettaloproteinase-7
- PCMI
Personal Clinical and Molecular mortality index
- PBMCs
Peripheral Blood Mononuclear Cells
- TFS
Transplant-free-survival
Footnotes
Disclosure statement
NK has been a paid consultant for Sanofi, Biogen, MMI, Pliant, Boehringer Ingelheim and was a grant recipient from Biogen and Gilead; He has also scientific collaborations with Miragen and Actelion. ELH has been a paid consultant for Boehringer Ingelheim and Pfizer and has received grants from Karos, Kolltan, Promedior, Biogen, Sanofi and Bristol Myers; NK and JDHM are inventors on patent applications for marker panels in IPF.
References
- 1.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, Brozek JL, Collard HR, Cunningham W, Homma S, Johkoh T, Martinez FJ, Myers J, Protzko SL, Richeldi L, Rind D, Selman M, Theodore A, Wells AU, Hoogsteden H, Schunemann HJ. American Thoracic S, European Respiratory s, Japanese Respiratory S, Latin American Thoracic A. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. American journal of respiratory and critical care medicine. 2015;192:e3–19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 2.Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, Loyd JE, Cosgrove GP, Lynch D, Groshong S, Collard HR, Wolters PJ, Bradford WZ, Kossen K, Seiwert SD, du Bois RM, Garcia CK, Devine MS, Gudmundsson G, Isaksson HJ, Kaminski N, Zhang Y, Gibson KF, Lancaster LH, Cogan JD, Mason WR, Maher TM, Molyneaux PL, Wells AU, Moffatt MF, Selman M, Pardo A, Kim DS, Crapo JD, Make BJ, Regan EA, Walek DS, Daniel JJ, Kamatani Y, Zelenika D, Smith K, McKean D, Pedersen BS, Talbert J, Kidd RN, Markin CR, Beckman KB, Lathrop M, Schwarz MI, Schwartz DA. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45:613–20. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, Lancaster L, Noble PW, Raghu G, Sahn SA, Szwarcberg J, Thomeer M, Valeyre D, King TE., Jr. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine. 2011;184:459–66. doi: 10.1164/rccm.201011-1790OC. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez IE, Eickelberg O. New cellular and molecular mechanisms of lung injury and fibrosis in idiopathic pulmonary fibrosis. Lancet. 2012;380:680–8. doi: 10.1016/S0140-6736(12)61144-1. [DOI] [PubMed] [Google Scholar]
- 5.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine. 2006;174:810–6. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 6.King TE, Jr., Albera C, Bradford WZ, Costabel U, Hormel P, Lancaster L, Noble PW, Sahn SA, Szwarcberg J, Thomeer M, Valeyre D, du Bois RM, Group IS. Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): a multicentre, randomised, placebo-controlled trial. Lancet. 2009;374:222–8. doi: 10.1016/S0140-6736(09)60551-1. [DOI] [PubMed] [Google Scholar]
- 7.Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE, Jr., Flaherty KR, Schwartz DA, Noble PW, Raghu G, Brown KK, Group IPFS. The clinical course of patients with idiopathic pulmonary fibrosis. Annals of internal medicine. 2005;142:963–7. doi: 10.7326/0003-4819-142-12_part_1-200506210-00005. [DOI] [PubMed] [Google Scholar]
- 8.King TE, Jr., Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. American journal of respiratory and critical care medicine. 2001;164:1171–81. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 9.Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, Poletti V, Buccioli M, Elicker BM, Jones KD, King TE, Jr., Collard HR. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Annals of internal medicine. 2012;156:684–91. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 10.Wells AU, Desai SR, Rubens MB, Goh NS, Cramer D, Nicholson AG, Colby TV, du Bois RM, Hansell DM. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. American journal of respiratory and critical care medicine. 2003;167:962–9. doi: 10.1164/rccm.2111053. [DOI] [PubMed] [Google Scholar]
- 11.Lindell KO, Liang Z, Hoffman LA, Rosenzweig MQ, Saul MI, Pilewski JM, Gibson KF, Kaminski N. Palliative care and location of death in decedents with idiopathic pulmonary fibrosis. Chest. 2015;147:423–9. doi: 10.1378/chest.14-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herazo-Maya JD, Noth I, Duncan SR, Kim S, Ma SF, Tseng GC, Feingold E, Juan-Guardela BM, Richards TJ, Lussier Y, Huang Y, Vij R, Lindell KO, Xue J, Gibson KF, Shapiro SD, Garcia JG, Kaminski N. Peripheral blood mononuclear cell gene expression profiles predict poor outcome in idiopathic pulmonary fibrosis. Sci Transl Med. 2013;5:205ra136. doi: 10.1126/scitranslmed.3005964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, Li K, Choi J, Vuga LJ, Lindell KO, Klesen M, Zhang Y, Gibson KF. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185:67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, Lindell KO, Cisneros J, Macdonald SD, Pardo A, Sciurba F, Dauber J, Selman M, Gochuico BR, Kaminski N. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008;5:e93. doi: 10.1371/journal.pmed.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song JW, Do KH, Jang SJ, Colby TV, Han S, Kim DS. Blood biomarkers MMP-7 and SP-A: predictors of outcome in idiopathic pulmonary fibrosis. Chest. 2013;143:1422–9. doi: 10.1378/chest.11-2735. [DOI] [PubMed] [Google Scholar]
- 16.Richards TJ, Park C, Chen Y, Gibson KF, Peter Di Y, Pardo A, Watkins SC, Choi AM, Selman M, Pilewski J, Kaminski N, Zhang Y. Allele-specific transactivation of matrix metalloproteinase 7 by FOXA2 and correlation with plasma levels in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;302:L746–54. doi: 10.1152/ajplung.00319.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peljto AL, Zhang Y, Fingerlin TE, Ma SF, Garcia JG, Richards TJ, Silveira LJ, Lindell KO, Steele MP, Loyd JE, Gibson KF, Seibold MA, Brown KK, Talbert JL, Markin C, Kossen K, Seiwert SD, Murphy E, Noth I, Schwarz MI, Kaminski N, Schwartz DA. Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA : the journal of the American Medical Association. 2013;309:2232–9. doi: 10.1001/jama.2013.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kropski JA, Pritchett JM, Zoz DF, Crossno PF, Markin C, Garnett ET, Degryse AL, Mitchell DB, Polosukhin VV, Rickman OB, Choi L, Cheng DS, McConaha ME, Jones BR, Gleaves LA, McMahon FB, Worrell JA, Solus JF, Ware LB, Lee JW, Massion PP, Zaynagetdinov R, White ES, Kurtis JD, Johnson JE, Groshong SD, Lancaster LH, Young LR, Steele MP, Phillips Iii JA, Cogan JD, Loyd JE, Lawson WE, Blackwell TS. Extensive phenotyping of individuals at risk for familial interstitial pneumonia reveals clues to the pathogenesis of interstitial lung disease. American journal of respiratory and critical care medicine. 2015;191:417–26. doi: 10.1164/rccm.201406-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ley B, Brown KK, Collard HR. Molecular biomarkers in idiopathic pulmonary fibrosis. American journal of physiology Lung cellular and molecular physiology. 2014;307:L681–91. doi: 10.1152/ajplung.00014.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szarvas T, Becker M, vom Dorp F, Gethmann C, Totsch M, Bankfalvi A, Schmid KW, Romics I, Rubben H, Ergun S. Matrix metalloproteinase-7 as a marker of metastasis and predictor of poor survival in bladder cancer. Cancer science. 2010;101:1300–8. doi: 10.1111/j.1349-7006.2010.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukuda A, Wang SC, Morris JPt, Folias AE, Liou A, Kim GE, Akira S, Boucher KM, Firpo MA, Mulvihill SJ, Hebrok M. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer cell. 2011;19:441–55. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spagnolo P, Tzouvelekis A, Maher TM. Personalized medicine in idiopathic pulmonary fibrosis: facts and promises. Curr Opin Pulm Med. 2015;21:470–8. doi: 10.1097/MCP.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 23.Greene KE, King TE, Jr., Kuroki Y, Bucher-Bartelson B, Hunninghake GW, Newman LS, Nagae H, Mason RJ. Serum surfactant proteins-A and -D as biomarkers in idiopathic pulmonary fibrosis. The European respiratory journal. 2002;19:439–46. doi: 10.1183/09031936.02.00081102. [DOI] [PubMed] [Google Scholar]
- 24.Kinder BW, Brown KK, McCormack FX, Ix JH, Kervitsky A, Schwarz MI, King TE., Jr. Serum surfactant protein-A is a strong predictor of early mortality in idiopathic pulmonary fibrosis. Chest. 2009;135:1557–63. doi: 10.1378/chest.08-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokoyama A, Kondo K, Nakajima M, Matsushima T, Takahashi T, Nishimura M, Bando M, Sugiyama Y, Totani Y, Ishizaki T, Ichiyasu H, Suga M, Hamada H, Kohno N. Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology. 2006;11:164–8. doi: 10.1111/j.1440-1843.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 26.Noth I, Zhang Y, Ma S-F, Flores C, Barber M, Huang Y, Broderick SM, Wade MS, Hysi P, Scuirba J, Richards TJ, Juan-Guardela BM, Vij R, Han MK, Martinez FJ, Kossen K, Seiwert SD, Christie JD, Nicolae D, Kaminski N, Garcia JGN. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. The Lancet Respiratory Medicine. 2013;1:309–17. doi: 10.1016/S2213-2600(13)70045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tzouvelekis A, Herazo-Maya J, Sakamoto K, Bouros D. Biomarkers in the Evaluation and Management of Idiopathic Pulmonary Fibrosis. Current topics in medicinal chemistry. 2016;16:1587–98. doi: 10.2174/1568026616666150930120959. [DOI] [PubMed] [Google Scholar]
- 28.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, Sheppard D, Pardo A, Selman M, Heller RA. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6292–7. doi: 10.1073/pnas.092134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujishima S, Shiomi T, Yamashita S, Yogo Y, Nakano Y, Inoue T, Nakamura M, Tasaka S, Hasegawa N, Aikawa N, Ishizaka A, Okada Y. Production and activation of matrix metalloproteinase 7 (matrilysin 1) in the lungs of patients with idiopathic pulmonary fibrosis. Archives of pathology & laboratory medicine. 2010;134:1136–42. doi: 10.5858/2009-0144-OA.1. [DOI] [PubMed] [Google Scholar]
- 30.Pardo A, Cabrera S, Maldonado M, Selman M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respiratory research. 2016;17:23. doi: 10.1186/s12931-016-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pardo A, Gibson K, Cisneros J, Richards TJ, Yang Y, Becerril C, Yousem S, Herrera I, Ruiz V, Selman M, Kaminski N. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005;2:e251. doi: 10.1371/journal.pmed.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison CJ, Butler GS, Rodriguez D, Overall CM. Matrix metalloproteinase proteomics: substrates, targets, and therapy. Current opinion in cell biology. 2009;21:645–53. doi: 10.1016/j.ceb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Morais A, Beltrao M, Sokhatska O, Costa D, Melo N, Mota P, Marques A, Delgado L. Serum metalloproteinases 1 and 7 in the diagnosis of idiopathic pulmonary fibrosis and other interstitial pneumonias. Respir Med. 2015;109:1063–8. doi: 10.1016/j.rmed.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Ulivi P, Casoni GL, Foschi G, Scarpi E, Tomassetti S, Romagnoli M, Ravaglia C, Mengozzi M, Zoli W, Poletti V. MMP-7 and fcDNA serum levels in early NSCLC and idiopathic interstitial pneumonia: preliminary study. Int J Mol Sci. 2013;14:24097–112. doi: 10.3390/ijms141224097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamai K, Iwamoto H, Ishikawa N, Horimasu Y, Masuda T, Miyamoto S, Nakashima T, Ohshimo S, Fujitaka K, Hamada H, Hattori N, Kohno N. Comparative Study of Circulating MMP-7, CCL18, KL-6, SP-A, and SP-D as Disease Markers of Idiopathic Pulmonary Fibrosis. Dis Markers. 2016;2016:4759040. doi: 10.1155/2016/4759040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White ES, Xia M, Murray S, Dyal R, Flaherty CM, Flaherty KR, Moore BB, Cheng L, Doyle TJ, Villalba J, Dellaripa PF, Rosas IO, Kurtis JD, Martinez FJ. Plasma Surfactant Protein-D, Matrix Metalloproteinase-7, and Osteopontin Index Distinguishes Idiopathic Pulmonary Fibrosis From Other Idiopathic Interstitial Pneumonias. American journal of respiratory and critical care medicine. 2016 doi: 10.1164/rccm.201505-0862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung K, Klotzek S, Stephan C, Mannello F, Lein M. Impact of blood sampling on the circulating matrix metalloproteinases 1, 2, 3, 7, 8, and 9. Clinical chemistry. 2008;54:772–3. doi: 10.1373/clinchem.2007.099937. [DOI] [PubMed] [Google Scholar]
- 38.Meisser A, Cohen M, Bischof P. Concentrations of circulating gelatinases (matrix metalloproteinase-2 and -9) are dependent on the conditions of blood collection. Clinical chemistry. 2005;51:274–6. doi: 10.1373/clinchem.2004.041707. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, Doyle TJ, Liu Y, Aggarwal R, Wang X, Shi Y, Ge SX, Huang H, Lin Q, Liu W, Cai Y, Koontz D, Fuhrman CR, Golzarri MF, Liu Y, Hatabu H, Nishino M, Araki T, Dellaripa PF, Oddis CV, Rosas IO, Ascherman DP. Biomarkers of rheumatoid arthritis-associated interstitial lung disease. Arthritis & rheumatology. 2015;67:28–38. doi: 10.1002/art.38904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doyle TJ, Patel AS, Hatabu H, Nishino M, Wu G, Osorio JC, Golzarri MF, Traslosheros A, Chu SG, Frits ML, Iannaccone CK, Koontz D, Fuhrman C, Weinblatt ME, El-Chemaly SY, Washko GR, Hunninghake GM, Choi AM, Dellaripa PF, Oddis CV, Shadick NA, Ascherman DP, Rosas IO. Detection of Rheumatoid Arthritis-Interstitial Lung Disease Is Enhanced by Serum Biomarkers. American journal of respiratory and critical care medicine. 2015;191:1403–12. doi: 10.1164/rccm.201411-1950OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.