Abstract
Much of the literature on the impact of male caffeine and alcohol intake on reproductive outcomes has utilized semen quality as a proxy for male fertility, although semen parameters have a limited predictive value for spontaneous pregnancy. The objective of this study was to investigate whether male caffeine and alcohol intakes are associated with semen parameters and assisted reproductive technologies (ART) outcome.
The Environment and Reproductive Health (EARTH) Study, an ongoing prospective cohort study, enrolls subfertile couples presenting for treatment at an academic fertility center (2007–2012). A total of 171 men with 338 semen analyses and 205 ART cycles were included in this analysis. Diet was assessed using a 131-item food frequency questionnaire (FFQ). Mixed models adjusting for potential confounders were used to evaluate the relationships of male caffeine and alcohol intakes with semen parameters and ART outcomes. There was no association between male caffeine and alcohol intake and semen quality. Male caffeine intake was negatively related to live birth after ART (P-trend<0.01), and male alcohol intake was positively related to live birth after ART (P-trend=0.04). Adjusted live birth rate among couples with a male partner in the highest quartile of caffeine intake (≥ 272mg/day) compared to couples with a male partner in the lowest quartile of intake (<99mg/day) was 19% versus 55%, respectively, p<0.01. In terms of alcohol intake, adjusted live birth rate among couples with a male partner in the highest quartile of alcohol intake (≥ 22g/day) compared to couples with a male partner in the lowest quartile of intake (< 3g/day) was 61% versus 28%, respectively, p=0.05. In conclusion, male pre-treatment caffeine and alcohol intakes were associated with live birth after ART, but not with semen parameters, among fertility patients.
Keywords: Caffeine, alcohol, male fertility, semen quality, assisted reproduction
INTRODUCTION
The relationships of male caffeine and alcohol intakes with reproductive function have been extensively investigated, but studies have yielded conflicting results. While most of the literature does not support a relationship of either moderate caffeine or alcohol intake with markers of male fertility (Curtis, et al., 1997; Jensen, et al., 2014; Jensen, et al., 1998; Jensen, et al., 2014; Klonoff-Cohen, et al., 2002; Li, et al., 2011; Olsen, et al., 1997), a few studies have reported positive and negative associations between both of these common exposures and markers of male fertility (Adelusi, et al., 1998; Florack, et al., 1994; Jensen, Gottschau, Madsen, Andersson, Lassen, Skakkebaek, Swan, Priskorn, Juul & Jorgensen, 2014; Klonoff-Cohen, et al., 2003; Sobreiro, et al., 2005). Much of the literature on beverage consumption and male fertility has utilized semen quality as a proxy, even though semen parameters have a limited predictive value for spontaneous pregnancy (Lewis, 2007; Sripada, et al., 2010) or infertility treatment outcomes (Nagy, et al., 1995). Moreover, most of the studies evaluating the relation of men’s alcohol or caffeine intake with direct markers of fertility, such as time to pregnancy, have found no evidence that these common exposures have a negative impact on fertility (Curtis, Savitz & Arbuckle, 1997; Hassan & Killick, 2004; Jensen, Hjollund, Henriksen, Scheike, Kolstad, Giwercman, Ernst, Bonde, Skakkebaek & Olsen, 1998; Olsen, Bolumar, Boldsen & Bisanti, 1997).
Although 40–60% of infertile couples have an identifiable reproductive abnormality in the male partner (Thonneau, et al., 1991), research on the potential role of nutrition and other modifiable factors in fertility has generally focused on female factors. Moreover, although nearly 2% of all births in the United States are the result of assisted reproductive technologies (ART)(Centers for Disease Control and Prevention, 2014), there are very limited data on the relationship between male caffeine and alcohol intakes and ART outcomes. Thus, the objective of this study was to investigate the relation of male caffeine and alcohol intakes with semen parameters and clinical outcomes after ART.
MATERIALS AND METHODS
Study population
Participants in this study are men from subfertile couples who presented at Massachusetts General Hospital Fertility Center (Boston, MA, USA) and enrolled in the Environment and Reproductive Health (EARTH) Study, an ongoing prospective cohort study investigating environmental factors and fertility. All men aged 18–55 with no history of vasectomy and in a couple anticipating use of their own gametes for infertility treatment are invited to participate. Approximately 78% of potential participants referred to the study and approached by the research nurses agree to join the study. Here, we report on men enrolled between January 2007 and December 2012 and followed through May 2013. For the semen quality analysis, 188 men with complete diet data and at least one semen analysis were available for investigation. There were a total of 485 semen samples available for review. From this group, azoospermic men (n=1), men with semen analyses produced prior to dietary assessment (n=27), and men with missing semen parameter data (n=5) were excluded. In order to minimize the possibility of exposure misclassification over time, semen analyses collected more than 18 months after dietary assessment were excluded (47 samples). This left a final sample size of 155 men contributing a total of 338 semen samples.
For the clinical outcomes analysis, only ART cycles with known cycle outcomes were included. Cycles that started prior to diet assessment (31 cycles) were excluded from analysis. A total of 121 men undergoing 205 ART cycles were included in the final analysis of clinical outcomes. Fifty men for whom there were data on semen analyses did not have eligible ART cycle data for analysis. Of these 50 men, the majority (n=30) were not included in the clinical analyses because they did not undergo ART treatment during the study period. Similarly, 16 men for whom data on ART outcomes were available did not have semen analysis data meeting criteria for inclusion for the evaluation of semen quality.
A trained research nurse measured participant height and weight at enrollment. Participants also completed a take-home questionnaire focusing on medical and reproductive history, as well as lifestyle factors. Research nurses abstracted clinical data, including IVF cycle characteristics and infertility diagnoses, from electronic medical records. Informed consent was obtained from all participants and the Institutional Review Boards of the Harvard T.H. Chan School of Public Health and the Massachusetts General Hospital approved the study.
Diet assessment
Diet was assessed using a previously validated 131-item food frequency questionnaire (FFQ)(Rimm, et al., 1992). Participants were asked how often, on average during the previous year, they consumed specific foods, beverages, and supplements. Nutrient contents for each item were obtained from a database based on the US Department of Agriculture nutrient database (United States Department of Agriculture & Agriculural Research Service, 2008). In a validation study, the de-attenuated correlation (i.e., observed correlation corrected for random within-person variability) between two, one-week diet records and FFQ reports were 0.93 for coffee, 0.88 for beer, 0.93 for red wine, 0.78 for white wine and 0.85 for liquor (Feskanich, et al., 1993). Specific caffeine-containing items were caffeinated coffee (137 mg caffeine/cup) and tea (47 mg caffeine/cup), caffeinated sodas (46 mg caffeine/bottle or can) and chocolate (7 mg caffeine/serving). The estimated alcohol content of each beverage was 11.3 g per bottle or can of light beer, 12.8 g per bottle or can of regular beer, 11.0 g per 4-ounce glass of wine, and 14.0 g per shot of liquor. We calculated the total intake of caffeine and alcohol by summing the caffeine and alcohol content for the specific items multiplied by weights proportional to the frequency of use of each item.
We identified dietary patterns using principal component analyses as previously described (Gaskins, et al., 2012). Two patterns were identified; the ‘Western’ pattern was characterized by high intakes of red meat, butter, high fat dairy, refined grains, pizza, snacks, energy drinks, mayonnaise and sweets, whereas the ‘Prudent’ pattern was characterized by high intakes of fish, chicken, fruit, cruciferous vegetables, tomatoes, leafy green vegetables, legumes, and whole grains. Participants were given a score for each pattern according to their adherence to these patterns. A higher score in each pattern indicates higher adherence to the respective pattern.
Semen analysis
Semen samples were produced on site by masturbation. Men were told to abstain from ejaculation for at least 48 hours prior to production. Samples were liquefied at 37°C for 20 minutes before analysis. Sperm count and motility were assessed with a computer-aided semen analysis system (CASA) (Hamilton-Thorne Biosciences, Ceros, Version 14). Sperm morphology was assessed using strict Kruger criteria (Kruger, et al., 1988). Per lab protocol, a total of 200 sperm per sample were analyzed. All analyses were performed within 45 minutes of collection and samples were maintained at 37°C during assessment.
Clinical outcomes
Clinical outcomes analyzed included % normal fertilization (number of 2PN embryos / number of M2 oocytes, calculated for conventional insemination, intracytoplasmic sperm injection (ICSI), and combined), embryo quality (proportion of slow cleaving embryos, accelerated cleavage embryos and poor quality embryos), implantation rate, clinical pregnancy, and live birth rate. Clinical pregnancy was defined as having at least one intrauterine gestational sac present on ultrasound. Live birth was defined as the birth of at least one living neonate at or after 24 weeks of gestation.
Statistical analysis
A total of 171 men were included in the analysis. Participants were divided into quartiles of caffeine and alcohol intakes. Intake quartiles were identified separately for the semen quality (n=155) and clinical outcomes (n=121) analyses. Univariate analyses were performed to assess demographic and nutritional characteristics by caffeine and alcohol intake quartile. The associations of caffeine and alcohol intake with semen quality and ART outcomes were evaluated using generalized linear mixed models with random intercepts to account for within-person correlations in repeated observations (semen analyses or treatment cycles) while adjusting for potential confounders. Specifically, we used linear mixed models when semen parameters were the outcome, and generalized linear mixed models with logit link function for fertilization rates and clinical outcomes. Sperm count and concentration were log transformed to normalize distributions. Results are presented as adjusted means or probabilities adjusted for confounders. We evaluated the presence of linear trends across categories of intake by entering the median intake in each quartile as continuous variables into the regression models. Factors related to alcohol or caffeine intake at p <0.20 were considered as potential confounders. We also decided a priori to include in the model terms for male and female body mass index (BMI) and female age, regardless of whether they met statistical properties of a confounder, to account for previously described relations in this cohort (Chavarro, et al., 2012; Colaci, et al., 2012). Statistical analyses were performed with SAS v9.3 (SAS Institute, Cary, NC). Testing for heterogeneity was carried out by adding cross-product interaction terms between alcohol and caffeine intake (dichotomized at the median) to our multivariate models.
RESULTS
Mean (standard deviation) age of the total male cohort at study entry was 37(5) years and the majority of men were Caucasian (85%). Caffeinated coffee accounted for 87% of total caffeine intake, and beer accounted for 79% of total alcohol intake. Median (min, max) daily intake of caffeine and alcohol was 161mg (2mg, 616mg) and 9.9g (0g, 151g), respectively. Alcohol and caffeine intakes were positively associated with each other (Spearman correlation coefficient 0.34, p<0.01), and alcohol intake was positively associated with current or past smoking (Table 1). Men with higher alcohol consumption had, on average, lower percent of calories from protein and carbohydrates and higher overall calorie intake. Male caffeine and alcohol intakes were not related to female partner age, BMI, or caffeine intake. The Spearman correlation coefficient for male and female alcohol intake was 0.52 (p<0.01), and for male and female caffeine intake was 0.16 (p=0.09). Both caffeine and alcohol intakes were positively related to greater adherence to the Western diet pattern, and unrelated to adherence to the Prudent diet pattern.
Table 1.
Characteristics of population based on male caffeine and alcohol intake
| Alcohol (g/day) | Caffeine (mg/day) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| < 3.0 | 3–9.9 | 10–18.9 | ≥19 | P-value | <67 | 67–160.9 | 161–271.9 | ≥272 | p-value | |
| n | 47 | 40 | 41 | 43 | 43 | 42 | 44 | 42 | ||
| Male partner characteristics | ||||||||||
| Alcohol, g/day* | 1.3(1.0) | 7.1(1.9) | 14.1(2.2) | 37.4(22.7) | <0.01 | 10.3(24.0) | 15.4(16.4) | 14.0(11.2) | 19.5(17.5) | 0.03 |
| Caffeine, g/d* | 119.3(126.7) | 169.7(114.4) | 223.7(123.1) | 231.0(137.8) | <0.01 | 29.4(20.5) | 115.1(17.5) | 232.4(32.4) | 361.3(84.0) | <0.01 |
| Age, years* | 37.8(5.8) | 36.4(5.1) | 35.9(4.1) | 36.6(4.8) | 0.21 | 37.4(5.9) | 35.2(4.8) | 37.5(4.2) | 36.7(4.9) | 0.93 |
| BMI* | 27.5(4.6) | 26.8(4.1) | 26.9(3.0) | 26.8(3.3) | 0.47 | 27.6(4.0) | 26.5(3.7) | 27.0(3.6) | 26.9(3.9) | 0.56 |
| Race, n(%) | 0.17 | 0.92 | ||||||||
| Caucasian | 36(77) | 33(83) | 38(93) | 38(88) | 36(84) | 37(88) | 37(84) | 35(83) | ||
| Other | 11(23) | 7(18) | 3(7) | 5(12) | 7(16) | 5(12) | 7(16) | 7(17) | ||
| Smoking, n(%) | <0.01 | 0.09 | ||||||||
| Never smoker | 37(79) | 26(65) | 27(66) | 17(40) | 30(70) | 30(71) | 27(61) | 20(48) | ||
| Current or Past smoker | 10(21) | 14(35) | 14(34) | 26(60) | 13(30) | 12(29) | 17(39) | 22(52) | ||
| Total calories* | 1835(633) | 1941(580) | 2058(499) | 2369(667) | <0.01 | 1930(664) | 2087(644) | 1916(545) | 2267(615) | 0.05 |
| Total fata | 31.4(7.9) | 30.8(5.2) | 33.7(4.8) | 31.5(5.9) | 0.45 | 32.0(7.5) | 32.1(5.7) | 31.8(5.4) | 31.5(6.3) | 0.70 |
| Saturated fata | 10.4(3.1) | 10.2(2.0) | 11.2(1.9) | 10.4(2.2) | 0.46 | 10.4(2.9) | 10.7(2.1) | 10.6(2.3) | 10.4(2.3) | 0.97 |
| Monounsaturated fata | 12.1(3.6) | 11.9(2.6) | 13.4(2.5) | 12.7(3.3) | 0.15 | 12.6(3.3) | 12.6(3.2) | 12.5(2.7) | 12.4(3.3) | 0.81 |
| Polyunsaturated fata | 6.1(1.8) | 5.9(1.2) | 6.1(1.6) | 5.7(1.6) | 0.44 | 6.2(1.8) | 5.9(1.5) | 5.9(1.6) | 5.9(1.4) | 0.41 |
| Total carbohydratesa | 53.4(9.9) | 51.3(7.4) | 46.3(5.2) | 43.6(7.0) | <0.01 | 50.6(10.3) | 48.3(8.3) | 48.3(6.8) | 47.7(8.4) | 0.14 |
| Total proteina | 16.7(3.3) | 16.8(2.9) | 16.6(2.4) | 15.2(2.5) | 0.02 | 16.2(3.0) | 16.3(2.6) | 16.5(2.8) | 16.3(3.2) | 0.72 |
| Prudent pattern score | 0.007(0.94) | 0.039(0.97) | −0.060(1.11) | −0.031(0.91) | 0.75 | −0.037(1.14) | −0.191(0.85) | −0.102(0.97) | 0.290(0.97) | 0.11 |
| Western pattern score | −0.497(0.89) | −0.198(0.74) | 0.209(0.82) | 0.698(1.16) | <0.01 | −0.206(0.99) | 0.142(1.10) | −0.136(0.79) | 0.385(1.10) | 0.03 |
| Female partner characteristics | ||||||||||
| Alcohol, g/day | 4.1(5.6) | 6.3(8.1) | 9.0(6.8) | 17.7(13.1) | <0.01 | 10.2(10.4) | 9.1(8.4) | 8.2(7.3) | 11.9(14.9) | 0.64 |
| Caffeine, mg/day | 113.0(103.5) | 129.7 (100.3) | 117.6(75.8) | 167.9(155.9) | 0.10 | 114.7 (113.2) | 111.9(76.5) | 149.9(137.5) | 147.2(117.1) | 0.17 |
| Age, years | 36.5(4.0) | 36.0(3.3) | 34.7(4.5) | 35.2(3.4) | 0.09 | 35.3(3.6) | 35.4(4.4) | 36.2(3.6) | 35.1(4.1) | 0.91 |
| BMI | 24.2(4.6) | 22.3(2.5) | 24.0(4.1) | 23.8(3.6) | 0.98 | 22.8(3.0) | 22.7(3.6) | 24.6(4.2) | 24.3(4.1) | 0.04 |
| Infertility Diagnosis, n(%) | 0.22 | 0.21 | ||||||||
| Diminished ovarian reserve | 7(15) | 3(8) | 4(10) | 1(2) | 6(14) | 4(10) | 3(7) | 2(5) | ||
| Endometriosis | 4(9) | 2(5) | 1(2) | 1(2) | 2(5) | 1(2) | 2(5) | 3(7) | ||
| Male factor | 16(34) | 13(33) | 11(27) | 12(28) | 16(37) | 14(33) | 9(20) | 13(31) | ||
| Ovulatory | 3(6) | 3(8) | 4(10 | 9(21) | 0(0) | 8(19) | 8(18) | 3(7) | ||
| Tubal factor | 6(12.8) | 2(5.0) | 2(4.9) | 2(4.7) | 5(12) | 2(5) | 2(5) | 3(7) | ||
| Unexplained | 11(23) | 16(40) | 19(46) | 18(42) | 14(33) | 12(29) | 20(45) | 18(43) | ||
| Uterine factor | 0(0) | 1(3) | 0(0) | 0(0) | 0(0) | 1(2) | 0(0) | 0(0) | ||
mean (standard deviation)
% total calories, mean (standard deviation)
Male alcohol (Figure 1) and caffeine intakes (Figure 2) were unrelated to various metrics of semen quality in analyses adjusted for smoking history, age, abstinence time, BMI, total calorie intake, dietary patterns, and each other. Similarly, there was no relation between caffeine intake and fertilization rate, although there was a suggestion of a higher fertilization rate with increasing alcohol intake in ICSI cycles but not in IVF cycles (Supplemental table). In terms of embryo quality, alcohol was not related to cleavage rate or proportion of poor quality embryos. Number of embryos transferred was not associated with alcohol or caffeine intake. Higher intake of caffeine was not related to proportion of slow-cleaving embryos or proportion of poor quality embryos, although it was associated with a lower proportion of embryos with accelerated cleavage (p-trend 0.03) (data not shown).
Figure 1.
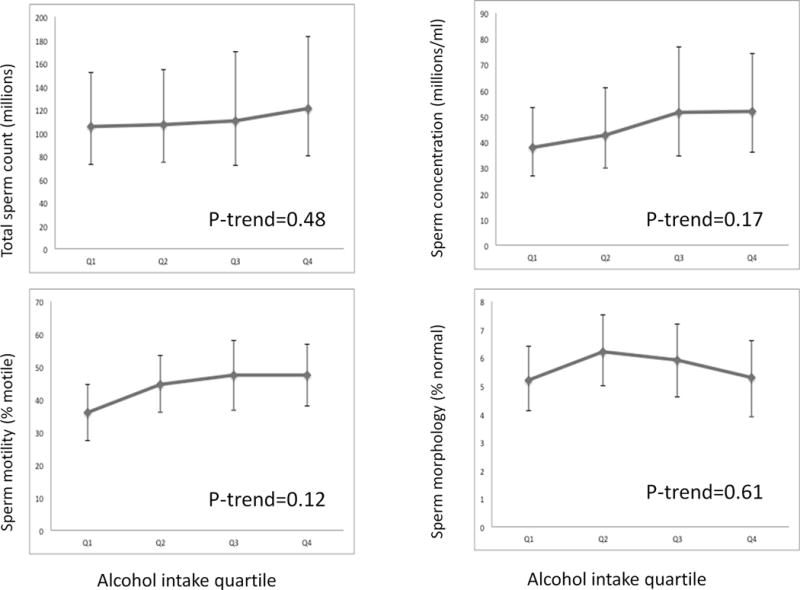
Alcohol and semen parameters. Results demonstrate the association between alcohol intake quartile and sperm count, concentration, motility, and morphology. Quartiles 1 through 4 include alcohol intakes of < 3g/day, 3–9.9g/day, 10–18.9g/day, and ≥ 19g/day, respectively. All results are adjusted for caffeine intake, smoking history, age, abstinence time, body mass index (BMI), total calorie intake, total protein intake, and total fat intake.
Figure 2.
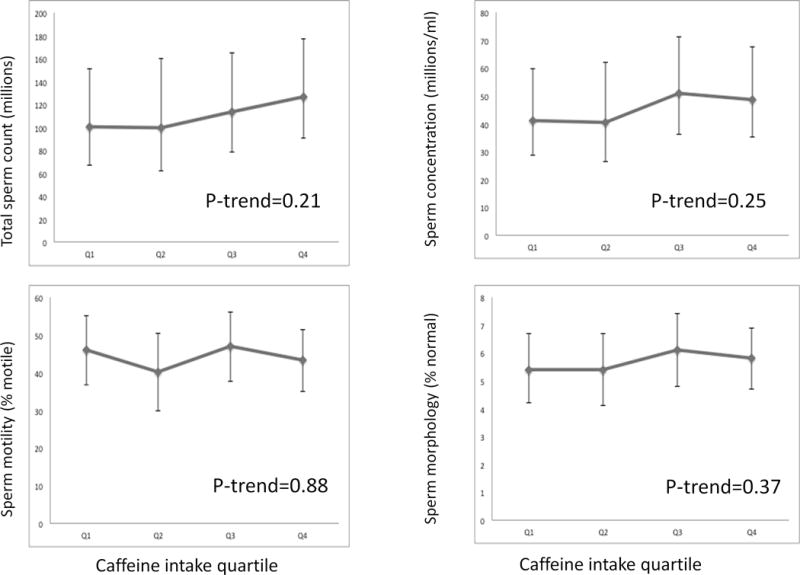
legend: Caffeine and semen parameters. Results demonstrate the association between caffeine intake quartile and sperm count, concentration, motility, and morphology. Quartiles 1 through 4 include caffeine intakes of < 55mg/day, 55–142mg/day, 143–264mg/day, and ≥ 265mg/day, respectively. All results are adjusted for alcohol intake, smoking history, age, abstinence time, body mass index (BMI), total calorie intake, total protein intake, and total fat intake.
Paternal intake of both alcohol and caffeine was associated with clinical outcomes after infertility treatment with ART. Alcohol was unrelated to the clinical pregnancy rate in crude and age-adjusted analyses. However, after adjusting for confounders (in particular, after adjustment for male caffeine intake), there was a positive association of marginal statistical significance between alcohol intake and clinical pregnancy (p=0.06) as well as alcohol intake and implantation (p=0.05) (Table 2). On the other hand, there was an inverse relation between male intake of caffeine and the probability of implantation and clinical pregnancy that was statistically significant in age-adjusted and multivariate-adjusted models (Table 2). The same pattern was observed for the probability of live birth (Figure 3). Specifically, the live birth rate was 36% lower among couples with a male partner in the highest quartile of caffeine intake (≥ 272mg/day) compared to couples with a male partner in the lowest quartile of intake (<99mg/day) (19% versus 55%, p<0.01). In terms of alcohol intake, live birth rate was 33% higher among couples with a male partner in the highest quartile of alcohol intake (≥ 22g/day) compared to couples with a male partner in the lowest quartile of intake (< 3g/day) (61% versus 28%, p=0.05).
Table 2.
Male beverage intake and probability of implantation and clinical pregnancy
| ALCOHOL | CAFFEINE | |||||
|---|---|---|---|---|---|---|
| Beverage intake quartile** | Crude rate | Age adjusted probability,% (95% CI) |
Multivariate adjusted* probability (95% CI) |
Crude rate | Age adjusted probability,% (95% CI) |
Multivariate adjusted* probability (95% CI) |
| Implantation | ||||||
| Q1 | 29/57 | 53.2(38.6–67.3) | 45.6(27.6–64.8) | 26/44 | 59.7(43.9–73.7) | 66.1(46.5–81.3) |
| Q2 | 28/47 | 61.3(45.5–75.0) | 59.1(40.1–75.8) | 35/47 | 74.9(59.8–85.7) | 76.2(58.8–87.7) |
| Q3 | 31/52 | 60.2(44.8–73.9) | 65.1(46.9–79.8) | 28/43 | 66.5(50.5–79.5) | 69.1(50.0–83.4) |
| Q4 | 32/49 | 66.2(50.6–78.9) | 76.3(56.3–88.9) | 31/71 | 44.0(31.9–56.8) | 42.3(28.0–58.0) |
| P-trend | 0.25 | 0.05 | 0.04 | 0.02 | ||
| Clinical Pregnancy | ||||||
| Q1 | 26/57 | 48.7(34.3–63.3) | 42.6(25.3–62.0) | 25/44 | 57.1(41.7–71.2) | 62.5(43.3–78.5) |
| Q2 | 25/47 | 54.9(39.3–69.7) | 49.1(31.7–66.8) | 31/47 | 66.3(51.3–78.7) | 68.4(50.9–81.9) |
| Q3 | 28/52 | 55.5(40.1–69.8) | 59.1(41.4–74.7) | 28/43 | 66.0(50.3–78.8) | 68.0(49.3–82.2) |
| Q4 | 29/49 | 60.8(45.2–74.5) | 70.9(50.2–85.4) | 24/71 | 34.1(23.6–46.4)*** | 32.7(20.4–47.9)*** |
| P-trend | 0.28 | 0.06 | 0.01 | 0.01 | ||
Adjusted for age, BMI, race, male caffeine or alcohol intake, smoking status, total calorie and dietary pattern, infertility diagnosis, and female caffeine intake, female alcohol intake, female age, female BMI
For alcohol, quartiles are: < 3g/day, 3–11.9g/day, 12–21.9g/day, and ≥ 22g/day. For caffeine, quartiles are: <99mg/day, 99–208.9mg/day, 209–271.9mg/day, and ≥ 272mg/day
P < 0.05 compared to Q1
Figure 3.
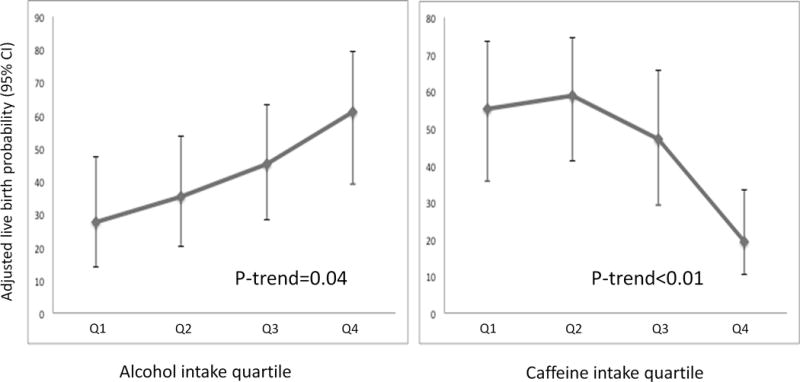
legend: Male caffeine and alcohol intake and adjusted live birth probability. Results demonstrate the association between caffeine and alcohol intake quartiles and probability of live birth. Caffeine intake quartiles 1 through 4 include caffeine intakes of <99mg/day, 99–208.9mg/day, 209–271.9mg/day, and ≥ 272mg/day, respectively. Alcohol intake quartiles 1 through 4 include alcohol intakes of < 3g/day, 3–11.9g/day, 12–21.9g/day, and ≥ 22g/day, respectively. All results are adjusted for age, race, BMI, male alcohol or caffeine intake, smoking status, total calorie intake, dietary pattern, infertility diagnosis, and female caffeine intake, female alcohol intake, female age, female BMI.
To further delineate the relationships between caffeine, alcohol, and live birth, we cross-classified caffeine and alcohol by their respective median intakes (Supplemental figure 1). Caffeine intakes < and ≥ 209mg/day were considered low and high caffeine intakes, respectively, and alcohol intakes < and ≥ 12g/day were considered low and high alcohol intakes, respectively. As expected, cycles with low male caffeine intake levels and high male alcohol intake levels had the highest adjusted live birth rate, and cycles with high male caffeine intake and low male alcohol intake had the lowest live birth rate (66% versus 26%, respectively, p <0.01). These differences notwithstanding, there was no evidence of significant super-additive interaction between caffeine and alcohol intake (p=0.76).
When ART cycles were stratified by insemination method (ICSI versus conventional insemination), the significant negative relationship between caffeine intake and live birth was observed among couples undergoing ICSI cycles but not in conventional insemination cycles (P-interaction=0.09, Supplemental figure 2). No such heterogeneity was observed when the relationship between alcohol and clinical pregnancy was stratified by insemination method.
Given the relationships between smoking and caffeine and alcohol intakes, we performed a sensitivity analysis by excluding male ever-smokers (N=46 men and 77 ART cycles). The overall patterns did not change, although the associations were attenuated. The adjusted probabilities of live birth for couples with men in increasing quartiles of caffeine intake were 38%, 71%, 36%, and 16%, (P- trend=0.07). The corresponding adjusted probabilities for couples with men in increasing quartiles of alcohol were 35%, 37%, 44%, and 53% (p, trend=0.50).
Last, we examined whether these relations were driven by intakes of specific alcoholic and caffeinated beverages. No clear patterns emerged when different alcoholic and caffeinated beverages were evaluated in their relation to clinical ART outcomes (data not shown).
DISCUSSION
Male partner intakes of alcohol or caffeine were unrelated to semen parameters in this prospective cohort of men presenting to a single fertility center. Despite the lack of association with these commonly used markers of male fertility, we found a positive association between male partner alcohol intake and the probability of achieving a live birth as a result of ART. On the other hand, caffeine intake was associated with a lower probability of achieving live birth after ART. The inverse association between caffeine intake and live birth appeared to be limited to ICSI cycles. These findings expand our understanding of how potentially modifiable diet and lifestyle factors impact male fertility in general and infertility treatment outcomes in particular. Furthermore, the inability to foresee the relationships of male caffeine and alcohol intakes with clinical outcomes based on their relation with semen analysis results calls into question the common practice of utilizing semen quality as a clinical and research marker of male fertility, especially in the setting of ART.
Our results showing no relation between alcohol intake and semen quality are in agreement with the preponderance of the evidence on this topic to date. While alcoholism and heavy drinking have been shown to negatively impact semen parameters and male reproductive hormone profiles (Muthusami & Chinnaswamy, 2005), moderate drinking does not appear to affect semen quality. A 2011 meta-analysis concluded that there is no association between alcohol intake and semen parameters, apart from an association with lower ejaculate volume (Li, Lin, Li & Cao, 2011). Moreover, a recent study including 8344 healthy male volunteers also did not demonstrate a relationship between alcohol and semen quality (Jensen, Swan, Jorgensen, Toppari, Redmon, Punab, Drobnis, Haugen, Zilaitiene, Sparks, Irvine, Wang, Jouannet, Brazil, Paasch, Salzbrunn, Skakkebaek & Andersson, 2014). A subsequent study by the same group did observe a negative association between sperm concentration and alcohol intake, although the magnitude was small and most pronounced in men who typically drank more than 25 servings of alcohol per week (Jensen, Gottschau, Madsen, Andersson, Lassen, Skakkebaek, Swan, Priskorn, Juul & Jorgensen, 2014). Overall, the men included in our study were not heavy drinkers, with men in the top alcohol intake quartile consuming ≥19g of alcohol daily or the equivalent of ≥1.4 U.S. standard drinks/day. Compared to results from the National Health and Nutrition Examination Survey (NHANES) 2003–2006 (Guenther, 2010), men included in our study drank significantly less alcohol than men aged 21–64 in the general population (median intake=0.7 drinks/day versus 1.4 drinks/day for our population versus the general population, respectively). Therefore, the null relationship we observed between semen quality and alcohol intake was not unexpected.
In contrast to these results, men who drank more alcohol had higher IVF success rates per cycle. The relationship between alcohol and IVF outcomes was strengthened after controlling for potential confounders, particularly after adjusting for caffeine intake. Although there are very limited data on the relationship between male alcohol intake and pregnancy rates after IVF, there are some studies evaluating male alcohol intake and natural fertility. In agreement with our findings, Florack et al. reported a positive association between moderate male alcohol intake and fecundity (Florack, Zielhuis & Rolland, 1994). However, most of the studies addressing this question have found no relation between men’s moderate alcohol intake and fecundity (Hassan & Killick, 2004; Jensen, Hjollund, Henriksen, Scheike, Kolstad, Giwercman, Ernst, Bonde, Skakkebaek & Olsen, 1998; Olsen, Bolumar, Boldsen & Bisanti, 1997). There are also few studies evaluating the relationship between male alcohol intake and ART outcomes. Klonoff-Cohen et al. found a negative relationship between male alcohol consumption and probability of live birth after IVF or gamete intrafallopian transfer (GIFT), likely due to a positive relationship with miscarriage (Klonoff-Cohen, Lam-Kruglick & Gonzalez, 2003). They did not observe an association with semen parameters or probability of clinical pregnancy. The reason for the disparity between these results and ours could be related to differences in technologies utilized and the overall population. As ART success rates have improved over time with fewer embryos transferred per cycle, environmental factors may be playing a larger role in pregnancy outcomes. Furthermore, 38% of 221 couples in the study by Klonoff-Cohen et al. (2003) underwent GIFT, a procedure not currently performed at our center. According to the authors, the relationship between alcohol and pregnancy outcomes was stronger among GIFT procedures. Moreover, success rates of the sites utilized in the aforementioned study were between 11% and 30%, in contrast to the overall live birth rate of 41% in our study. In contrast to our findings, a study of 250 men who underwent ICSI cycles found that alcohol intake was negatively related to fertilization rate but unrelated to pregnancy outcome (Braga, et al., 2012). Clearly, further evaluation of the relation between male partner intake of alcohol and couple fecundity in natural and assisted reproduction is necessary.
Similar to our results with alcohol, we found no relation between male caffeine intake and semen parameters; a finding in line with those reported in a recent meta-analysis (Li, Lin, Li & Cao, 2011) and elsewhere (Jensen, et al., 2010). However, high caffeine intake was associated with a lower probability of clinical pregnancy and live birth per cycle, especially for men who consumed ≥272mg of caffeine daily. These results remained significant after controlling for potential confounders such as female partner caffeine consumption. The median caffeine intake of our population (161mg/day) was lower than that of the general male population in the US (211mg/day) (Fulgoni, et al., 2015). Although many studies report a null association between caffeine intake and male reproductive function, a few did observe a relationship between high caffeine intake in males and decreased natural fecundity (Florack, Zielhuis & Rolland, 1994; Jensen, et al., 1998). Data on male caffeine intake and IVF outcome are limited. Klonoff-Cohen et al. did not find an association between male caffeine and live birth after IVF, although similar to their previously described manuscript regarding alcohol, technologies and success rates were different in comparison to our current study (Klonoff-Cohen, Bleha & Lam-Kruglick, 2002). Braga et al. reported a negative relationship between caffeine intake and ICSI fertilization rate, but no relationship was seen with pregnancy outcomes (Braga, Halpern, Figueira Rde, Setti, Iaconelli & Borges, 2012).
Further studies are needed to elucidate the physiologic mechanisms involved in these associations, particularly in the absence of a relation with traditional semen parameters. Sperm DNA damage may play a role, as drinking > 3 cups of coffee per day (~ 300 mg/day of caffeine) has been previously described as a risk factor for sperm DNA damage, independent of age (Schmid, et al., 2007). Caffeine has also been reported to affect Sertoli cell metabolism in a dose-dependent manner (Dias, et al., 2014). A recent study in rats demonstrated changes in testicular cyto-architecture with higher doses of caffeine (Oluwole, et al., 2016). However, whether these changes could result in poorer ART outcomes is unknown.
Our findings suggest that a relationship between an environmental or dietary factor and semen quality cannot be interpreted as applying directly to fertility or ART outcomes. This underscores the fact that there are other aspects of sperm structure and function, such as its genome, epigenome, transcriptome, and membrane composition, which impact reproduction but are not clinically assessed (Casas & Vavouri, 2014; Rahman, et al., 2013). Along these lines, the observation that the association between caffeine and pregnancy outcome was most relevant for ICSI cycles may suggest that current sperm selection procedures fail to weed out sperm which have been negatively impacted by environmental factors in ways not visible on routine microscopy. Alternatively, the heterogeneity between ICSI and conventional insemination cycles could be due to confounding by an unmeasured indication for ICSI.
It is important to consider the strengths and limitations of this study. First, diet was assessed only once and misclassification of true intake over time may have occurred. However, the expected effect of this type of error would be to attenuate the observed relations. In this study, alcohol intake levels are based on self-reported drinking habits over the course of one year, as opposed to a shorter time period. Hence, it is not possible to make conclusions regarding the effect of acute alcohol consumption preceding ART on treatment outcomes. In addition, while we controlled for many possible male and female confounders, residual and unmeasured confounding cannot be excluded, as is the case in all observational studies. This problem may be more important for the observed association with alcohol intake, which was more sensitive to inclusion of potential confounders. Our findings may not be generalizable to couples trying to conceive naturally, although they may still apply to couples attempting conception through ART. Last, men who participate in the EARTH study may have different demographic characteristics and nutritional intakes as compared to those who choose not to participate. Although this raises issues surrounding the generalizability of our results, the live birth rates of women with male partners who chose to participate versus those of women with partners who chose not to participate in the study were similar (64% versus 60%, respectively, at time of last ART cycle, p=0.58). These results must be validated in other populations before any clinical recommendations can be made.
Strengths of our study include its prospective design, which limits the possibility of reverse causation, the use of a previously validated diet assessment questionnaire, and the ability to adjust for multiple demographic and lifestyle factors, both in male and female partners. The fact that we accounted for and cross-classified caffeine and alcohol intakes which can be correlated such as in our data, allowed us to assess the independent association of these intakes with clinical outcomes, even though their potential impacts are in opposite directions. Moreover, our results add to the extremely limited data on male alcohol and caffeine intake and ART outcomes.
In summary, habitual caffeine intake in males from couples undergoing fertility treatment was associated with a lower probability of achieving a live birth after ART, whereas alcohol intake was related to a higher probability of live birth. Neither alcohol nor caffeine was related to semen parameters. Given the paucity of data, it is important that these associations are further evaluated in large prospective cohort studies. In addition, due to the disconnect between the relations with semen quality and with clinical outcomes following ART, future studies should consider evaluating direct markers of (couple) fertility rather than solely relying on conventional semen quality parameters as a proxy for male fertility potential.
Supplementary Material
Supplemental figure 1 legend: Cross-classification of caffeine and alcohol and adjusted live birth probability. Caffeine intakes < and ≥ 209mg/day were considered low and high caffeine intakes, respectively, and alcohol intakes < and ≥ 12g/day were considered low and high alcohol intakes, respectively. All results are adjusted for age, race, BMI, smoking status, total calorie intake, dietary pattern, infertility diagnosis, and female caffeine intake, female alcohol intake, female age, female BMI.
Supplemental figure 2 legend. Male caffeine and alcohol intake and adjusted live birth probability stratified by insemination type. Results demonstrate the association between caffeine intake quartiles and probability of live birth, separately for conventional insemination and ICSI cycles. Caffeine intake quartiles 1 through 4 include caffeine intakes of <99mg/day, 99–208.9mg/day, 209–271.9mg/day, and ≥ 272mg/day, respectively. All results are adjusted for age, race, BMI, male alcohol intake, smoking status, total calorie intake, dietary pattern, infertility diagnosis, and female caffeine intake, female alcohol intake, female age, female BMI.
Acknowledgments
We acknowledge all members of the EARTH study team, specifically the Harvard T. H. Chan School of Public Health research nurses Jennifer B. Ford and Myra G. Keller, research staff Ramace Dadd and Patricia Morey, physicians and staff at Massachusetts General Hospital Fertility Center and a special thanks to all the study participants.
Study funding: The authors are supported by NIH grants ES009718, ES022955, ES000002, P30 DK046200 and T32 DK007703-16.
Support: Supported by National Institutes of Health grants ES009718, ES022955, ES000002, P30 DK046200 and T32 DK007703-16.
Footnotes
Conflict of interest: None of the authors have any conflict of interest related to this manuscript.
Declaration of Authors Roles
1) Study conception and design; 2) data acquisition; 3) data analysis; 4) interpretation of data; 5) drafting of manuscript; 6) critically revising manuscript; 7) final approval of manuscript.
Anatte E. Karmon, MD – 1, 2, 3, 4, 5, 6, 7
Thomas L. Toth, MD- 2, 6, 7
Yu-Han Chiu, MD, MPH- 3, 4, 6, 7
Audrey J. Gaskins, ScD- 4, 6, 7
Cigdem Tanrikut, MD- 2, 6, 7
Diane L. Wright, MD- 2, 6, 7
Russ Hauser, MD, ScD- 1, 4, 6, 7
Jorge E. Chavarro, MD, ScD- 1, 3, 4, 6, 7
References
- Adelusi B, al-Twaijiri MH, al-Meshari A, Kangave D, al-Nuaim LA, Younnus B. Correlation of smoking and coffee drinking with sperm progressive motility in infertile males. African journal of medicine and medical sciences. 1998;27:47–50. [PubMed] [Google Scholar]
- Braga DP, Halpern G, Figueira Rde C, Setti AS, Iaconelli A, Jr, Borges E., Jr Food intake and social habits in male patients and its relationship to intracytoplasmic sperm injection outcomes. Fertility and sterility. 2012;97:53–59. doi: 10.1016/j.fertnstert.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Casas E, Vavouri T. Sperm epigenomics: challenges and opportunities. Frontiers in genetics. 2014;5:330. doi: 10.3389/fgene.2014.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention A S f R M, Society for Assisted Reproductive Technology. 2012 Assisted Reproductive Technology Fertility Clinic Success Rates Report 2014 [Google Scholar]
- Chavarro JE, Ehrlich S, Colaci DS, Wright DL, Toth TL, Petrozza JC, Hauser R. Body mass index and short-term weight change in relation to treatment outcomes in women undergoing assisted reproduction. Fertil Steril. 2012;98:109–116. doi: 10.1016/j.fertnstert.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaci DS, Afeiche M, Gaskins AJ, Wright DL, Toth TL, Tanrikut C, Hauser R, Chavarro JE. Men’s body mass index in relation to embryo quality and clinical outcomes in couples undergoing in vitro fertilization. Fertil Steril. 2012;98:1193–1199 e1191. doi: 10.1016/j.fertnstert.2012.07.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis KM, Savitz DA, Arbuckle TE. Effects of cigarette smoking, caffeine consumption, and alcohol intake on fecundability. American journal of epidemiology. 1997;146:32–41. doi: 10.1093/oxfordjournals.aje.a009189. [DOI] [PubMed] [Google Scholar]
- Dias TR, Alves MG, Bernardino RL, Martins AD, Moreira AC, Silva J, Barros A, Sousa M, Silva BM, Oliveira PF. Dose-dependent effects of caffeine in human Sertoli cells metabolism and oxidative profile: Relevance for male fertility. Toxicology. 2014;328C:12–20. doi: 10.1016/j.tox.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. Journal of the American Dietetic Association. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- Florack EI, Zielhuis GA, Rolland R. Cigarette smoking, alcohol consumption, and caffeine intake and fecundability. Preventive medicine. 1994;23:175–180. doi: 10.1006/pmed.1994.1024. [DOI] [PubMed] [Google Scholar]
- Fulgoni VL, 3rd, Keast DR, Lieberman HR. Trends in intake and sources of caffeine in the diets of US adults: 2001–2010. The American journal of clinical nutrition. 2015;101:1081–1087. doi: 10.3945/ajcn.113.080077. [DOI] [PubMed] [Google Scholar]
- Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Human reproduction. 2012;27:2899–2907. doi: 10.1093/humrep/des298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther PM, Bowman SA, Goldman JD. Alcoholic Beverage Consumption by Adults 21 years and Over in the Unites States: Results from the National Health and Nutrition Examination Survey, 2003–2006: Technical Report. Center for Nutrition Policy and Promotion, and Agriculture Research Service, U.S. Department of Agriculture; 2010. [Google Scholar]
- Hassan MA, Killick SR. Negative lifestyle is associated with a significant reduction in fecundity. Fertility and sterility. 2004;81:384–392. doi: 10.1016/j.fertnstert.2003.06.027. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Gottschau M, Madsen JO, Andersson AM, Lassen TH, Skakkebaek NE, Swan SH, Priskorn L, Juul A, Jorgensen N. Habitual alcohol consumption associated with reduced semen quality and changes in reproductive hormones; a cross-sectional study among 1221 young Danish men. BMJ Open. 2014;4:e005462. doi: 10.1136/bmjopen-2014-005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TK, Henriksen TB, Hjollund NH, Scheike T, Kolstad H, Giwercman A, Ernst E, Bonde JP, Skakkebaek NE, Olsen J. Caffeine intake and fecundability: a follow-up study among 430 Danish couples planning their first pregnancy. Reproductive toxicology. 1998;12:289–295. doi: 10.1016/s0890-6238(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Hjollund NH, Henriksen TB, Scheike T, Kolstad H, Giwercman A, Ernst E, Bonde JP, Skakkebaek NE, Olsen J. Does moderate alcohol consumption affect fertility? Follow up study among couples planning first pregnancy. Bmj. 1998;317:505–510. doi: 10.1136/bmj.317.7157.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TK, Swan S, Jorgensen N, Toppari J, Redmon B, Punab M, Drobnis EZ, Haugen TB, Zilaitiene B, Sparks AE, Irvine DS, Wang C, Jouannet P, Brazil C, Paasch U, Salzbrunn A, Skakkebaek NE, Andersson AM. Alcohol and male reproductive health: a cross-sectional study of 8344 healthy men from Europe and the USA. Human reproduction. 2014;29:1801–1809. doi: 10.1093/humrep/deu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TK, Swan SH, Skakkebaek NE, Rasmussen S, Jorgensen N. Caffeine intake and semen quality in a population of 2,554 young Danish men. Am J Epidemiol. 2010;171:883–891. doi: 10.1093/aje/kwq007. [DOI] [PubMed] [Google Scholar]
- Klonoff-Cohen H, Bleha J, Lam-Kruglick P. A prospective study of the effects of female and male caffeine consumption on the reproductive endpoints of IVF and gamete intra-Fallopian transfer. Human reproduction. 2002;17:1746–1754. doi: 10.1093/humrep/17.7.1746. [DOI] [PubMed] [Google Scholar]
- Klonoff-Cohen H, Lam-Kruglick P, Gonzalez C. Effects of maternal and paternal alcohol consumption on the success rates of in vitro fertilization and gamete intrafallopian transfer. Fertility and sterility. 2003;79:330–339. doi: 10.1016/s0015-0282(02)04582-x. [DOI] [PubMed] [Google Scholar]
- Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertility and sterility. 1988;49:112–117. doi: 10.1016/s0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- Lewis SE. Is sperm evaluation useful in predicting human fertility? Reproduction. 2007;134:31–40. doi: 10.1530/REP-07-0152. [DOI] [PubMed] [Google Scholar]
- Li Y, Lin H, Li Y, Cao J. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertility and sterility. 2011;95:116–123. doi: 10.1016/j.fertnstert.2010.06.031. [DOI] [PubMed] [Google Scholar]
- Muthusami KR, Chinnaswamy P. Effect of chronic alcoholism on male fertility hormones and semen quality. Fertility and sterility. 2005;84:919–924. doi: 10.1016/j.fertnstert.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Nagy ZP, Liu J, Joris H, Verheyen G, Tournaye H, Camus M, Derde MC, Devroey P, Van Steirteghem AC. The result of intracytoplasmic sperm injection is not related to any of the three basic sperm parameters. Human reproduction. 1995;10:1123–1129. doi: 10.1093/oxfordjournals.humrep.a136104. [DOI] [PubMed] [Google Scholar]
- Olsen J, Bolumar F, Boldsen J, Bisanti L. Does moderate alcohol intake reduce fecundability? A European multicenter study on infertility and subfecundity. European Study Group on Infertility and Subfecundity. Alcoholism, clinical and experimental research. 1997;21:206–212. [PubMed] [Google Scholar]
- Oluwole OF, Salami SA, Ogunwole E, Raji Y. Implication of caffeine consumption and recovery on the reproductive functions of adult male Wistar rats. J Basic Clin Physiol Pharmacol. 2016;27:483–491. doi: 10.1515/jbcpp-2015-0134. [DOI] [PubMed] [Google Scholar]
- Rahman MS, Lee JS, Kwon WS, Pang MG. Sperm proteomics: road to male fertility and contraception. International journal of endocrinology. 2013;2013:360986. doi: 10.1155/2013/360986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. American journal of epidemiology. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- Schmid TE, Eskenazi B, Baumgartner A, Marchetti F, Young S, Weldon R, Anderson D, Wyrobek AJ. The effects of male age on sperm DNA damage in healthy non-smokers. Human reproduction. 2007;22:180–187. doi: 10.1093/humrep/del338. [DOI] [PubMed] [Google Scholar]
- Sobreiro BP, Lucon AM, Pasqualotto FF, Hallak J, Athayde KS, Arap S. Semen analysis in fertile patients undergoing vasectomy: reference values and variations according to age, length of sexual abstinence, seasonality, smoking habits and caffeine intake. Sao Paulo medical journal = Revista paulista de medicina. 2005;123:161–166. doi: 10.1590/S1516-31802005000400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada S, Townend J, Campbell D, Murdoch L, Mathers E, Bhattacharya S. Relationship between semen parameters and spontaneous pregnancy. Fertility and sterility. 2010;94:624–630. doi: 10.1016/j.fertnstert.2009.02.085. [DOI] [PubMed] [Google Scholar]
- Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, Lopes P, Tabaste JM, Spira A. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions 1988–1989. Human reproduction. 1991;6:811–816. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture & Agriculural Research Service. USDA National Nutrient Database for Standard Reference, Release 21. 2008 URL: http://www.ars.usda.gov/ba/bhnrc/ndl.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1 legend: Cross-classification of caffeine and alcohol and adjusted live birth probability. Caffeine intakes < and ≥ 209mg/day were considered low and high caffeine intakes, respectively, and alcohol intakes < and ≥ 12g/day were considered low and high alcohol intakes, respectively. All results are adjusted for age, race, BMI, smoking status, total calorie intake, dietary pattern, infertility diagnosis, and female caffeine intake, female alcohol intake, female age, female BMI.
Supplemental figure 2 legend. Male caffeine and alcohol intake and adjusted live birth probability stratified by insemination type. Results demonstrate the association between caffeine intake quartiles and probability of live birth, separately for conventional insemination and ICSI cycles. Caffeine intake quartiles 1 through 4 include caffeine intakes of <99mg/day, 99–208.9mg/day, 209–271.9mg/day, and ≥ 272mg/day, respectively. All results are adjusted for age, race, BMI, male alcohol intake, smoking status, total calorie intake, dietary pattern, infertility diagnosis, and female caffeine intake, female alcohol intake, female age, female BMI.


