Abstract
Aim
To investigate the association between changes in oestradiol and follicle-stimulating hormone levels during the menopausal transition and incident diabetes.
Methods
We followed 1407 pre-menopausal women, aged 42–52 years at baseline, who experienced natural menopause, from baseline to the 12th annual follow-up visit in the Study of Women’s Health Across the Nation (SWAN). Diabetes was defined based on fasting glucose level, medication use and self-report of physician diagnosis. Cox proportional hazards regression was used to evaluate the associations of incident diabetes with three components of the rate of change in hormones: the intercept (pre-menopausal levels) and two piece-wise slopes representing change during the early and late transition, respectively.
Results
During 15 years of follow-up, 132 women developed diabetes. After adjusting for potential confounders, a higher oestradiol intercept, but not its rate of change, was borderline significantly associated with lower risk of diabetes [hazard ratio for an interquartile range increase (75.2 pmol/L) 0.53, 95% CI 0.27–1.06]. For follicle-stimulating hormone, a greater rate of increase in the early transition, but not the intercept or late transition, was significantly associated with lower risk of diabetes [hazard ratio for an interquartile range increase (5.9 IU/L/year) 0.31, 95% CI 0.10–0.94].
Conclusions
Lower pre-menopausal oestradiol levels and a slower rate of follicle-stimulating hormone change during the early transition were associated with higher risk of developing diabetes. Given that obesity plays an important role in diabetes risk and in the levels and changes in oestradiol and follicle-stimulating hormone over the menopausal transition, weight control in earlier mid-life is important to prevent future diabetes development.
Introduction
Endogenous oestrogens may reduce the risk of developing diabetes in pre-menopausal women by lowering body fat and promoting insulin sensitivity [1]. Studies of oral contraceptives and menopausal hormone therapy support the notion that oestrogens play a preventive role in diabetes risk [2–4]. As women transition through the menopause, oestradiol levels drop considerably. Some cross-sectional studies have shown that oestradiol levels are higher in post-menopausal women with diabetes [5–7], but others report no association [8,9]. Only a few prospective studies have examined the association between oestradiol and incident diabetes. Two studies in post-menopausal women (the Women’s Health Study [10] and the Multi-Ethnic Study of Atherosclerosis [11]) reported a significant association between higher levels of oestradiol and higher risk of diabetes, whereas the Rancho Bernardo Study found a non-significant positive association between oestradiol (both total and bioavailable levels) and incident diabetes in older women (aged 55–89 years) [12]; however, a recent report from the Diabetes Prevention Program failed to find an association between oestradiol and incident diabetes in either pre- or post-menopausal women [13].
Prospective studies to date have been limited by assessing only hormone levels measured at baseline. Women’s hormone levels, especially oestradiol and follicle-stimulating hormone (FSH), change dramatically during the menopausal transition. Oestradiol levels remain stable until ~ 2 years before the final menstrual period, then decline rapidly until ~2 years after the final menstrual period, and then stabilize once again; FSH gradually increases beginning ~7 years before the final menstrual period and then accelerates from 2 years before the final menstrual period until 1 year after the final menstrual period [14]. To date, however, no study has examined how change in sex steroid hormone levels during the menopausal transition influences diabetes risk in mid-life. The Study of Women’s Health Across the Nation (SWAN), a multi-site, multi-ethnic longitudinal study of the natural history of the menopausal transition includes annual or biannual hormone measurements over a 15-year period, as well as longitinal follow-up for incident diabetes. These data allow us to address the aforementioned limitation.
In the present study, we investigated the association between change in serum sex steroid hormone levels during the menopausal transition including the late transition, the stage during which the most dramatic changes in the endogenous endocrine environment occur, and incident diabetes. We focused on oestradiol and FSH because our previous studies in SWAN have shown that testosterone, dehydroepiandrosterone and sex hormone-binding globulin are relatively stable over the menopausal transition stages [15,16].
Methods
Study population
In the present analysis we used data from SWAN, details of which have been reported elsewhere [17]. Between 1996 and 1997, 3302 women were enrolled into the longitudinal cohort at seven study sites (Boston, MA; Chicago, IL; Detroit, MI; Los Angeles, CA; Newark, NJ; Oakland, CA; and Pittsburgh, PA). Each site recruited representative samples of at least 450 women: white women and women from one specified minority group (black in Boston, Chicago, Detroit, and Pittsburgh; Chinese in Oakland; Japanese in Los Angeles; Hispanic in Newark). Eligibility criteria included: age 42–52 years; having an intact uterus; having at least one menstrual period and not taking hormone medications (e.g. birth control pills, oestrogen or progesterone preparations) in the 3 months before the baseline survey; and having self-identified with the site’s designated race/ethnic groups. Institutional review board approval was obtained at each study site, and all participants provided signed informed consent at each study visit.
Menopausal status and date of the final menstrual period were determined from questions about menstrual bleeding on the annual visit questionnaires. Women who reported bleeding in the 3 months before an annual visit with no decrease in the predictability of menses in the year before the visit were categorized as pre-menopausal. Women who reported no menses for ≥12 months were classified as post-menopausal. The final menstrual period was defined as the first day of the bleeding period which was followed by at least 12 months of amenorrhoea [18,19].
Among the 3302 participants in SWAN, 1555 had an observed natural final menstrual period not masked by hormone use between the enrolment visit and the 12th follow-up visit (2009–2010) and thus were potentially eligible for this analysis. We excluded women who were ascertained to have diabetes at baseline (n=87); developed diabetes in the early peri-menopause (n=23) before marked changes in hormone levels; were pregnant during the follow-up (n=2); or were missing information on oestradiol and FSH (n=1). We further excluded the 35 women who were found to be in late peri-menopause (defined as a self-report of >3 but <12 months of amenorrhoea) at baseline. Thus, the final eligible analytical sample included 1407 women.
Outcome ascertainment
Serum glucose levels were measured at all clinic visits except visits 9 and 10 using a hexokinase-coupled reaction (Boehringer Mannheim Diagnostics, Indianapolis, IN, USA). Diabetes was ascertained at each visit if a participant met at least one of the following: (1) a fasting serum glucose level ≥7 mmol/L; (2) current use of insulin or oral hypoglycaemic medication; and (3) self-report of physician diagnosis of diabetes. Almost all of the diabetes cases in this life stage can be assumed to be Type 2 diabetes.
Sex hormone assessment
In SWAN, oestradiol and FSH were assayed from serum samples obtained at each follow-up visit. The oestradiol assay was conducted in duplicate using the automated Ciba Corning Diagnostics ACS:180 analyzer (Bayer Diagnostics Corp., Norwood, MA, USA). The average inter- and intra-assay coefficients of oestradiol variation were 10.6% and 6.4%, respectively, over the oestradiol assay range, and the lower limit of detection was 3.67 pmol/L. A single FSH assay was conducted using a two-site chemiluminometric immunoassay. Inter- and intra-assay coefficients of FSH variation were 12.0% and 6.0%, respectively, and the lower limit of detection was 1.1 IU/L.
Other covariates
Sociodemographic factors including age, race/ethnicity and educational attainment (less than high school, high school, some college, college or postgraduate) were obtained at the SWAN baseline examination. Self-reported smoking status (never, former, current) was collected at each examination. Height and weight were measured in women without shoes and in light clothing using a stadiometer and calibrated scale. BMI was calculated as weight in kilograms divided by height in meters squared. Obesity was defined as BMI ≥ 30 kg/m2.
Statistical methods
Variable distributions were examined for normality, the presence of non-plausible outliers and changing variability over time. A two-stage approach was used to evaluate the associations of changes in hormones (oestradiol and FSH) with incident diabetes. In stage 1, among the 1407 women in the analytical sample, all of whom had an observable natural final menstrual period, 13 122 hormone observations were collected over the 12-year follow-up period (mean of 9.3 observations/woman) for data analyses. To describe the trajectories of mean serum hormone levels over time in relation to the time before and after the final menstrual period, a multiple-step process was used to organize these data into trajectories of mean hormone levels and rates of change profiles in relation to the final menstrual period [20,21]. Piece-wise linear mixed effect modelling was used to estimate segmented rate of changes in hormones for three time periods based on previous modelling of the hormone trajectories [20,21], > 2 years before the final menstrual period, from 2 years before to 2 years after the final menstrual period, and > 2 years after the final menstrual period. The rate of changes in hormones corresponding to the first two time periods characterizes the hormonal changes occurring in pre-/early transition stage, and the late transition to early post-menopausal stage. Thus we captured the effect of longitudinal change in hormone levels using trajectory models for each hormone that constituted their intercept and two piece-wise slopes. These subject-specific annualized slopes were used as independent variables in the next stage of analysis.
In stage 2, Cox proportional hazards regression analysis was used to model the association of the rate of change in hormone levels with the development of diabetes. Person-years were computed from baseline to the date of the visit at which incident diabetes was ascertained, loss to follow-up or the date of the 12th visit, whichever came first. Each hormone variable has three components: the intercept, which indicates baseline levels, and two piece-wise coefficients for time, which represent the rate of change during the early and late transitions, respectively. These three components were fit as continuous variables into the survival models. Hazard ratios and 95% CIs for the incident diabetes were calculated. Potential confounding factors considered include baseline age, baseline BMI, baseline smoking status, race/ethnicity, education and study site. Model goodness of fits were assessed using Akaike information criteria (AIC). In the final model, study site was not included because it is collinear with race/ethnicity and the results with and without study site remain unchanged.
We also evaluated whether the effect of hormones on diabetes risk differed according to baseline obesity status by including interaction terms between each component of the rate of change and obesity. As the analytical design evaluated the effect of rate of change in sex hormone levels beginning from the baseline examination, we were able to evaluate effect modification by baseline obesity status but not by weight gain during the menopausal transition. Analyses were implemented in sas version 9.3 (SAS Institute, Cary, NC, USA). A two-sided P value <0.05 was taken to indicate statistical signficance.
Results
The mean (sd) age of the participants at baseline was 46.4 (2.7) years (Table 1). On average, women experienced the final menstrual period at 51.8 (2.7) years of age. During a median of 11 years of follow-up, 132 of 1407 women developed diabetes in late peri-menopause or post-menopause (9.4%; incidence of 9 per 1000 person-years). Baseline age and age at final menstrual period were not different between women who did and did not develop diabetes; however, women who developed diabetes during follow-up had lower baseline oestradiol levels than those who did not (median oestradiol 160.4 vs 213.6 pmol/L; median FSH 16.2 vs. 16.5 IU/L). Women with incident diabetes were also more likely to be black or Hispanic, early peri-menopausal, obese and current smokers at baseline than those without incident diabetes.
Table 1.
Baseline characteristics of 1407 women in the Study of Women’s Health Across the Nation with an observed natural final menstrual period and without diabetes at baseline
| All | Incident diabetes | ||
|---|---|---|---|
| No | Yes | ||
| Number of women | 1407 | 1275 | 132 |
| Mean ± sd age, years | 46.4 ± 2.7 | 46.4 ± 2.7 | 46.8 ± 2.8 |
| Mean ± sd age at FMP, years | 51.8 ± 2.7 | 51.8 ± 2.8 | 51.8 ± 2.5 |
| Menopausal status, %* | |||
| Pre-menopause | 57.3 | 58.1 | 49.2 |
| Early peri-menopause | 42.7 | 41.9 | 50.8 |
| Race/ethnicity‡, % | |||
| White | 43.6 | 45.0 | 29.6 |
| Black | 28.8 | 26.6 | 50.0 |
| Hispanic | 5.9 | 5.5 | 9.9 |
| Chinese | 10.3 | 11.0 | 3.8 |
| Japanese | 11.4 | 11.9 | 6.8 |
| Education‡, % | |||
| < High school | 6.7 | 5.8 | 15.4 |
| High school | 16.6 | 15.9 | 23.1 |
| Some college | 31.3 | 31.1 | 33.1 |
| 4-year college | 21.9 | 22.6 | 15.4 |
| >4-year college | 23.5 | 24.6 | 13.1 |
| Site‡, % | |||
| Detroit, MI | 16.4 | 14.9 | 30.3 |
| Boston, MA | 15.2 | 15.2 | 15.2 |
| Chicago, IL | 14.9 | 14.6 | 17.4 |
| Oakland, CA | 16.2 | 17.2 | 6.8 |
| Los Angeles, CA | 16.8 | 17.5 | 9.9 |
| Newark, NJ | 8.8 | 8.6 | 10.6 |
| Pittsburgh, PA | 11.8 | 12.0 | 9.9 |
| Smoking status†, % | |||
| Never | 59.8 | 60.5 | 53.0 |
| Former | 23.7 | 24.1 | 20.5 |
| Current | 16.4 | 15.4 | 26.5 |
| Obesity, % | 28.5 | 25.3 | 59.1 |
| Sex hormone, median (Q1, Q3) | |||
| Oestradiol*, pmol/L | 211.4 (121.1, 322.2) | 213.6 (124.4, 324.4) | 160.4 (113.8, 274.9) |
| FSH, IU/L | 16.5 (11.4, 27.6) | 16.6 (11.3, 27.6) | 16.2 (11.6, 27.1) |
P<0.05,
P<0.01,
P<0.0001 based on chi-squared test for categorical variables and Wilcoxon rank-sum test for continuous variables for comparing those with and without incident diabetes.
In age-adjusted models (Table 2, Model 1), the estimated oestradiol intercept (i.e. baseline – pre-menopausal – oestradiol) was significantly and inversely associated with incident diabetes, but the rate of change in oestradiol levels during the early and late menopausal transition were not associated with the risk of diabetes. Adjustment for BMI attenuated the associations (Table 2, Model 2). In the fully adjusted model with age, BMI, smoking status, education and race/ethnicity, each interquartile range increase (comparing the 25th percentile with the 75th percentile) in the oestradiol intercept (75.2 pmol/L) was borderline significantly associated with lower risk of incident diabetes (hazard ratio 0.53, 95% CI 0.27–1.06; P = 0.07). For FSH, a greater rate of increase > 2 years before the final menstrual period (early transition) was significantly associated with lower risk of incident diabetes, but the baseline (pre-menopausal) FSH level or the rate of change in FSH between 2 years before and 2 years after the final menstrual period (late transition) were not associated with incident diabetes. The fully adjusted hazard ratio of diabetes for an interquartile range increase (5.9 IU/L/year) in early transition FSH rate of change was 0.31 (95% CI 0.10–0.94; P = 0.04). We also compared the goodness-of-fits between the model with only covariates included in Model 3 (AIC=1869.73) and the model with covariates and each hormone (AIC=1693.77 for oestradiol; AIC=1683.01 for FSH). Both oestradiol and FSH significantly improved the model goodness-of-fit (P<0.0001).
Table 2.
Regression coefficients from Cox models and hazard ratios for incident diabetes for an interquartile range increase* in variables of the longitudinal change in hormone levels among 1407 women, Study of Women’s Health Across the Nation
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| β (se) | HR (95% CI) | β (se) | HR (95% CI) | β (se) | HR (95% CI) | |
| Oestradiol | ||||||
| Intercept (pre-menopausal) | −0.0316 (0.0144) | 0.52 (0.29, 0.93)‡ | −0.0218 (0.0160) | 0.64 (0.34, 1.22) | −0.0309 (0.0171) | 0.53 (0.27, 1.06)† |
| Slope for early transition | 0.0377 (0.0659) | 1.07 (0.84, 1.37) | 0.0178 (0.0699) | 1.03 (0.80, 1.34) | 0.0539 (0.0764) | 1.11 (0.83, 1.47) |
| Slope for late transition | −0.0321 (0.0184) | 0.61 (0.35, 1.06) | −0.0196 (0.0213) | 0.74 (0.39, 1.41) | −0.0311 (0.0223) | 0.62 (0.32, 1.21) |
| FSH | ||||||
| Intercept (pre-menopausal) | 0.0054 (0.0139) | 1.20 (0.48, 3.00) | 0.0117 (0.0141) | 1.48 (0.58, 3.77) | 0.0121 (0.0141) | 1.51 (0.59, 3.81) |
| Slope for early transition | −0.2360 (0.0910) | 0.25 (0.09, 0.71)¶ | −0.1787 (0.0904) | 0.35 (0.12, 0.99)‡ | −0.1953 (0.0947) | 0.31 (0.10, 0.94)‡ |
| Slope for late transition | 0.0261 (0.0276) | 1.33 (0.74, 2.39) | 0.0134 (0.0265) | 1.16 (0.66, 2.03) | 0.0257 (0.0288) | 1.32 (0.72, 2.44) |
FSH, follicle-stimulating hormone; HR, hazard ratio.
Model 1: adjusted for baseline age; Model 2: Model 1 + baseline BMI; Model 3: Model 2 + race/ethnicity, education and smoking status.
Interquartile ranges for the oestradiol model: 75.2 pmol/L for the intercept; 7.0 pmol/L/year for the early transition slope; 56.5 pmol/L/year for the late transition slope. IQRs for the FSH model: 33.7 IU/L for the intercept; 5.9 IU/L/year for the early transition slope; 10.8 IU/L/year for the late transition slope.
P<0.1,
P<0.05,
P<0.01.
In a model that included both oestradiol and FSH, the associations remained unchanged (data not shown). We also evaluated potential effect modification by baseline obesity status but the associations were similar, independently of baseline obesity status (data not shown).
Discussion
This is the first study to prospectively examine the associations between levels of and change in oestradiol and FSH over the menopausal transition and incident diabetes in a cohort of women. We observed that independent of age, women with higher endogenous oestradiol levels at baseline (i.e. 7 years before the final menstrual period) in the pre-menopause and early peri-menopause had a lower risk of developing diabetes in mid-life as they transitioned through the menopause. The rate of change in oestradiol during the menopausal transition did not appear to be associated with diabetes risk. By contrast, a steeper increase in FSH from ~7 to 2 years before the final menstrual period (i.e. during the early menopausal transition) was associated with a lower risk of developing diabetes. Neither baseline FSH levels nor change in FSH in the later menopausal transition were associated with incident diabetes. These findings suggest that pre-menopausal levels of oestradiol rather than the rate of change during the menopausal transition, and the rate of FSH change in the early stage of the menopausal transition rather than pre-menopausal levels or the rate of change in late perimenopause, are associated with diabetes risk during mid-life.
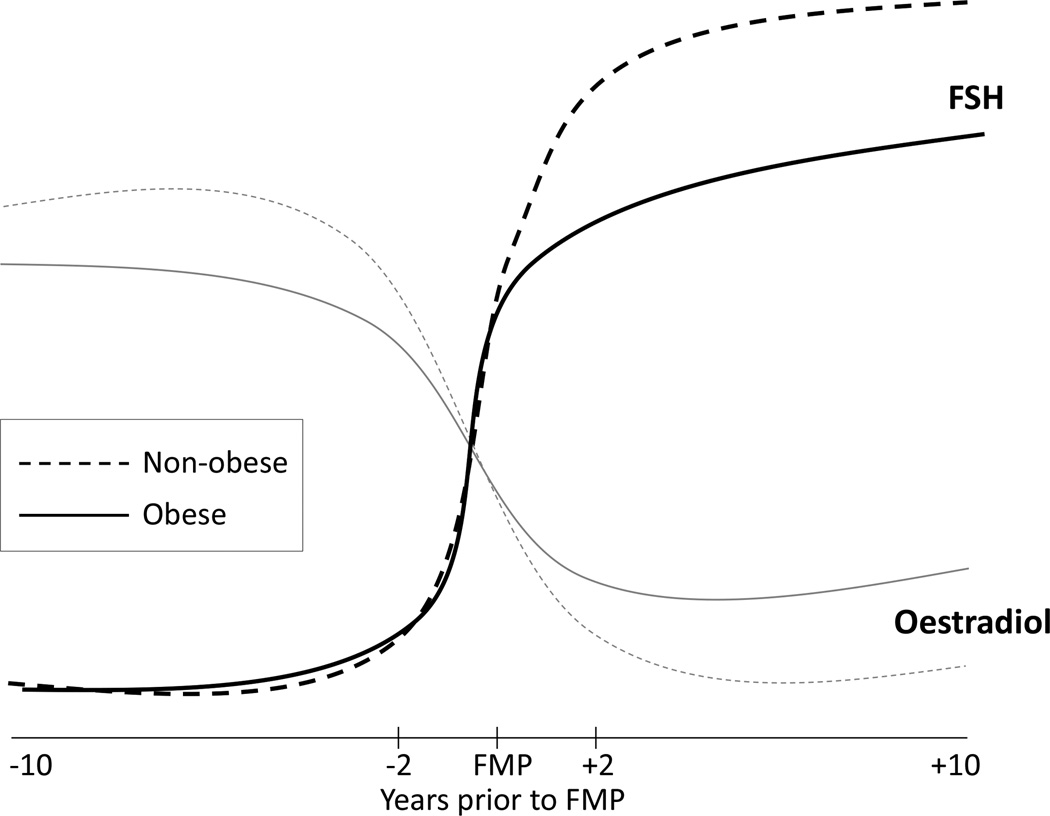
The observed association between pre-menopausal oestradiol levels and incident diabetes may be partially explained by different patterns of oestradiol by the presence of obesity. Randolph et al. [14] reported that obese women compared with non-obese women had lower pre-menopausal oestradiol levels but higher post-menopausal oestradiol levels in SWAN (Fig. 1). Obesity has a direct inhibitory effect on the ovarian oestradiol production in pre-menopausal women [22,23]. Adipose tissue is an important source of oestrogens by aromatization of androgens after menopause [24,25]. Such body fat distribution changes as well as increased proinflammatory cytokines during the menopausal transition have been associated with glucose homeostasis [26,27]. Slower oestradiol change in obese women during the menopausal transition may in part be attributable to this production of oestrogens in adipose tissue [14] and may offset the oestradiol effect on diabetes risk. This may be why we found a significant association of diabetes risk with pre-menopausal oestradiol levels but not the rate of oestradiol change during the menopausal transition. Despite a post-menopausal shift of oestrogen production from the ovary to adipose tissue, as endogenous oestradiol levels substantially decline through the menopausal transition, obesity-related insulin resistance may play a larger role in diabetes risk.
FIGURE 1.
Schematic trajectories of oestradiol and follicle stimulating hormone (FSH) around the final menstrual period (FMP) by obesity status.
Our finding of no association between the rates of change in oestradiol during early and late transition and diabetes risk does not necessarily mean that oestradiol has no impact on diabetes risk in post-menopausal women because previous studies in post-menopausal women observed that higher oestradiol levels were associated with increased risk of diabetes even after adjusting for an indicator of adiposity (e.g. BMI) [10,11]. In a nested case–control study of 359 incident diabetes cases and 359 age-, race-, duration of follow-up- and fasting status-matched controls among post-menopausal women in the Women’s Health Study, Ding et al. [10] observed adjusted relative risks of diabetes comparing the highest with the lowest quintile of 12.6 (95% CI 2.8–56.3) for total oestradiol and 13.1 (95% CI 4.2–40.8) for free oestradiol [10]. In a prospective cohort study with 1612 diabetes-free post-menopausal women in the Multi-Ethnic Study of Atherosclerosis, the highest quartile of oestradiol had an adjusted hazard ratio of 1.92 (95% CI 1.10–3.35) compared with the lowest quartile [11]. A relatively small study with 233 women aged 55–85 years in the Rancho Bernardo Study reported that women in the top quartile of total oestradiol and bioavailable oestradiol compared with the bottom three quartiles had odds ratios of incident diabetes of 1.3 (95% CI 0.4–4.6) and 1.9 (95% CI 0.7–5.3), respectively [12].
Obesity-related patterns of relative change in FSH during the menopausal transition may also explain the observed association between FSH change and incident diabetes. Pre-menopausal FSH levels do not seem to differ between obese and non-obese women but obese women have a markedly slower rate of FSH increase during the late transition and have lower FSH levels after menopause (Fig. 1) [14,23]. In the present study, risk of developing diabetes was associated with the rate of FSH change during the early transition, but not during the late transition. This finding suggests that, although the obesity–FSH trajectory association indicates the period from late transition to early post-menopause is a critical window of obesity-related difference in FSH levels, diabetes risk may begin earlier. Underlying biological mechanisms are unclear as roles of FSH in glucose metabolism and diabetes risk are poorly understood. Epidemiological evidence is also scant. A recent study conducted in East China in 1610 post-menopausal women not using hormone therapy (aged 55–89 years) reported that FSH was significantly inversely associated with fasting glucose and HbA1c [28]. Women in the lowest quartile of FSH compared with the highest quartile had a threefold greater odds of diabetes (95% CI 1.10–8.31) in the fully adjusted model, including measures of adiposity and insulin resistance and oestradiol [28]. It is difficult to compare our findings directly with those of the previous studies on FSH and oestradiol and diabetes because none of these studies examined pre-menopausal oestradiol and FSH levels or changes in oestradiol and FSH during the menopausal transition. Further longitudinal studies need to be conducted to replicate our findings.
Although cigarette smoking may also play a role in diabetes risk, the observed associations appear to be independent of cigarette smoking. Cigarette smoking can influence ovarian function and reduce endogenous oestrogen production by accelerating regression of follicles in the ovary and blocking aromatase [29]; however, Randolph et al. [14] found no difference by smoking status in trajectories of oestradiol and FSH across the menopausal transition in SWAN. The association of pre-menopausal oestradiol level or change in FSH during the early menopausal transition with incident diabetes remained unchanged after adjustment for cigarette smoking. In the present analysis, cigarette smoking was significantly associated with incident diabetes, as suggested in a recent meta-analysis [30].
The present study did not observe significant effect modification by baseline obesity. Given our research question, we were unable to test if weight gain during the menopausal transition (i.e. time-varying obesity status) modified the association between changes in oestradiol or FSH and incident diabetes risk; therefore, the present finding of no effect modification by baseline obesity does not necessarily imply that weight gain during the menopausal transition does not confer susceptibility to sex-hormone-related diabetes risk. A more sophisticated model, such as a marginal structural model, may be useful to better understand the role of weight gain in the association between sex hormones and diabetes.
The present study has numerous strengths. Annualized measures of sex steroid hormones for up to 15 years allow us to separate out the effect of baseline levels vs rate of change in hormone levels during the menopausal transition. Repeated measures of sex hormones account for within-individual variations and thus minimize a potential exposure measurement error attributable to a single cross-sectional measurement. The study design enabled us to identify an important window of susceptibility to diabetes risk related to endogenous hormone levels that may inform appropriate timing for clinical screening and intervention. The ethnically diverse population in the SWAN cohort increases the generalizability of our findings. This paper also has some limitations. Measurement of diabetes onset at visits 9 and 10 was based only on medication use and self-report. Thus, some cases ascertained at visit 12 may have had an earlier onset; however, this delay in ascertainment would only have affected our results if cases were misclassified as being in late peri-menopause when they were in fact in early peri-menopause. As <15% of the participants were still pre- or early peri-menopausal at visit 9, such misclassification would be minimal. In stage 1 where the hormone trajectory models were estimated, we did not completely restrict hormone data only before the diabetes onset. This may have biased our findings in stage 2 if diabetes influenced the rates of change in sex hormones across the menopausal transition. The impact of this would be minimal, however, because only 1.7% of the hormone data were gathered after the diabetes onset. We also cannot exclude the possibility of unmeasured or residual confounding.
In conclusion, in the present study we found that lower pre-menopausal oestradiol levels and a slower rate of FSH increase during the early transition were associated with a higher risk of developing diabetes as women transition through the menopause, independently of age and obesity. Given that obesity and excess fat play an important role in not only diabetes risk itself but the changes in oestradiol and FSH over the menopausal transition, these findings provide support for the relevance of an important strategy for preventive intervention: weight control in earlier mid-life is important to prevent future diabetes development. These results suggest the importance of monitoring for diabetes during the menopausal transition as well as the importance of adopting healthy diets and increasing exercise during this life stage.
What’s new?
This is the first study to examine prospectively the associations between levels of and change in oestradiol and follicle-stimulating hormone (FSH) during the menopausal transition and incident diabetes in a cohort of women.
The study shows that, independent of age and other important risk factors of diabetes, women with lower pre-menopausal oestradiol levels and a slower rate of FSH change during the early menopause transition had a higher risk of developing diabetes.
Given that obesity plays an important role in diabetes risk as well as in the levels and changes in oestradiol and FSH over the menopausal transition, weight control in earlier mid-life is important to prevent future diabetes development.
Acknowledgments
We thank the study staff at each site and all the women who participated in SWAN. Clinical centres: University of Michigan, Ann Arbor (Siobán Harlow, PI 2011 – present, Mary Fran Sowers, PI 1994–2011); Massachusetts General Hospital, Boston, MA (Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994–1999); Rush University, Rush University Medical Center, Chicago, IL (Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009); University of California, Davis/Kaiser (Ellen Gold, PI); University of California, Los Angeles (Gail Greendale, PI); Albert Einstein College of Medicine, Bronx, NY (Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010); University of Medicine and Dentistry, New Jersey Medical School, Newark (Gerson Weiss, PI 1994–2004); and the University of Pittsburgh, Pittsburgh, PA (Karen Matthews, PI). NIH Program Office: National Institute on Aging, Bethesda, MD (Winifred Rossi 2012 – present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001); National Institute of Nursing Research, Bethesda, MD (Program Officers). Central Laboratory: University of Michigan, Ann Arbor (Daniel McConnell, Central Ligand Assay Satellite Services). Coordinating Center: University of Pittsburgh, Pittsburgh, PA (Maria Mori Brooks, PI 2012 – present; Kim Sutton-Tyrrell, PI 2001 – 2012); New England Research Institutes, Watertown, MA (Sonja McKinlay, PI 1995–2001).
Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair
Funding sources
SWAN has grant support from the National Institutes of Health (NIH), Department of Health and Human Services, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495 and U01AG017719). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Footnotes
Competing interests
None declared.
References
- 1.Jelenik T, Roden M. How estrogens prevent from lipid-induced insulin resistance. Endocrinology. 2013;154:989–992. doi: 10.1210/en.2013-1112. [DOI] [PubMed] [Google Scholar]
- 2.Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, et al. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138:1–9. doi: 10.7326/0003-4819-138-1-200301070-00005. [DOI] [PubMed] [Google Scholar]
- 3.Perseghin G, Scifo P, Pagliato E, Battezzati A, Benedini S, Soldini L, et al. Gender factors affect fatty acids-induced insulin resistance in nonobese humans: effects of oral steroidal contraception. J Clin Endocrinol Metab. 2001;86:3188–3196. doi: 10.1210/jcem.86.7.7666. [DOI] [PubMed] [Google Scholar]
- 4.Sites CK, L’Hommedieu GD, Toth MJ, Brochu M, Cooper BC, Fairhurst PA. The effect of hormone replacement therapy on body composition, body fat distribution, and insulin sensitivity in menopausal women: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2005;90:2701–2707. doi: 10.1210/jc.2004-1479. [DOI] [PubMed] [Google Scholar]
- 5.Chearskul S, Charoenlarp K, Thongtang V, Nitiyanant W. Study of plasma hormones and lipids in healthy elderly Thais compared to patients with chronic diseases: diabetes mellitus, essential hypertension and coronary heart disease. J Med Assoc Thai. 2000;83:266–277. [PubMed] [Google Scholar]
- 6.Golden SH, Dobs AS, Vaidya D, Szklo M, Gapstur S, Kopp P, et al. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007;92:1289–1295. doi: 10.1210/jc.2006-1895. [DOI] [PubMed] [Google Scholar]
- 7.Phillips GB, Tuck CH, Jing TY, Boden-Albala B, Lin IF, Dahodwala N, et al. Association of hyperandrogenemia and hyperestrogenemia with type 2 diabetes in Hispanic postmenopausal women. Diabetes Care. 2000;23:74–79. doi: 10.2337/diacare.23.1.74. [DOI] [PubMed] [Google Scholar]
- 8.Andersson B, Marin P, Lissner L, Vermeulen A, Bjorntorp P. Testosterone concentrations in women and men with NIDDM. Diabetes Care. 1994;17:405–411. doi: 10.2337/diacare.17.5.405. [DOI] [PubMed] [Google Scholar]
- 9.Goodman-Gruen D, Barrett-Connor E. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care. 2000;23:912–918. doi: 10.2337/diacare.23.7.912. [DOI] [PubMed] [Google Scholar]
- 10.Ding EL, Song Y, Manson JE, Rifai N, Buring JE, Liu S. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007;50:2076–2084. doi: 10.1007/s00125-007-0785-y. [DOI] [PubMed] [Google Scholar]
- 11.Kalyani RR, Franco M, Dobs AS, Ouyang P, Vaidya D, Bertoni A, et al. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab. 2009;94:4127–4135. doi: 10.1210/jc.2009-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh JY, Barrett-Connor E, Wedick NM, Wingard DL. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25:55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- 13.Mather KM, Kim C, Christophi CA, Aroda VR, Knowler WC, Edelstein SE, et al. Steroid sex hormones, sex hormone binding globulin and diabetes incidence in the Diabetes Prevention Program. J Clin Endocrinol Metab. 2015:jc20152328. doi: 10.1210/jc.2015-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randolph JF, Jr, Zheng H, Sowers MR, Crandall C, Crawford S, Gold EB, et al. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab. 2011;96:746–754. doi: 10.1210/jc.2010-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crandall CJ, Tseng CH, Karlamangla AS, Finkelstein JS, Randolph JF, Jr, Thurston RC, et al. Serum sex steroid levels and longitudinal changes in bone density in relation to the final menstrual period. J Clin Endocrinol Metab. 2013;98:E654–E663. doi: 10.1210/jc.2012-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, et al. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocrinol Metab. 2009;94:2945–2951. doi: 10.1210/jc.2009-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sowers MR, Crawford SL, Sternfeld B, Morganstein D, Gold EB, Greendale GA, et al. SWAN: A Multicenter, Multiethnic, Community-Based Cohort Study of Women and the Menopausal Transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathology. San Diego, CA: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 18.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab. 2007;92:3060–3067. doi: 10.1210/jc.2007-0066. [DOI] [PubMed] [Google Scholar]
- 19.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–1168. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sowers MR, Zheng H, McConnell D, Nan B, Harlow S, Randolph JF., Jr Follicle stimulating hormone and its rate of change in defining menopause transition stages. J Clin Endocrinol Metab. 2008;93:3958–3964. doi: 10.1210/jc.2008-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sowers MR, Zheng H, McConnell D, Nan B, Harlow SD, Randolph JF., Jr Estradiol rates of change in relation to the final menstrual period in a population-based cohort of women. J Clin Endocrinol Metab. 2008;93:3847–3852. doi: 10.1210/jc.2008-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Pergola G, Maldera S, Tartagni M, Pannacciulli N, Loverro G, Giorgino R. Inhibitory effect of obesity on gonadotropin, estradiol, and inhibin B levels in fertile women. Obesity (Silver Spring) 2006;14:1954–1960. doi: 10.1038/oby.2006.228. [DOI] [PubMed] [Google Scholar]
- 23.Freeman EW, Sammel MD, Lin H, Gracia CR. Obesity and reproductive hormone levels in the transition to menopause. Menopause. 2010;17:718–726. doi: 10.1097/gme.0b013e3181cec85d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45:S116–S124. doi: 10.1067/mjd.2001.117432. [DOI] [PubMed] [Google Scholar]
- 25.Szymczak J, Milewicz A, Thijssen JH, Blankenstein MA, Daroszewski J. Concentration of sex steroids in adipose tissue after menopause. Steroids. 1998;63:319–321. doi: 10.1016/s0039-128x(98)00019-1. [DOI] [PubMed] [Google Scholar]
- 26.Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 27.Sites CK, Calles-Escandon J, Brochu M, Butterfield M, Ashikaga T, Poehlman ET. Relation of regional fat distribution to insulin sensitivity in postmenopausal women. Fertil Steril. 2000;73:61–65. doi: 10.1016/s0015-0282(99)00453-7. [DOI] [PubMed] [Google Scholar]
- 28.Wang N, Kuang L, Han B, Li Q, Chen Y, Zhu C, et al. Follicle-stimulating hormone associates with prediabetes and diabetes in postmenopausal women. Acta Diabetol. 2016;53:227–236. doi: 10.1007/s00592-015-0769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanko LB, Christiansen C. An update on the antiestrogenic effect of smoking: a literature review with implications for researchers and practitioners. Menopause. 2004;11:104–109. doi: 10.1097/01.GME.0000079740.18541.DB. [DOI] [PubMed] [Google Scholar]
- 30.Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:958–967. doi: 10.1016/S2213-8587(15)00316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]