Abstract
Dietary-associated diseases have increased tremendously in our current population, yet key molecular changes associated with high-fat diets that cause clinical prediabetes, obesity, hyperglycemia, and peripheral neuropathy remain unclear. This study examines molecular and metabolic aspects altered by voluntary exercise and a high-fat diet in the mouse dorsal root ganglion. Mice were examined for changes in mRNA and proteins encoding anti-inflammatory mediators, metabolic-associated molecules, and pain associated ion channels. Proteins involved in the synaptosomal complex and pain associated TRP ion channels decrease in the dorsal root ganglion of high-fat exercise animals relative to their sedentary controls. Exercise reversed high-fat diet induced mechanical allodynia without affecting weight gain, elevated blood glucose, and utilization of fat as a fuel source. Independent of weight or fat mass changes, high-fat exercised mice display reduced inflammation associated mRNAs. The benefits of exercise on abnormal peripheral nerve function appear to occur independent of systemic metabolic changes, suggesting that the utilization of fats and inflammation in the peripheral nervous system may be key for diet-induced peripheral nerve dysfunction and the response to exercise.
Keywords: Exercise, Fat, Pain, Inflammation, TRP Channel
Introduction
Diabetes and prediabetes are a continuing rising epidemic in the US and worldwide, with patients experiencing symptoms of neuropathy in both per and over diabetic patients (Groover et al., 2013; Services, 2014). A high fat diet fed to rodents mimics prediabetes and negatively alters peripheral sensation (Guilford et al., 2011; Groover et al., 2013). Our previous work in mice has shown that exercise reduces most but not all diet-induced metabolic abnormalities (Groover et al., 2013). This is consistent with clinical studies that reveal exercise benefits individuals with neuropathy (Li and Hondzinski, 2012) and neuropathic pain (Balducci et al., 2006; Dobson et al., 2014). However, the mechanisms by which exercise exerts its benefits remain poorly understood.
We demonstrate that a high-fat diet fed to mice alters molecular pathways involved in inflammation and pain. Our results suggest that exercise provides select benefits that occur independent of alterations in body weight, fat mass, and traditional mitochondrial and metabolic signals, suggesting that exercise benefits sensory function in specific and unpredicted ways.
Methods and Materials
Seven-week-old male C57/BL6 #027 mice were purchased from Charles River (Wilmington, Mass) and maintained on a 12:12h light/dark cycle. All mice were given ad libitum access to food and water and were fed a chow diet (8604; Envigo, Madison Wisconsin; 14% kcals from fat, 32% protein, and 54% carbohydrate) or a high fat diet (07011; Envigo; 54% kcals from vegetable shortening (hydrogenated) and corn oil fat, 21% protein and 24% carbohydrate). At 8 weeks of age, all mice were fed the chow diet through all baseline testing. After baseline behavioral testing was complete, animals were separated and the groups were given different diets. All animal use was in accordance with NIH guidelines and conformed to the principles specified by the Institutional Animal Care and Use Committee.
Following baseline testing, animals were separated into sedentary or exercise groups (Groover et al., 2013). Treatment groups are identified throughout the study as: chow-fed sedentary (CF-Sed), chow-fed exercise (CF-Ex), high-fat sedentary (HF-Sed), and high-fat exercise (HF-Ex).
Animal weight and blood glucose (glucose diagnostic reagents; Sigma, St. Louis, MO) was measured weekly after a 3-hour fast. At sacrifice, blood was drawn from the chest cavity and allowed to clot for 30 min on ice, then spun at 3,000g for 30 min at 4 °C and serum was frozen at −80 °C for bradykinin and β-hydroxybutyrate analysis. Animals were acclimatized and tested for mechanical sensitivity as previously described (Chaplan et al., 1994; Groover et al., 2013).
Fat and lean mass was measured by MRI using an EchoMRI-100 (EchoMRI, Houston, TX). Body composition was measured before sacrifice at completion of the 12 week study.
A metabolic monitoring system (Promethion, Sable Systems Int., Las Vegas, NV) was used to measure total energy expenditure (TEE) and respiratory quotient (RQ) over a 48-hr period 10 weeks into the study. Mice were singly housed in metabolic chambers and acclimated for 2 days prior to data collection. Data were analyzed as two 12-hour cycle averages (12 hours ambient light [07:00–19:00] and 12 hours of dark [19:00–07:00]) and were calculated per animal; light and dark averages were used to calculate group means.
RNA was extracted and cDNA synthesized as previously described (Jack et al., 2011). Primer sequences for TFAM, HIF1-α, DGKB, BDH1, HSP60, HSP70, TRPV1, TRPA1, AKAP150, SNAP25, CGRP, TrkA, and GAPD, were created for mouse sequences by Integrated DNA Technologies (Coralvile, IA). A custom PCR plate from Bio-Rad was used to analyze mRNAs for tumor necrosis factor (TNF-α), interleukin 1 Beta (IL-1β), interleukin 6 (IL-6), mitogen-activated protein kinase 14 (p38), mitogen-activated protein kinase 8 (JNK), nerve growth factor (NGF), synaptosomal-associated protein, 23kDa (SNAP23), syntaxin binding protein 4 (STXBP4), vesicle-associated membrane protein 2 (VAMP2). All reactions were performed in triplicate and mRNA levels were normalized to GAPDH. ΔΔCT values were used to calculate fold change and relative expression levels.
Lumbar and thoracic DRGs were flash frozen in liquid nitrogen and stored at −80 °C. Western blot analysis was performed as previously described (Grote et al., 2013). Additional samples were used for analysis of oxidative phosphorylation (OXPHOS) of electron transport complexes. Antibodies include Anti-Total OXPHOS (1:333, Abcam, Cambridge, MA); Anti-p38 MAPK and Anti-Phospho-p38 MAPK (1:1000, Cell Signaling, Danvers, MA); Anti-Phospho-AMPK (1:1000, Cell Signaling, Danvers, MA); Anti-AMPK (1:1000, Cell Signaling, Danvers, MA); Anti-PPAR-α (1:500. Abcam, Cambridge, MA); Anti-Snap23 (1:1000, Abcam, Cambridge, MA); Anti-Snap25 (1:1000, Abcam, Cambridge, MA); Anti-Tubulin (1:1000, Abcam, Cambridge, MA); Anti-PPAR-γ-Coactivator 1 (PGC1-α) (1:500, Calbiochem EDM Millipore, San Diego, CA); and Anti-β-Actin (1:2500, Abcam, Cambridge, MA).
Results are presented as means ± SEM. Data was analyzed using a two-factor ANOVA with post hoc comparisons analyzed using Fisher’s test of least square difference. Statistical significance was set at P < 0.05 and analyses were performed using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA).
Results
By 6 weeks, both high-fat fed groups had decreases in withdrawal thresholds compared to chow-fed exercised and sedentary (HF-Sed, p=0.072; HF-Ex, p=0.068) mice, although thresholds of high-fat fed mice were only statistically significantly decreased relative to chow-fed exercised mice (HF-Ex, p = 0.049; HF-Sed, p = 0.051). At the conclusion of the study, high-fat sedentary mice maintained decreased thresholds relative to the chow-fed sedentary controls (p = 0.018), whereas thresholds of high fat exercised mice were rescued and fell between chow sedentary mice and high fat sedentary mice (p = 0.008) (Fig. 1).
Figure 1.
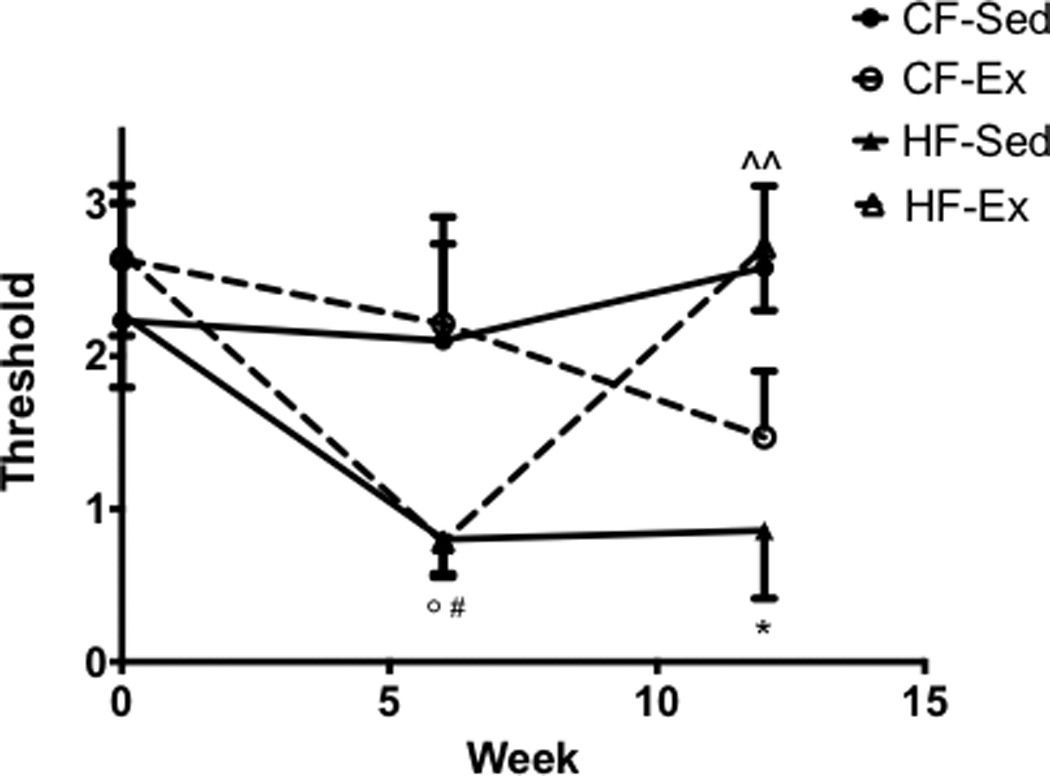
A High-Fat Diet Induces Mechanical Allodynia and Is Rescued by Exercise Von Frey mechanical sensitivity examination shows sensitivity in both HF-Ex and HF-Sed compared to control animals after 6 weeks of a high-fat diet. HF-Ex mice return to baseline levels at 12 weeks of physical activity. (n=8 for all groups) All data presented as mean ± SEM *:CF-Sed vs. HF-Sed #: CF-Ex vs. HF-Sed ^: HF-Sed vs.HF-Ex °: CF-Ex vs.HF-EX!!.
High-fat sedentary and high-fat exercised mice gained more weight compared to both chow-fed groups (Fig. 2a). Additionally, measurements of body weight due to changes in fat vs. lean mass revealed a main effect of diet on fat mass (p <0.0001, Fig. 2b). Both high-fat sedentary and high-fat exercise mice gained significantly more fat mass compared to chow-fed groups (CF-Sed vs. HF-Sed, p = 0.001; CF-Sed vs. HF-Ex, p = 0.025; CF-Ex vs. HF-Sed, p = 0.0002; CF-Ex vs. HF-Ex, p = 0.005, Fig. 2b). No alterations in lean mass were noted between any groups.
Figure 2.
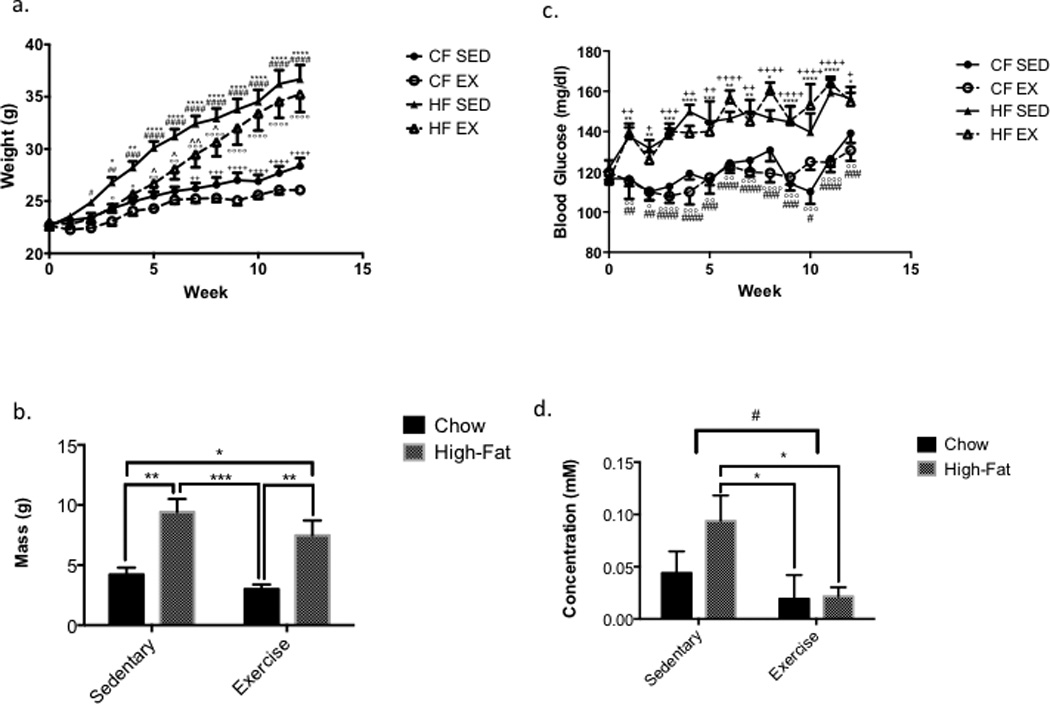
A High-Fat Diet Increases Body Weight and Fat Mass (A) A high-fat diet causes weight gain in both HF-Sed and HF-Ex mice compared to their standard diet controls (n=8 for all groups). (B) A high-fat diet causes fat mass gains in both HF-Sed and HF-Ex mice compared to their standard controls (n=4 for all groups). All data presented as mean ± SEM; *:CF-Sed vs. HF-Sed #: CF-Ex vs. HF-Sed +: CF-Sed vs. HFEx ^: HF-Sed vs. HF-Ex °: CF-Ex vs. HF-Ex. (C) A high-fat diet slightly increases blood glucose levels in both exercised and sedentary mice, though hyperglycemia does not develop in either cohort. (D) High-fat sedentary mice show increased ketones in the blood compared to both chow-fed and high-fat fed mice. A main effect of exercise in reducing the level of blood ketones is present (p < 0.05) (n=8 for all groups) All data presented as mean ± SEM; *:CF-Sed vs. HF-Sed #: CF-Ex vs. HF-Sed +: CF-Sed vs. HF-Ex °: CF-Ex vs. HF-Ex!!.
High-fat sedentary and high-fat fed exercised mice had elevated blood glucose levels compared to chow-fed mice two weeks after diet and exercise initiation (Fig. 3a). Both high-fat diet groups’ blood glucose levels remained below 250 mg/dl.
Figure 3.
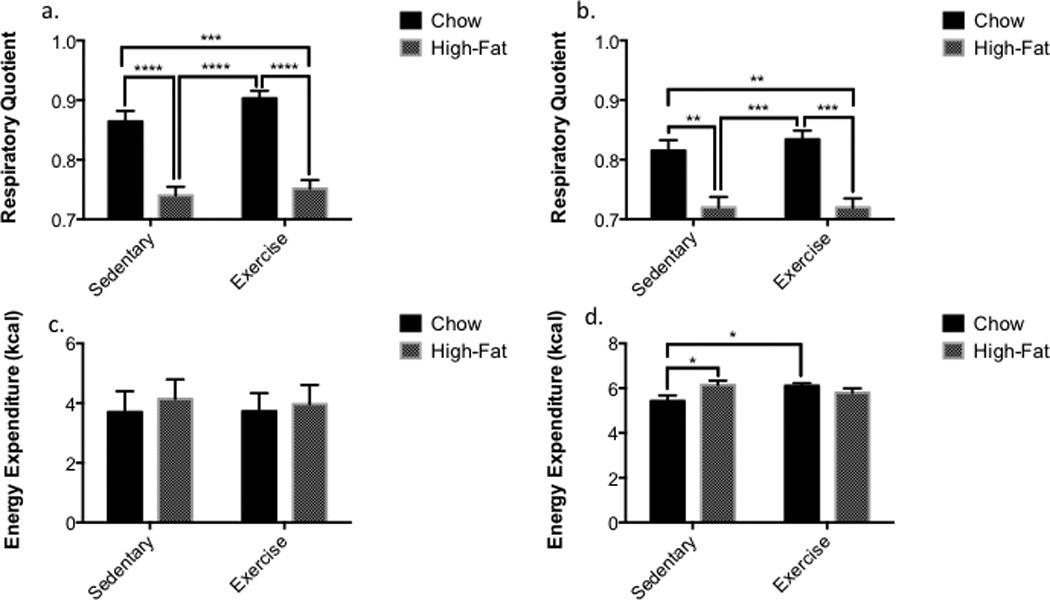
A High-Fat Diet Alters Fuel Source Utilization (A) Dark cycle respiratory quotient (RQ) has a main effect of diet as seen with high-fat fed mice as compared to chow-fed controls (p < 0.0001). (B) A decreased RQ corresponding with diet is still present (p < 0.0001) during light cycle hours though the difference in RQ is less significant. (C) Light cycle total energy expenditure (TEE) is unaltered by diet or exercise (D) Dark cycle TEE is increased with exercise in chow-fed mice but not high-fat fed mice as compared to chow-fed controls. A high-fat diet did increase TEE in sedentary mice as compared to their chow fed counterpart. (n=4 for all groups) All data presented as mean ± SEM.
Serum bradykinin was not altered by diet or exercise when compared to all groups or by main effects. Serum β–hydroxybutyrate displayed a main effect of exercise (p = 0.021) and was significantly elevated in high fat sedentary mice compared to both exercised groups (HF-Sed vs. CF-Ex, p = 0.016; HF-Sed vs. HF-Ex, p = 0.0119), though not different compared to chow-fed sedentary (HF-Sed vs. CF-Sed, p= 0.081, Fig. 3b).
Regardless of exercise training, mice on a high-fat diet had a reduced RQ (dark cycle p<0.0001; light cycle p < 0.0001); while mice fed a chow diet had an RQ of ~0.8 (Figs. 4a, b). Analysis of fuel source utilization in relation to dark-light cycles displayed significant differences (CF-Sed vs. HF-Sed, p <0.0001; CF-Sed vs. HF-Ex, p = 0.0002; CF-Ex vs. HF-Sed, p <0.0001; CF-Ex vs. HF-Ex, p <0.0001). The light cycle showed similar albeit less significant alterations (CF-Sed vs. HF-Sed, p = 0.001; CF-Sed vs. HF-Ex, p = 0.0013; CF-Ex vs. HF-Sed, p = 0.0003; CF-Ex vs. HF-Ex, p = 0.0003).
Figure 4.
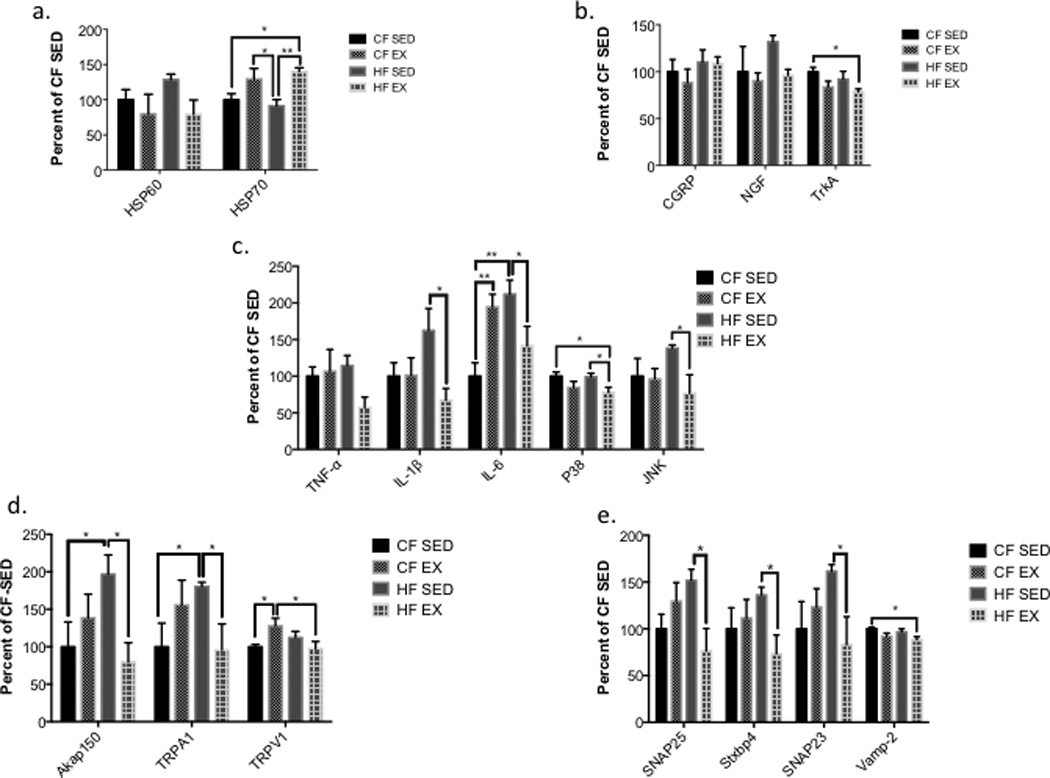
Exercise Reduces High-Fat Diet Altered Gene Expression (A) Exercise increases gene expression of HSP70, but not HSP60, relative to sedentary controls. (B) Exercise reduces gene expression of inflammatory cytokines (TNF-α, IL-1β, IL-6, p38, and JNK) induced with a highfat diet and sedentary environment. (C) Traditional pain related genes are unaltered by diet or exercise, except TrkA, which is reduced in HF-Ex mice relative to CF-Sed controls. (D) Exercise reduces gene expression of synaptosomal complex components (SNAP23, SNAP25, STXBP4) in high-fat diet fed mice. (E) A high-fat diet increases gene expression of AKAP150 (the kinase for TRPV1) and TRPA1 relative to chow-fed controls, and exercise reduces gene expression back to levels not different from chow fed controls. (n=4 for all groups) All data presented as mean ± SEM.
Analysis of energy expenditure was divided into 12-hour dark and light cycle quantifications and revealed no differences between any groups (Fig. 4c). Dark cycle analysis displays increased energy expenditure of both high-fat sedentary (p = 0.0120) and chow-fed exercised mice (p = 0.0254) compared to chow-fed sedentary mice (Fig. 4d).
The expression of mRNA encoding TFAM, DGKB, BDH1, HSP60 and HIF1-α was unaltered by diet or exercise. HSP70 mRNA levels were increased by exercise compared to sedentary mice (CF-Sed vs. HF-Ex, p = 0.014; HF-Sed vs. CF-Ex, p = 0.014; HF-Sed vs. HF-Ex p = 0.003, Fig. 5a). TrkA mRNA was reduced in high fat fed exercised mice compared to chow-fed sedentary mice (p = 0.024). Diet or exercise did not alter the expression of mRNAs encoding CGRP and NGF (Fig. 5b).
Figure 5.
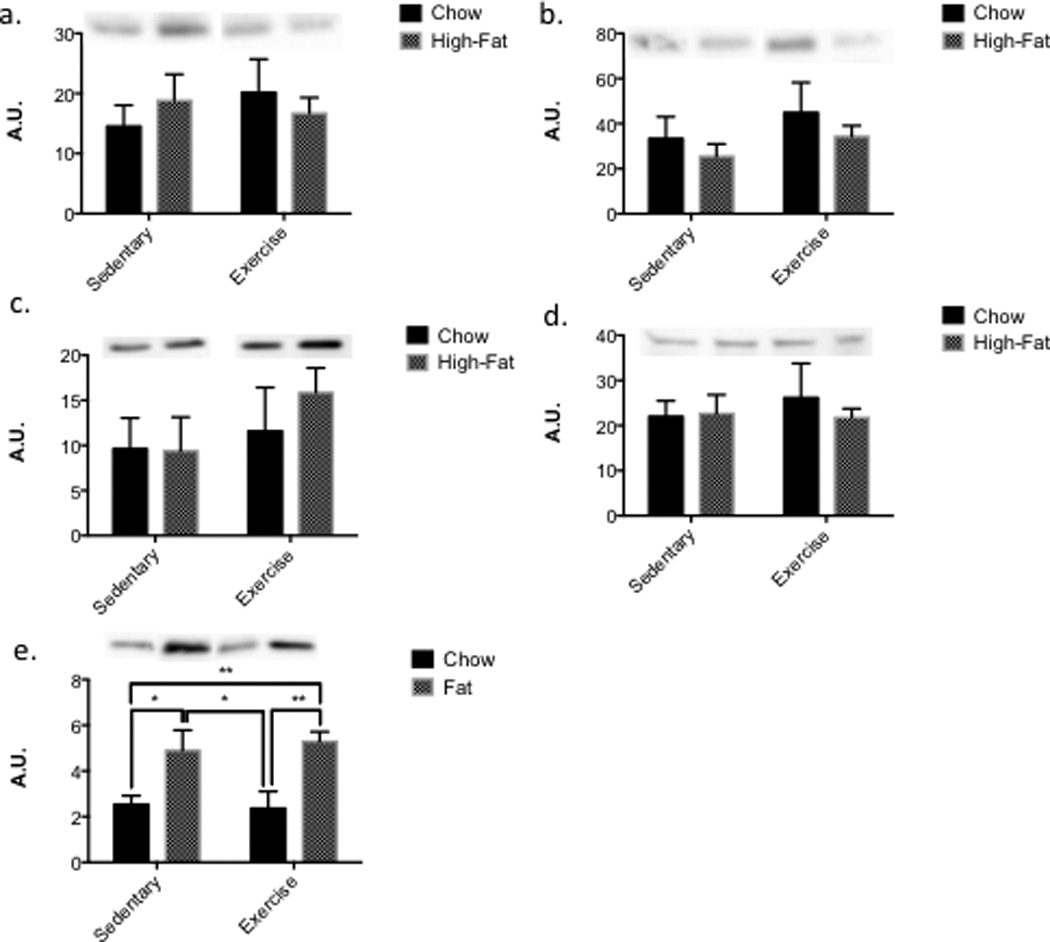
Diet and exercise does not alter mitochondrial, inflammatory or synaptosomal proteins, but does alter ketone processing signal!! (A, B, C, D) Electron complexes II, III, IV, and V are unaltered by either diet or exercise in the DRG of mice (E) The main effect of a high-fat diet increases PPAR-α in both exercise and sedentary mice as compared to their chow-fed counterparts (p < 0.001). (CF-Sed n=6, CF-Ex n=5, HFSed n=7, HF-Ex n=8) All data presented as mean ± SEM.
Exercise significantly decreased IL-1β (p = 0.011) and IL-6 (p = 0.032) levels in high fat-fed exercised mice compared to high-fat sedentary mice. IL-6 levels were elevated relative to chow-fed sedentary mice in chow-fed exercised (p = 0.007) and high-fat sedentary mice (p = 0.002, Fig. 5c). TNF-α was not significantly reduced in high-fat fed exercised mice relative to high fat sedentary mice (p = 0.053). In addition, p38 was decreased in high-fat fed exercised mice relative to chow-fed sedentary mice (p = 0.032) and high-fat sedentary mice (p = 0.0376). JNK was significantly decreased in high-fat fed exercised mice relative to high fat sedentary mice (p = 0.039).
TRPV1 mRNA expression was increased in chow-fed exercised mice relative to chow-fed sedentary (p = 0.037) and high-fat exercised mice (p = 0.026, Fig. 5d). AKAP150 was significantly increased relative to chow-fed sedentary (p = 0.030) and high-fat exercised mice (p = 0.012). TRPA1 was increased in high-fat fed sedentary mice compared to chow-fed sedentary (p = 0.049) and high-fat exercised mice (p = 0.040). mRNAs encoding SNAP23 (p = 0.035), SNAP25 (p = 0.009), and Stxbp4 (p = 0.033) were increased in high-fat fed sedentary mice compared to high-fat fed exercised mice. VAMP2 mRNA was slightly decreased in high-fat fed exercised mice compared to high-fat sedentary (p = 0.084). VAMP2 was statistically different in high-fat exercised mice compared to chow-fed sedentary mice (p = 0.017, Fig. 5e).
Western blot analyses revealed no changes in phosphorylation of p38 protein in the DRG due to diet or exercise. There were no significant alterations in any of the electron complexes analyzed between groups (Fig. 5a, 5b, 5c, 5d). PGC1-α, phosphorylated and total AMPK protein levels were not different across groups. Analysis of SNAP23 and SNAP25 protein levels also revealed no differences in response to diet or exercise.
Analysis of PPAR-α protein expression revealed increases in high-fat sedentary and high-fat exercised mice compared to chow-fed sedentary and chow-fed exercised mice (HF-Sed vs. CF-Sed, p = 0.0188; HF-Sed vs. CF-Ex p = 0.0184; HF-Ex vs. CF-Sed p = 0.0076; HF-Ex vs. CF, p = 0.0079, Fig. 5e).
Discussion
Our results demonstrate that mice fed a high-fat diet develop mechanical allodynia (Guilford et al., 2011; Groover et al., 2013). Mice with access to running wheels have a slow attenuation of mechanical allodynia after 6 weeks of exercise. We investigated potential molecular mediators that may explain how exercise benefits allodynia caused by a high-fat diet and revealed that exercise can correct sensory impairments without inducing changes in several key metabolic parameters.
In previous studies using high-fat diets, sedentary mice increase fat storage and weight gain, while exercised mice fail to gain weight and increase fat mass similar to sedentary mice (Bradley et al., 2008). Exercised mice fed a high-fat diet gained weight similar to high-fat fed sedentary mice. It is plausible to suggest these metabolic changes are due to exercise-induced increases in muscle mass as opposed to fat. Our results suggest changes other than fat mass reduction may be important in influencing allodynia based on the result that high-fat fed exercised mice did not differ in lean and fat mass compared to sedentary high-fat fed mice.
Since fat mass and weight changes may not explain the benefits associated with exercise, differences in utilization of fat as an energy source could be important. All our metabolic measures were obtained in cages where mice did not have access to running wheels to avoid acute metabolic changes associated with exercise. High-fat diets induce whole body lipid oxidation as evidenced by respiratory quotients (RQ) that approach a ratio of 0.7 (VCO2/VO2) (Krogh and Lindhard, 1920). Exercised and a high-fat fed sedentary mice appeared to utilize fats as a primary fuel source, suggesting that exercise does not lead to greater utilization of fat as an energy source. This is supported by our findings that sedentary high-fat fed mice had a greater resting energy expenditure compared to high-fat exercised mice. The sedentary high-fat fed mice had the highest fat pad mass, the greatest weight gains, and burned the most energy, indicating resting energy output may not be a key factor in the amount and utilization of fat related to allodynia. One caveat is that the current study performed the calorimetric analysis in caging without running wheels which could be argued to dampen the effects of 10 prior weeks of training.
We found increased blood levels of β-hydroxybutyrate in sedentary high-fat fed mice and that exercise significantly reduced β-hydroxybutyrate levels. Normally when utilizing fat as a key fuel source, ketone levels are elevated (McGarry and Foster, 1980). Exercised high-fat fed mice may process fat more efficiently, leading to a decrease in ketones. We analyzed PPAR-α, a fatty acid-activated transcription factor increased during ketogenesis (Lemberger et al., 1996). While PPAR-α protein levels were increased by the high-fat diet, exercise was unable restore elevated levels of PPAR-α in the DRG. The role of PPAR-α in DRG neurons is complex, as it has been shown to play a role in both analgesia and pain (Piomelli and Sasso, 2014).
Our results suggest the benefits of exercise are not mediated via increased fat metabolism alone, since high fat exercised mice have increased fat pad mass and burn fats as a primary fuel source, while expending less resting energy than high-fat fed sedentary mice. Exercised mice fed a high-fat diet displayed improved ketone processing and similar utilization of fat, while at the same time displaying decreased expression of mediators associated with inflammation. This suggests that exercise exerts benefits by affecting inflammatory pathways that influence the peripheral nervous system compared to the whole body fat storage and utilization.
The reduction of inflammatory mRNAs (TNF-α, IL-1β, IL-6, p38, JNK) in high-fat exercised mice as compared to sedentary high-fat mice is consistent with exercise’s anti-inflammatory role. Based on the exercise-induced changes in inflammatory gene expression, we hypothesized that this could lead to changes in TRP ion channels associated with pain (Chung et al., 2011). Our results revealed that TRPV1 mRNA was unaltered by diet or exercise. A kinase involved in the translocation and insertion of TRPV1 into membranes, AKAP150, was significantly decreased in high-fat exercised mice. Modification of TRPV1 trafficking and translocation are consistent with alterations in the synaptosomal complex. SNAP23/25, STXBP4, and VAMP2 were all decreased in high-fat diet exercised mice. These results point to a possible mechanism related to TRP channel trafficking and membrane insertion.
TRPA1 was also examined as it often co-localizes with TRPV1 and is co-activated through TRPV1 signaling (Bautista et al., 2006; Akopian et al., 2007). TRPA1 mRNA was elevated in high-fat sedentary mice and decreased in high-fat exercised mice. Changes in TRPA1 levels provide a strong link to the mechanical allodynia that occurs in response to a high-fat diet, and also suggests that future studies of both TRPV1 and TRPA1 are needed to fully understand the role of diet and exercise on sensory function.
Acknowledgments
This work was supported by the NIH grants P20GM1033418 and RO1NS43314 (D.E.W.) as well as core facility support from NICHD P30 HD002528 (Kansas IDDRC).
References
- Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol. 2007;583:175–193. doi: 10.1113/jphysiol.2007.133231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F, Fallucca F. Exercise training can modify the natural history of diabetic peripheral neuropathy. Journal of diabetes and its complications. 2006;20:216–223. doi: 10.1016/j.jdiacomp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bradley RL, Jeon JY, Liu FF, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. American journal of physiology. Endocrinology and metabolism. 2008;295:E586–E594. doi: 10.1152/ajpendo.00309.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chung MK, Jung SJ, Oh SB. Role of TRP channels in pain sensation. Advances in experimental medicine and biology. 2011;704:615–636. doi: 10.1007/978-94-007-0265-3_33. [DOI] [PubMed] [Google Scholar]
- Dobson JL, McMillan J, Li L. Benefits of exercise intervention in reducing neuropathic pain. Frontiers in cellular neuroscience. 2014;8:102. doi: 10.3389/fncel.2014.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groover AL, Ryals JM, Guilford BL, Wilson NM, Christianson JA, Wright DE. Exercise-mediated improvements in painful neuropathy associated with prediabetes in mice. Pain. 2013;154:2658–2667. doi: 10.1016/j.pain.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote CW, Ryals JM, Wright DE. In vivo peripheral nervous system insulin signaling. Journal of the peripheral nervous system : JPNS. 2013;18:209–219. doi: 10.1111/jns5.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford BL, Ryals JM, Wright DE. Phenotypic changes in diabetic neuropathy induced by a high-fat diet in diabetic C57BL/6 mice. Experimental diabetes research. 2011;2011:848307. doi: 10.1155/2011/848307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack MM, Ryals JM, Wright DE. Characterisation of glyoxalase I in a streptozocin-induced mouse model of diabetes with painful and insensate neuropathy. Diabetologia. 2011;54:2174–2182. doi: 10.1007/s00125-011-2196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A, Lindhard J. The Relative Value of Fat and Carbohydrate as Sources of Muscular Energy: With Appendices on the Correlation between Standard Metabolism and the Respiratory Quotient during Rest and Work. The Biochemical journal. 1920;14:290–363. doi: 10.1042/bj0140290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemberger T, Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: a nuclear receptor signaling pathway in lipid physiology. Annual review of cell and developmental biology. 1996;12:335–363. doi: 10.1146/annurev.cellbio.12.1.335. [DOI] [PubMed] [Google Scholar]
- Li L, Hondzinski JM. Select exercise modalities may reverse movement dysfunction because of peripheral neuropathy. Exercise and sport sciences reviews. 2012;40:133–137. doi: 10.1097/JES.0b013e31825f7483. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annual review of biochemistry. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Sasso O. Peripheral gating of pain signals by endogenous lipid mediators. Nature neuroscience. 2014;17:164–174. doi: 10.1038/nn.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Services USDoHH. 2014 National Diabetes Statistics Report. Center for Disease Control and Prevention. 2014 [Google Scholar]


