Abstract
Background
Preeclampsia (PE), small for gestational age (SGA), and spontaneous preterm birth (PTB) each may be complications of impaired placental function in pregnancy. Although their exact pathogenesis is still unknown, certain infectious agents seem to play a role. Helicobacter pylori (H. pylori) colonization has been associated with increased risk for PE. Our aim was to assess the association between H. pylori colonization and PE, SGA, and PTB.
Material and methods
We measured IgG anti-H. pylori and CagA-antibodies in serum of pregnant women (median 20.5 weeks, range 16.5–29.4) who participated in a population-based prospective cohort study. Delivery and medical records were assessed. Information on demographics, education, and maternal risk factors was collected by questionnaire. We used multivariate logistic regression analyses to assess associations between H. pylori colonization and PE, SGA, and PTB.
Results
In total, 6348 pregnant women were assessed. H. pylori-positivity was found in 2915 (46%) women, of whom 1023 (35%) also were CagA-positive. Pregnancy was complicated by PE, SGA or PTB in 927 (15%) women. H. pylori colonization was associated with PE (aOR 1.51; 95%CI 1.03–2.25). Differentiation according to CagA-status revealed the same risk. H. pylori was positively related with SGA, mainly explained by CagA-positive strains (aOR 1.34; 1.04–1.71). No association was observed between H. pylori and PTB.
Conclusions
Our data suggest that H. pylori colonization may be a risk factor for PE and SGA. If these associations are confirmed by future studies and shown to be causal, H. pylori eradication may reduce related perinatal morbidity and mortality.
Keywords: H. pylori colonization, virulence factor CagA, preeclampsia, small for gestational age, spontaneous preterm birth
INTRODUCTION
The involvement of systemic inflammatory responses in pregnancies complicated by pre-eclampsia (PE), small for gestational age (SGA), and spontaneous preterm birth (PTB) has led to the hypothesis that maternal infections may play a role in the etiology and pathogenesis of these pregnancy complications (1, 2). Although the exact causes of these complications are still unknown, one hypothesis for their origin is that they each are related to suboptimal placentation in early pregnancy (3–5). In this respect, colonization with Helicobacter pylori (H. pylori) may be of interest as it might be involved in the pathogenesis of impaired trophoblast invasiveness (6).
H. pylori is a Gram-negative bacterium that colonizes the stomach of about half of the world’s population. After its re-discovery in 1982, extensive research demonstrated that H. pylori is an important risk factor for peptic ulcer disease, gastric adenocarcinoma, and mucosa associated lymphoid tissue (MALT)-lymphoma (7). An important host-interaction factor of H. pylori is the cytotoxin-associated gene A (cagA). The CagA protein is directly injected by H. pylori into the cytoplasm of gastric epithelial cells and subsequently affects cell morphology, proliferation and apoptosis (8). Colonization with CagA-positive strains is associated with higher levels of inflammatory cells and mediators compared to CagA-negative strains, both locally and systemically (9).
As such, recent studies have focused on extra-gastric manifestations of H. pylori colonization, including cardiovascular, hematologic, respiratory, and pregnancy-related diseases, including PE, SGA, and PTB (10). However, only few studies, each with a small number of cases, assessed the associations between H. pylori colonization and PE (11–14), and SGA (12, 15). These studies yielded conflicting results. Therefore, we examined the association between H. pylori colonization and each of these pregnancy-related complications in pregnant women participating in a large population-based prospective cohort study. As colonization with a CagA-positive strain is associated with higher levels of inflammatory mediators (16), we also assessed the effects of CagA-positive H. pylori strains on the risk of having these illnesses.
MATERIALS AND METHODS
Design and setting
This study was embedded in The Generation R Study, a population-based prospective cohort study among women and their children in Rotterdam, The Netherlands. In total 8879 pregnant women were included between April 2002 and January 2006. Assessments consisted of physical examinations, fetal ultrasounds, biological samples, and questionnaires (17, 18). Approval was obtained from the Medical Ethics Committee of the Erasmus Medical Center. All participants provided written informed consent. H. pylori status could be measured in 6837 women. For the present study, women with maternal comorbidity known to be associated with an increased risk for the occurrence of these three illnesses (i.e. chronic hypertension, heart disease, diabetes, high cholesterol, thyroid disease and systemic lupus erythematosus) were excluded (n=179). Twin pregnancies, and women without data on PE, SGA, and PTB were also excluded. This left a study population of 6348 pregnant women with available information on both H. pylori status and pregnancy complications (Figure 1).
Figure 1.
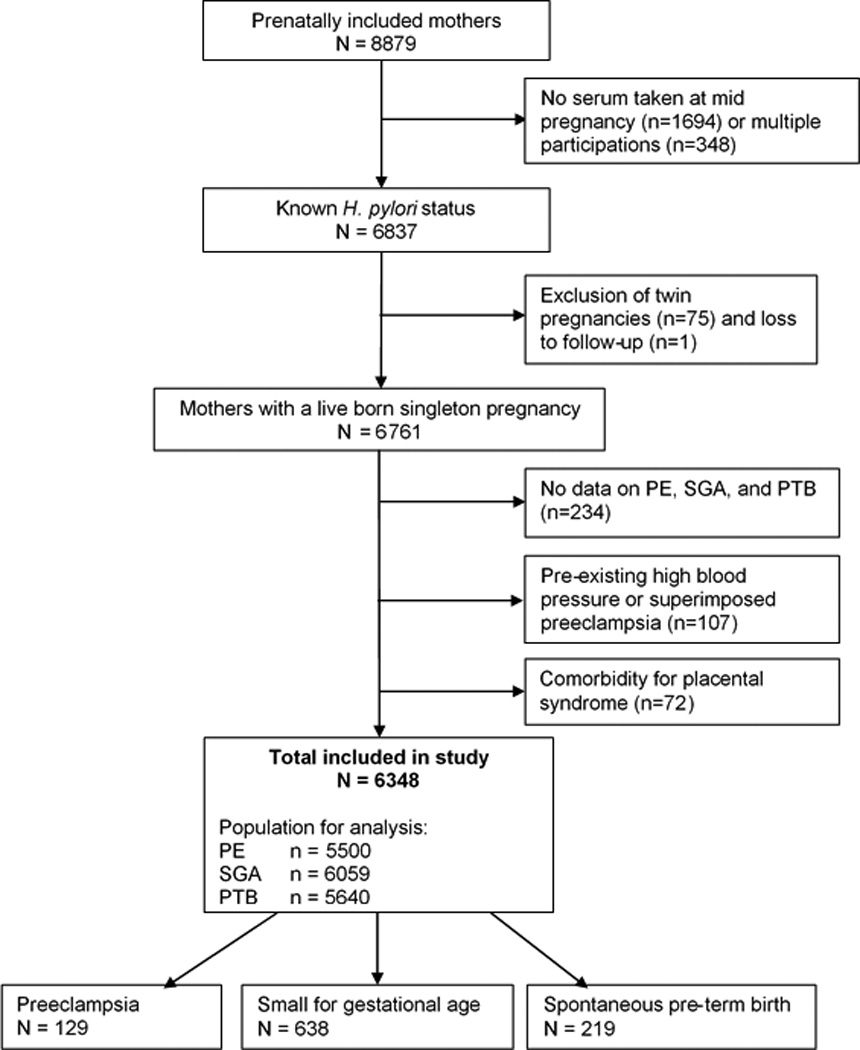
Study design
H. pylori colonization during pregnancy
Mid-pregnancy serum samples (median 20.5 weeks, range 16.5–29.4) were examined for IgG antibodies against H. pylori and the cytotoxin-associated gene A (CagA) protein using two separate enzyme-linked immunosorbent assays (ELISA), as described (19, 20). All samples were measured at least in duplicate. For each sample, the optical density ratio (ODR) was calculated by dividing the optical density (OD) by the mean OD of the positive controls. H. pylori positivity was defined as either an ODR≥1 or CagA positivity. The cut-off for CagA positivity was an ODR value ≥0.35. Details regarding H. pylori colonization in this cohort of pregnant women have been described (21). Both ELISAs were validated locally.
Pregnancy complications: PE, SGA, and PTB
Information on the pregnancy complications PE, SGA and PTB was obtained from medical records. For women who had suspected PE, the records were cross-checked with the original hospital charts (22). PE was defined as the development of systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg after 20 weeks of gestation in previously normotensive women, with concurrent proteinuria (≥0.3 g in a 24-hour urine specimen or ≥2+ [1 g/L] from a voided specimen, or ≥1+ [0.3 g/L] from a catheterized specimen) (23). Pregnancies complicated by hemolysis, elevated liver enzymes, and low platelet (HELLP) syndrome (n=22) were defined as cases of preeclampsia (23). PTB was defined as spontaneous onset of birth <37 weeks of gestation. We defined SGA as birth weight under the 10th percentile based on reference curves from our own cohort (24). Birth weight was adjusted for gestational age and transformed resulting in a normal distribution, which allowed use of means and standard deviations (SD) (25).
Covariates
Data on maternal age, ethnicity, educational level, parity, smoking during pregnancy, and maternal comorbidity (chronic hypertension, heart disease, diabetes, high cholesterol, thyroid disease and systemic lupus erythematosus) were obtained from questionnaires repeatedly applied during pregnancy. Height and weight were measured at enrolment and body mass index (BMI, kg/m2) was calculated. In Rotterdam, the largest ethnic groups are of Dutch, Surinamese, Turkish, Moroccan, Dutch-Antilles, and Cape Verdean descent. Women with another ethnic background were categorized into ‘other Western’ (European, North American, Oceanean) or ‘other non-Western’ (African, Asian, South- and Central American). The highest educational level of the mother was defined as completion of university or higher vocational training. Mothers were categorized as having a middle/low level of education if they had completed intermediate vocational training, or had completed education below that level.
Statistical analysis
Firstly, with respect to missing data on BMI (0.6%), ethnicity (6.5%), educational level (8.1%), parity (0.8%) and smoking (11.9%), values were imputed using multiple imputation (26). In the present study, five draws for each missing value were performed providing five substituted data points, which in turn created five completed data sets. Analyses were performed separately on each completed dataset and thereafter combined into one global result (26). Supplementary Table 1 provides the percentages of missing values per covariate. Secondly, the frequency distributions between risk factors for pregnancy complications and H. pylori colonization were examined using the Independent Students’ t-test (normally distributed continuous data) and the Chi-square test (categorical data). Then, univariate and multivariate logistic regression analyses were applied to assess the associations between H. pylori colonization and each separate pregnancy outcome (i.e. PE, PTB, and SGA). A number of cases was diagnosed with more than one of these pregnancy complications. The inclusion of potential confounders was based on earlier literature (4, 27) and/or if they changed the effect estimates with ≥10%. We used three regression models to explore the effect of potential confounders on the association between H. pylori and PE, PTB, and SGA. In model 1, we adjusted for maternal age, ethnicity and parity. Model 2 was additionally adjusted for body mass index and smoking during pregnancy. The third model was also adjusted for maternal education level as a proxy for socio-economic status. Analyses regarding H. pylori and SGA were also adjusted for fetal gender in all three models. Lastly, to examine effect modification we evaluated statistical interaction. Effect modification occurs when the magnitude of the effect of H. pylori on the outcome (PE, SGA, or PTB) differs depending on the level of a third covariate (i.e. maternal educational level, parity, smoking, body mass index, and fetal gender). The latter may be involved with sex specific associations regarding placentation (28). We evaluated this statistical interaction, by adding a new variable to the analysis, which is the product term of H. pylori status and the covariate. If the p-value for interaction was <0.05, a stratified analysis according to the specific covariate was performed. All measures of associations are presented as Odds Ratios (OR) with their 95% Confidence Intervals (CI). Statistical analyses were performed using IBM SPSS Statistics 21.0 for Windows (SPSS IBM, Armonk, New York, USA).
RESULTS
Population characteristics
In total, 6348 women were included in the study. Their baseline characteristics are shown in Table 1. Mean age at enrolment was 29.7 years (SD 5.3), and 51.0% were of non-Dutch ethnic background. Overall, the H. pylori colonization rate was 45.9%, of whom 35.1% carried CagA-positive strains. Nine-hundred and twenty-seven (14.6%) developed either PE, SGA or PTB. Compared to women without one of the indicator conditions, women with a complicated pregnancy were younger, had attained a lower level of education, were more often of non-Dutch ethnicity, had a lower BMI, did smoke more often during pregnancy, and were more often pregnant with their first child. (Table 1, p<0.001 for univariate comparisons; see supplementary table 1 for observed and imputed data). In total, 129 (2.0%) women were diagnosed with PE, 638 (10.1%) with SGA, and 219 (3.4%) with PTB (Figure 1). Fifty-eight women (0.9%) had more than one of these complications, and contribute to both complication groups.
Table 1.
Characteristics of mothers (n = 6348)
| Imputed | Complicated pregnancy1 | p-value | ||
|---|---|---|---|---|
| No (n=5421) | Yes (n=927) | |||
| Age, years (SD) | 29.7 (5.3) | 29.9 (5.2) | 28.9 (5.6) | <0.001 |
| Body mass index, kg/m2 (range) | 23.7 (15.2–51.2) | 23.8 (15.2–51.2) | 23.3 (15.8–50.2) | <0.001 |
| Data missing | ||||
| Ethnicity (%) | <0.001 | |||
| Dutch | 3031 (47.7) | 2656 (49.0) | 375 (40.1) | |
| Turkish | 654 (10.3) | 546 (10.1) | 88 (9.5) | |
| Surinamese | 569 (9.0) | 433 (8.0) | 143 (15.4) | |
| Moroccan | 458 (7.2) | 421 (7.8) | 45 (4.9) | |
| Cape Verdean | 302 (4.8) | 235 (4.3) | 68 (7.3) | |
| Dutch Antillean | 246 (3.9) | 190 (3.5) | 59 (6.4) | |
| Other Western | 689 (10.9) | 605 (11.2) | 86 (9.3) | |
| Other non-Western | 399 (6.3) | 335 (6.2) | 63 (6.8) | |
| Data Missing | ||||
| Education level (%) | <0.001 | |||
| Low/Middle | 3807 (60.0) | 3178 (58.6) | 628 (67.7) | |
| High | 2541 (40.0) | 2243 (41.4) | 299 (32.3) | |
| Data missing | ||||
| Parity (%) | <0.001 | |||
| Nulli parity | 3503 (55.2) | 2865 (52.9) | 638 (68.8) | |
| Multi parity | 2845 (44.8) | 2556 (47.1) | 289 (31.2) | |
| Data missing | ||||
| Smoking during pregnancy (%) | <0.001 | |||
| No | 5136 (80.9) | 4462 (82.3) | 675 (72.8) | |
| Yes | 1211 (19.1) | 959 (17.7) | 252 (27.2) | |
| Data missing | ||||
| Children’s gender | 0.65 | |||
| Female | 3209 (50.5) | 2733 (50.4) | 475 (51.2) | |
| Male | 3139 (49.5) | 2687 (49.6) | 452 (48.8) | |
| Data missing | ||||
| H. pylori and CagA | ||||
| H. pylori-negative | 3433 (54.1) | 2979 (55.0) | 454 (49.0) | <0.001 |
| H. pylori-positive | 2915 (45.9) | 2442 (45.0) | 473 (51.0) | |
| CagA-negative | 1892 (64.9) | 1604 (65.7) | 288 (60.9) | <0.001 |
| CagA-positive | 1023 (35.1) | 838 (34.3) | 185 (39.1) | |
Values are means (SD), medians (range) or absolute numbers (percentages).
Pregnancy complicated by PE, SGA, or PTB
H. pylori colonization and PE, SGA, and PTB
Women with one of these pregnancy complications were more often H. pylori positive (51.0%) than women with an uncomplicated pregnancy (45.0%) (p<0.001). Among those women with H. pylori, the CagA-positivity rate was higher in those with a complicated pregnancy (39.1% vs. 34.3%, p<0.001) (Table 1).
Supplementary table 2 shows the prevalence of PE, SGA, and PTB according to H. pylori and CagA-status. Univariate logistic regression analyses showed an increased risk of PE and SGA in H. pylori-positive mothers (OR 1.49; 95% CI 1.05–2.12 and OR 1.32; 1.12–1.56, respectively) (Supplementary table 3). Parallel results for H. pylori and SGA were observed when using an ethnic-specific 10th percentile definition for SGA (data not shown). Differentiation into CagA-negative and CagA-positive strains revealed a positive association between Hp+CagA+ mothers and SGA (OR 1.59; 95% CI 1.28–1.97). No association was observed between H. pylori and PTB. Multivariate analyses revealed an association with PE (final OR 1.51; 95%CI 1.03–2.25), but not with SGA or PTB (Table 2). Differentiation into CagA-negative or CagA-positive strains showed an association between CagA-positive strains and SGA (final OR 1.34; 95% CI 1.04–1.71). Increased risk of PE was observed in mothers with a CagA-negative strain (Model 2, OR 1.58; 95% CI 1.03–2.40). The association attenuated slightly after additional adjustment for educational level. We did not observe an interaction between H. pylori status and fetal gender, maternal educational level, parity, smoking, and body mass index (data not shown).
Table 2.
Associations of H. pylori and CagA status with PE, SGA, and PTB.
| Preeclampsia (n = 5550) | Small for gestational age (n = 6059) | Spontaneous preterm birth (n = 5640) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Hp− |
Reference n = 58 / 3037 |
Reference | Reference |
Reference n = 306 / 3285 |
Reference | Reference |
Reference n = 113 / 3092 |
Reference | Reference |
| Hp+ |
1.59 (1.07, 2.35)* n = 71 / 2513 |
1.57 (1.06, 2.32)* |
1.51 (1.03, 2.25)* |
1.20 (0.99, 1.46) n = 332 / 2774 |
1.18 (0.98, 1.43) |
1.16 (0.96, 1.41) |
1.18 (0.87, 1.61) n = 106 / 2548 |
1.17 (0.86, 1.60) |
1.15 (0.84, 1.57) |
| Hp+CagA− |
1.58 (1.04, 2.40)* n = 46 / 1650 |
1.58 (1.03, 2.40)* |
1.53 (1.00, 2.33) |
1.12 (0.91, 1.38) n = 195 / 1799 |
1.09 (0.89, 1.35) |
1.08 (0.87, 1.33) |
1.21 (0.86, 1.68) n = 70 / 1674 |
1.19 (0.86, 1.67) |
1.18 (0.84, 1.64) |
| Hp+CagA+ | 1.61 (0.95, 2.73) n = 25 / 863 |
1.56 (0.92, 2.64) |
1.50 (0.89, 2.55) |
1.38 (1.08, 1.76)* n = 137 / 975 |
1.36 (1.06, 1.74)* |
1.34 (1.04, 1.71)* |
1.13 (0.73, 1.73) n = 36 / 874 |
1.11 (0.72, 1.70) |
1.08 (0.71, 1.66) |
Values are odds ratios for PE, SGA, and PTB (95% confidence interval) from logistic regression models. n = number of cases per total group.
Model 1 was adjusted for maternal age, parity, ethnicity.
Model 2: model 1 additionally adjusted for body mass index and smoking.
Model 3: model 2 additionally adjusted for educational level.
Analyses regarding H. pylori and SGA were also adjusted for fetal gender in all three models.
p<0.05
When excluding cases with more than one pregnancy complication from each group, multivariate analyses showed only CagA-positivity independently associated with SGA (OR 1.33; 95% CI 1.03–1.72) (Supplementary table 4). Multivariate analysis of pregnancies complicated by both PE and SGA (n = 38) showed a trend to increased risk in H. pylori positive women (OR 1.71; 95% 0.83–3.53).
DISCUSSION
This large population-based prospective cohort study showed that H. pylori colonization is associated with an increased risk on PE and that carriage of a CagA+ H. pylori strain is a risk factor for SGA. These findings may be helpful for a better understanding of the pathogenesis of these pregnancy complications and support the link with chronic inflammatory conditions. The potential association between H. pylori and these gestational disorders has been studied before. Previous studies however were considerably smaller, thereby limiting power. Furthermore, not all studies included separate analyses of CagA data.
The observed association between H. pylori and PE is consistent with prior epidemiological studies (11–14). In a small Italian study of 47 PE cases and 47 controls, Ponzetto et al. found a higher H. pylori seropositivity in mothers with PE (51.1%) compared to women with an uncomplicated pregnancy (31.9%) (OR 2.67; 95% CI 1.08–6.57) (11). In contrast to our observation, they observed greater differences between those having CagA-positive and CagA-negative strains (80.9% vs. 14.9%). Another study from the same group investigated the association of several H. pylori virulence factors with fetal growth retardation (FGR) (FGR, n = 13), PE (n = 17), and both (PE and FGR, n = 32) compared with controls (n = 49) (12). In PE women with or without FGR, the H. pylori-positivity rate was higher while there was no difference in prevalence between FGR-only and controls. They observed that CagA-strains were more prevalent in PE pregnant women compared to controls, while there was no difference between FGR cases and controls. Besides the small number of cases compared to our study, the study differs in case definition as they used FGR and we SGA as proxy for FGR. An Australian study determined the association between H. pylori colonization and SGA in 448 pregnant women (15), of whom 34 (7.5%) had SGA. Multivariate analysis revealed that H. pylori seropositivity was associated with SGA (OR 2.59; 95% CI 1.12–5.95; p = 0.025). A similar trend was observed in our study, but only for those with CagA-positive strains. CagA status data were not available in the prior study. We found no association between H. pylori and PTB, regardless of CagA-status. One other study, assessing this relation in 416 pregnant women, did not observe a significant association between H. pylori colonization and PTB (29).
The overall H. pylori prevalence in this cohort may be higher than for other populations in a Western country. This difference is mainly explained by the high colonization rates in women with a non-European ethnic background. Studies evaluating the H. pylori colonization in multi-ethnic populations all showed higher prevalence among immigrant groups, compared to the original population (30, 31). Although PE, SGA, and PTB are different clinical entities, all three may be caused by suboptimal deep placentation in early pregnancy (5) (32, 33) (34, 35). Large numbers of non-transformed spiral arteries are frequently observed in PE patients with or without SGA (5), in patients with SGA without gestational hypertension (32, 33), and in patients with preterm labor with or without preterm pre-labor rupture of membranes (PROM) (34, 35). Impaired remodeling of the spiral arteries may lead to insufficient uteroplacental arterial flow and episodes of irregular placental perfusion (3). Such impaired remodeling might be due to failure of appropriate uterine preconditioning (36), and excessive or atypical maternal immune responses to trophoblasts (37). Franceschi et al. have shown that anti-CagA antibodies were able to recognize β-actin on the surface of trophoblast cells in a dose-dependent binding assay in vitro (6). This binding resulted in impaired cytotrophoblast invasiveness, which is characteristic for the development of the placental syndrome; however, we observed no association between CagA-positive strains and PE. The association between H. pylori and PE disappeared when excluding women with more than one of the studied pregnancy complications (i.e. with both PE and SGA), which suggests that the significant result was based on those cases. PTB is a syndrome caused by multiple pathologic processes with placental involvement as one of the possibilities (38). In our study, although all spontaneous in onset, we were not able to distinguish between different causes of PTB.
Our data add further evidence to the associations between H. pylori and PE and SGA. Although association does not imply causation, epidemiological findings should stimulate biological studies. If the association between H. pylori and these illnesses is causal, eradication of H. pylori may be part of an effective intervention for reducing related perinatal morbidity and mortality. As the overall H. pylori prevalence in Western countries is declining, screening for H. pylori may be most efficient in pregnant women with increased H. pylori prevalence, like a low socioeconomic status, or a non-Western ethnic background (21). Nevertheless, we think that more evidence is needed, before organizing eradication programs in pregnant or non-pregnant women.
The strengths of this study include the large number of subjects participating in a population-based cohort, with detailed prospectively collected data on socioeconomic, and sociodemographic characteristics, together with other potential confounding factors. However, it cannot be excluded that the findings may partly result from unmeasured confounding. Our study may be limited by the fact that we measured IgG antibodies against H. pylori and CagA, indicating present or recent colonization. However, it is not clear whether any effect of H. pylori is dependent on active colonization or the presence of circulating anti-H. pylori and CagA antibodies. The use of one general definition for SGA in all ethnic groups may limit our results, as a prior study has shown differences in birth weights between ethnic populations of this cohort (39). However, additional analyses, using the ethnic-specific 10th percentile revealed parallel results for H. pylori and SGA. Since validated ethnic-specific growth curves are lacking, we continued to use the population-specific 10th percentile. Although this study is population-based, selective participation occurred since participating women were generally higher educated, and were more often from Dutch ethnic background (17). Missing data for several characteristics and potential confounders may have biased the outcome. Therefore, we performed the final analyses after a multiple imputation procedure. This is considered useful to deal with missing data, since it requires the fewest assumptions and reduces potential bias when missing data are not random (26).
In summary, H. pylori colonization is positively associated with PE. In addition, we confirmed the important role of CagA-positive strains in SGA, as the association was determined by colonization with these strains.
Supplementary Material
Acknowledgments
Sources of financial support:
The Generation R Study is made possible by financial support from the Erasmus Medical Center, Rotterdam, the Erasmus University Rotterdam and the Netherlands Organization for Health Research and Development, and by R01 DK090989 from the National Institutes of Health, and the Diane Belfer Program for Human Microbial Ecology. The researchers are independent from the funders. The study sponsors had no role in the study design, data analysis, interpretation of data, or writing of this report.
Footnotes
Contribution of authors to the study:
WH, SS, LH, ES, EK contributed to the conception and design, acquisition of data, analyses and interpretation of the data, drafted the article, revised it critically for important intellectual content and gave final approval of the version to be published. VJ, AH, GP, MB contributed to the conception and design and acquisition of data, revised it critically for important intellectual content and gave final approval of the version to be published.
REFERENCES
- 1.Herrera JA, Chaudhuri G, Lopez-Jaramillo P. Is infection a major risk factor for preeclampsia? Med Hypotheses. 2001;57(3):393–397. doi: 10.1054/mehy.2001.1378. [DOI] [PubMed] [Google Scholar]
- 2.von Dadelszen P, Magee LA. Could an infectious trigger explain the differential maternal response to the shared placental pathology of preeclampsia and normotensive intrauterine growth restriction? Acta Obstet Gynecol Scand. 2002;81(7):642–648. [PubMed] [Google Scholar]
- 3.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 4.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 5.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The "Great Obstetrical Syndromes" are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franceschi F, Di Simone N, D'Ippolito S, Castellani R, Di Nicuolo F, Gasbarrini G, et al. Antibodies anti-CagA cross-react with trophoblast cells: a risk factor for pre-eclampsia? Helicobacter. 2012;17(6):426–434. doi: 10.1111/j.1523-5378.2012.00966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19(3):449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atherton JC, Blaser MJ. Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J Clin Invest. 2009;119(9):2475–2487. doi: 10.1172/JCI38605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham DY, Yamaoka Y. Disease-specific Helicobacter pylori virulence factors: the unfulfilled promise. Helicobacter. 2000;5(Suppl 1):S3–S9. doi: 10.1046/j.1523-5378.2000.0050s1003.x. discussion S27–31. [DOI] [PubMed] [Google Scholar]
- 10.Franceschi F, Tortora A, Gasbarrini G, Gasbarrini A. Helicobacter pylori and Extragastric Diseases. Helicobacter. 2014;19(Suppl 1):52–58. doi: 10.1111/hel.12159. [DOI] [PubMed] [Google Scholar]
- 11.Ponzetto A, Cardaropoli S, Piccoli E, Rolfo A, Gennero L, Kanduc D, et al. Pre-eclampsia is associated with Helicobacter pylori seropositivity in Italy. J Hypertens. 2006;24(12):2445–2449. doi: 10.1097/HJH.0b013e3280109e8c. [DOI] [PubMed] [Google Scholar]
- 12.Cardaropoli S, Rolfo A, Piazzese A, Ponzetto A, Todros T. Helicobacter pylori's virulence and infection persistence define pre-eclampsia complicated by fetal growth retardation. World J Gastroenterol. 2011;17(47):5156–5165. doi: 10.3748/wjg.v17.i47.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aksoy H, Ozkan A, Aktas F, Borekci B. Helicobacter pylori seropositivity and its relationship with serum malondialdehyde and lipid profile in preeclampsia. J Clin Lab Anal. 2009;23(4):219–222. doi: 10.1002/jcla.20330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UstUn Y, Engin-UstUn Y, Ozkaplan E, Otlu B, Sait TekerekoGlu M. Association of Helicobacter pylori infection with systemic inflammation in preeclampsia. J Matern Fetal Neonatal Med. 2010;23(4):311–314. doi: 10.3109/14767050903121456. [DOI] [PubMed] [Google Scholar]
- 15.Eslick GD, Yan P, Xia HH, Murray H, Spurrett B, Talley NJ. Foetal intrauterine growth restrictions with Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16(9):1677–1682. doi: 10.1046/j.1365-2036.2002.01333.x. [DOI] [PubMed] [Google Scholar]
- 16.Bhat N, Gaensbauer J, Peek RM, Bloch K, Tham KT, Blaser MJ, et al. Local and systemic immune and inflammatory responses to Helicobacter pylori strains. Clinical and diagnostic laboratory immunology. 2005;12(12):1393–1400. doi: 10.1128/CDLI.12.12.1393-1400.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaddoe VW, van Duijn CM, Franco OH, van der Heijden AJ, van Iizendoorn MH, de Jongste JC, et al. The Generation R Study: design and cohort update 2012. Eur J Epidemiol. 2012;27(9):739–756. doi: 10.1007/s10654-012-9735-1. [DOI] [PubMed] [Google Scholar]
- 18.Jaddoe VW, Bakker R, van Duijn CM, van der Heijden AJ, Lindemans J, Mackenbach JP, et al. The Generation R Study Biobank: a resource for epidemiological studies in children and their parents. Eur J Epidemiol. 2007;22(12):917–923. doi: 10.1007/s10654-007-9209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Perez GI, Dworkin BM, Chodos JE, Blaser MJ. Campylobacter pylori antibodies in humans. Ann Intern Med. 1988;109(1):11–17. doi: 10.7326/0003-4819-109-1-11. [DOI] [PubMed] [Google Scholar]
- 20.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55(10):2111–2115. [PubMed] [Google Scholar]
- 21.den Hollander WJ, Holster IL, den Hoed CM, van Deurzen F, van Vuuren AJ, Jaddoe VW, et al. Ethnicity is a strong predictor for Helicobacter pylori infection in young women in a multi-ethnic European city. J Gastroenterol Hepatol. 2013;28(11):1705–1711. doi: 10.1111/jgh.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coolman M, de Groot CJ, Jaddoe VW, Hofman A, Raat H, Steegers EA. Medical record validation of maternally reported history of preeclampsia. J Clin Epidemiol. 2010;63(8):932–937. doi: 10.1016/j.jclinepi.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Hypertens Pregnancy. 2001;20(1):IX–XIV. doi: 10.1081/PRG-100104165. [DOI] [PubMed] [Google Scholar]
- 24.Verburg BO, Steegers EA, De Ridder M, Snijders RJ, Smith E, Hofman A, et al. New charts for ultrasound dating of pregnancy and assessment of fetal growth: longitudinal data from a population-based cohort study. Ultrasound Obstet Gynecol. 2008;31(4):388–396. doi: 10.1002/uog.5225. [DOI] [PubMed] [Google Scholar]
- 25.Niklasson A, Ericson A, Fryer JG, Karlberg J, Lawrence C, Karlberg P. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977–1981) Acta paediatrica Scandinavica. 1991;80(8–9):756–762. doi: 10.1111/j.1651-2227.1991.tb11945.x. [DOI] [PubMed] [Google Scholar]
- 26.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCowan L, Horgan RP. Risk factors for small for gestational age infants. Best Pract Res Clin Obstet Gynaecol. 2009;23(6):779–793. doi: 10.1016/j.bpobgyn.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Scott NM, Hodyl NA, Murphy VE, Osei-Kumah A, Wyper H, Hodgson DM, et al. Placental cytokine expression covaries with maternal asthma severity and fetal sex. Journal of immunology. 2009;182(3):1411–1420. doi: 10.4049/jimmunol.182.3.1411. [DOI] [PubMed] [Google Scholar]
- 29.McKenna D, Watson P, Dornan J. Helicobacter pylori infection and dyspepsia in pregnancy. Obstet Gynecol. 2003;102(4):845–849. doi: 10.1016/s0029-7844(03)00766-x. [DOI] [PubMed] [Google Scholar]
- 30.De Vries AC, Van Driel HF, Richardus JH, Ouwendijk M, Van Vuuren AJ, De Man RA, et al. Migrant communities constitute a possible target population for primary prevention of Helicobacter pylori-related complications in low incidence countries. Scand J Gastroenterol. 2008;43(4):403–409. doi: 10.1080/00365520701814077. [DOI] [PubMed] [Google Scholar]
- 31.Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol. 2012;175(1):54–59. doi: 10.1093/aje/kwr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. British journal of obstetrics and gynaecology. 1986;93(10):1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 33.Gerretsen G, Huisjes HJ, Elema JD. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. British journal of obstetrics and gynaecology. 1981;88(9):876–881. doi: 10.1111/j.1471-0528.1981.tb02222.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002;187(5):1137–1142. doi: 10.1067/mob.2002.127720. [DOI] [PubMed] [Google Scholar]
- 35.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189(4):1063–1069. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 36.Brosens JJ, Parker MG, McIndoe A, Pijnenborg R, Brosens IA. A role for menstruation in preconditioning the uterus for successful pregnancy. Am J Obstet Gynecol. 2009;200(6):615 e1–615 e6. doi: 10.1016/j.ajog.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 37.Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12(6):747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Troe EJ, Raat H, Jaddoe VW, Hofman A, Looman CW, Moll HA, et al. Explaining differences in birthweight between ethnic populations. The Generation R Study. BJOG. 2007;114(12):1557–1565. doi: 10.1111/j.1471-0528.2007.01508.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


