Abstract
Human tissues are remarkably adaptable and robust, harboring the collective ability to detect and respond to external stresses while maintaining tissue integrity. Following injury, many tissues have the capacity to repair the damage - and restore form and function - by deploying cellular and molecular mechanisms reminiscent of developmental programs. Indeed, it is increasingly clear that cancer and chronic conditions that develop with age arise as a result of cells and tissues re-implementing and deregulating a selection of developmental programs. Therefore, understanding the fundamental molecular mechanisms that drive cell and tissue responses is a necessity when designing therapies to treat human conditions. Extracellular matrix stiffness synergizes with chemical cues to drive single cell and collective cell behavior in culture and acts to establish and maintain tissue homeostasis in the body. This review will highlight recent advances that elucidate the impact of matrix mechanics on cell behavior and fate across these length scales during times of homeostasis and in disease states.
Keywords: Biomechanics, tissue homeostasis, development, cancer, mechanotransduction, mechanosensing, cell contractility, tissue tension, intracellular tension, mechanical force, matrix stiffness, mechanical memory, actin-myosin contractility, EMT
Introduction
Early two-dimensional (2D) cell culture studies focused on cellular responses to gases, charged ions (e.g. Ca2+, K+, etc), and simple compounds made by living organisms, or ‘biochemicals’ as they are most commonly called. From this work came an expansive body of knowledge on cell receptor-ligand interactions, the process of signal transduction, hypoxic response, and cellular activities such as growth, proliferation, survival, and motility. However, tissues are multicellular entities, and it became clear that biochemicals alone were not sufficient to generate tissues from cells. Biomechanical forces, an integrated feature of tissues that are initiated and dynamically controlled by cells, were often overlooked [1].
We now know that cell and tissue shape is defined by Type II myosin, which establishes inter- and intracellular tension through motor contractility along the actin cytoskeleton [2-4]. Actin filaments are anchored to cell-cell and cell-extracellular matrix (ECM) attachment points, and via cell surface receptors (e.g. integrins, cadherins), and the actin-myosin system is responsive to counter forces transferred from the ECM and other cells [5-7]. Iterative interactions between cells and the surrounding environment modifies tissue tension and relays cell-cell and cell-ECM forces across a tissue, resulting in adaptations in the size, shape, and position of cells during development and tissue regeneration. Biomolecules that can respond to changes in mechanical forces are called mechanosensors. As an example, integrin receptors can respond to extra- or intracellular forces with changes in conformation. This then drives recruitment of “inside-out” or “outside-in signal” transduction complexes, in addition to altering cytoskeletal dynamics, that then modify protein activity and gene expression [5,8-10]. In this way, cells possess an elaborate mechanism to integrate external biochemical cues together with physical interactions with neighboring cells and changes in the ECM to control tissue growth and morphology, and maintain tissue homeostasis. When this biochemical–biomechanical balance is disrupted, chronic disease and cancer often follows [11].
Cells possess the machinery to recognize and respond to many types of mechanical forces such as shear, tensile, and compressive stress, and environmental stiffness [12]. This focused review will highlight recent insights into the impact of matrix stiffness on single cell, multicellular sheet, and three-dimensional tissue tension, molecular signaling, behavior and fate. A mechanistic comprehension of how stiffness influences cellular systems across length scales then allows greater resolution of the processes maintaining tissue homeostasis that are hijacked in disease states (Figure 1).
Figure 1. The influence of force across length scales.
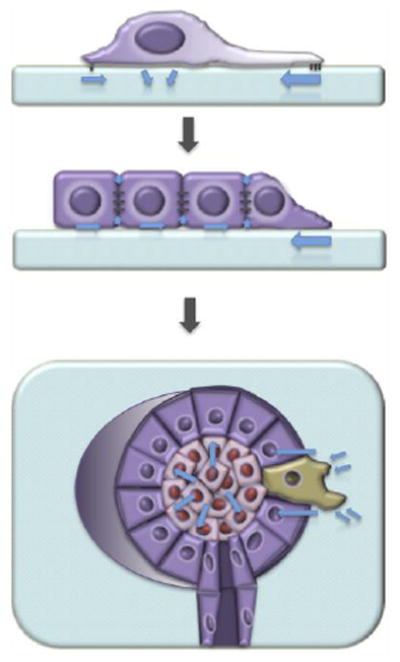
Distinct mechanical stresses influence mammary epithelial cell behavior and fate at the single cell (top), multicellular (middle), and tissue (bottom) levels.
Tension at the single cell level
Scientific discovery is driven in part by technological advance. With the advent of methods to dissociate single cells from normal (or transformed) tissues and maintain them for many generations outside of the body in culture dishes [13], there was an unprecedented opportunity to evaluate cellular responses to mechanical probing and biochemical cues . In essence, cell culture deconstructs cellular environments and enables a reductionist evaluation of cell responses to single or combinations of parameters. The concept that cells can mechanosense - and that mechanosensing controls fundamental cell behaviors - was first proposed in just this way. When embedded within soft agar, cancer cells, but not normal cells, could survive and divide to produce colonies - this observation introduced the notion that normal cells might require environmental anchor points for cellular functions and a integral feature of mechanosensing [14,15]. This observation further implied that hijacking molecular pathways downstream of mechanosensing could be a key feature of cell transformation, a premise supported by numerous subsequent studies [16] tying human health to the importance of understanding cellular mechanotransduction.
Focal adhesions and cell contractility
Mechanotransduction, or the ability to convert mechanical stress into chemical signals to drive cellular activity, begins with the capacity of cells to exert force on the surrounding environment [10,17,18]. This concept was first demonstrated in a paradigm shifting study by Harris et al, in which cell-induced wrinkling of flexible silicone-rubber culture substrates offered early evidence that cells generate attachment points that anchor and support force generation, which are then used to propel cells forward during migration [19]. Around the same time, the integrin family was discovered and became prime ‘mechanosensor’ candidates owing to their intracellular engagement with signaling complexes, extracellular attachment to the ECM, and conformation- and clustering-dependent activity [20]. Since then, our comprehension of how cells communicate with the ECM has flourished. More specifically, large multiprotein complexes termed ‘focal adhesions’ have been discovered, which nucleate from the intracellular domains of integrins in response to force and act as a mechanical link with the ECM to propagate inside-out and outside-in signal transduction pathways [5,8,9]. Focal adhesion proteins include focal adhesion kinase (FAK), Src family kinases (SFK), paxillin, α-actinin, vinculin, and talin, most of which bind directly to the actin cytoskeleton [21] (Figure 2).
Figure 2. Focal adhesions and cell contractility.
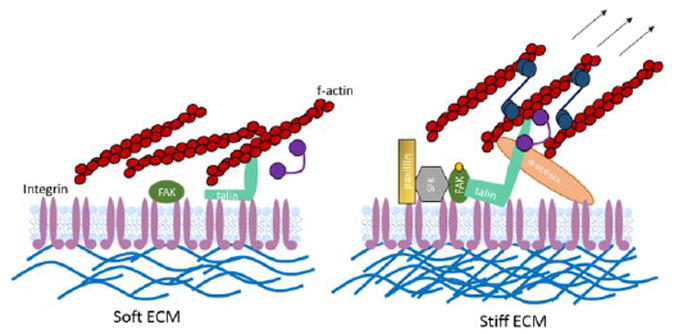
Immature focal adhesions form when cells are in contact with soft extracellular matrices (ECM; left), and therefore intracellular force generation does not occur. Focal adhesion maturation (right) is supported by cellular interactions with stiff matrices, which engage the actin-myosin network and ultimately initiate mechanotransduction events that drive cell behaviors in response to extracellular cues.
The increased load bearing of stiff cell substrates supports higher intracellular tension through the actin-myosin system as compared with soft substrates, which in turns fuels the growth and stability of cell-matrix adhesions and drives the activity of another mechanosensitive biomolecule; talin [22]. Talin binds directly to integrins and to actin. When talin bound actin undergoes rearward-flow in stiff environments, talin is stretched to open up binding sites for vinculin. This stabilizes the growing adhesion and increases the affinity for talin binding to integrins, thereby reinforcing the downstream mechanotransduction signaling events [17]. The very nature of proteins recruited to maturing focal adhesions, many of which are kinases, highlights one straightforward mechanism to explain how mechanical force contributes to dynamic changes in intracellular signaling, and allows cells to respond appropriately to the extracellular environment by promoting survival, proliferation, migration, or fate transitions [23].
Through the implementation of synthetic hydrogel culture substrates, which are cross-linked to varying degrees to adjust matrix stiffness and modified to present an adhesive interface, it is now accepted that cellular forces established through cell-ECM interactions and modified by the stiffness of the matrix are critical to cell spreading and motility [24]. Technologies such as the traction force microscopy technique [25] further validated this conclusion by quantifying the magnitude, position, and direction of cell generated forces in response to different matrices.
Matrix stiffness and cell fate
Early mechanotransduction research focused primarily on the fibroblast since this contractile cell type is intimately involved in depositing and modifying the extracellular environment and establishing tissue tension. However, the fact that many other cell types express the mechanosensing and transducing machinery gave way to the concept that perhaps mechanotransduction is a universal property of all cell types, governing an even broader set of cell biological responses. Groundbreaking work from Engler et al provided the first demonstration that substrate stiffness modifies the directed differentiation of multipotent mesenchymal stromal cells (MSCs; [26]). MSCs were cultured on synthetic substrates that were crosslinked to varying degrees to modify the elastic modulus of the surface (while maintaining ligand density constant). When the substrate was matched to the bulk mechanical properties of native tissues, an enrichment of cells expressing transcripts and proteins consistent with neuronal, skeletal muscle, or bone progenitors was observed. Pharmacological agents to manipulate cell contractility and GTPase activity suggested that actin-myosin force generation and Rho signaling plays an integral role in mechano-sensitive directed differentiation. Subsequent studies stressed that culture substrate stiffness does not induce effects in isolation, but rather that chemical cues in the culture media synergize with matrix elasticity to elicit changes in gene expression [27].
These findings have important implications for overcoming a long-standing regenerative medicine hurdle – constructing defined protocols to robustly and efficiently direct the differentiation of multipotent MSCs, embryonic stem cells, and pluripotent stem cells to a desired fate. Indeed, matrix mechanics is now a common consideration in specification protocols alongside the biochemical approach. On the flip side of that coin is the challenge of maintaining stem cell self-renewal potential in culture. Adult stem cells, such as skeletal muscle stem cells, quickly lose self-renewal properties and differentiate to generate post-mitotic multinucleated muscle fibers when removed from the body and cultured in plastic dishes [28-31]. If instead freshly-isolated muscle stem cells are cultured on substrates matching the softness of the native tissue, and in conjunction are treated with the fibroblast growth factor-2 mitogen, it becomes possible to support self-renewal divisions ex vivo [31,32]. Furthermore, this can produce a therapeutic population of cells that contribute to muscle repair and repopulate the stem cell niche when transplanted into recipient muscles [31,32]. Tuning substrate stiffness appears to support the self-renewal of stem cells isolated from a variety of tissues, highlighting the universality of the principle [33].
Mechanical memory
If mechanotransduction in response to matrix stiffness drives normal processes, then it follows that progressive conditions characterized by stiff fibrotic scarring might also be influenced by mechanics. In normal repair, fibroblasts play a critical role in resolving tissue injury by depositing and organizing ECM, as well as establishing a balance of tissue forces, or ‘tensional homeostasis’ [34]. In chronic conditions a subpopulation of fibroblasts transition to the myofibroblast fate, as characterized by high-level expression of α-smooth muscle actin (α-SMA), a protein that stabilizes stress fibers to supercharge contractility and boost extracellular matrix production. The downstream effect of myofibroblast conversion is the propagation of fibrotic conditions that characterize a number of conditions including cancer. It also appears that this is a self-propagating cycle, owing to the phenomena of heritable changes in gene expression and/or protein activity that are elicited by culture on stiff substrates, or “mechanical memory”, that is emerging in the literature [35-38].
MSCs maintain a malleable fate when cultured on substrates within a tight range of stiffnesses, but when exposed to surfaces above that range, MSCs are irreversibly biased to generate cartilage cells [38]. Since the vast majority of MSC maintenance culture utilizes rigid polystyrene dishes, this is a cautionary tale warning against making bold conclusions about lineage decisions toward the cartilage fate. A similar trend dictates fibroblast fate - fibroblasts born into mechanically homeostatic environments are conditioned to maintain the fibroblast phenotype, even if they transiently contact a stiffer environment, as would be expected to occur during the normal process of wound repair. However, fibroblasts born into mechanically stiff environments transition to the contractile myofibroblast fate, and even when challenged with a soft environment, will act as though they are still in a stiff environment [39]. As a result, converted myofibroblasts further stiffen the environment and convert future generations of fibroblasts to a similar fate. These culture findings have important implications for mesenchymal stromal cell transplantation therapies, as well for understanding cancer progression, and might warrant consideration when implanting rigid biomaterials or devices into soft tissues. It also highlights another level of understanding that is required of the molecular mechanisms driving irreversible fate changes in response to rigid matrices.
Towards this therapeutic goal, α-SMA also appears to be required for the fate of the cell, such that diminished expression of α-SMA converts myofibroblasts back to a multipotent MSC-like cell [37]. If α-SMA dictates myofibroblast mechanical memory, then identifying molecular mediators that control α-SMA expression might target and erase the mechanical memory. Indeed, a recent report showed that NKX2.5, an α-SMA repressor, is driven out of the nucleus when cells are cultured on stiff substrates. By overexpressing NKX2.5 it is possible to both prevent the α-SMA response to stiff matrices, as well as to erase the α-SMA-induced mechanical memory that is characteristic of myofibroblasts [35] (Figure 3).
Figure 3. Erasing a mechanical memory.
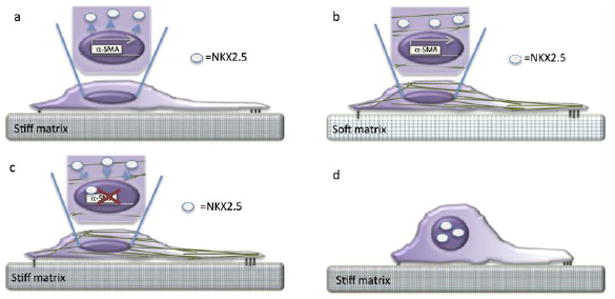
(a) Mesenchymal stromal cell culture on rigid substrates induces expression of α-SMA, which in turn transitions the cells from a more rounded morphology (as portrayed in d) to that of a contractile myofibroblast-like fate characterized by actin stress fiber formation (green fibrillar structures as seen in b and c) and cell spreading (as seen in a-c). On stiff culture substrates, α-SMA expression is reinforced by the nuclear deportation of NKX2.5 (white circles outside of dark purple nucleus), a potent inhibitor of α-SMA transcription. NKX2.5 is then either degraded or retained in the cytoplasm in association with stress fibers (as seen in b). (b) Typically, mesenchymal stromal cells propagated on soft substrates retain a rounded shape (as seen in d). If, however, mesenchymal stromal cells exposed to a stiff culture environment are then transitioned to a soft substrate, the ‘mechanical memory’ of the stiff environment prevails; NKX2.5 is excluded from the nucleus, α-SMA expression is retained, and the contractile morphology is observed. (c) Notably, by enforcing NKX2.5 expression and nuclear import, α-SMA expression is abolished and (d) it is possible convert a myofibroblast-like cell back to the original mesenchymal stromal cell fate, even if cultured on a stiff substrate. This indicates that the mechanical memory can be erased and cell fate reverted by understanding the molecular mechanisms at play when cell contact a stiff culture substrate.
The implication of mechanical memory is that it is engrained within the cell and is difficult to overturn. In the case of α-SMA, once expressed at high levels, endogenous self-enforcing mechanisms exist to ensure persistent expression of the protein [40]. Another proposed mechanism by which a mechanical memory is imprinted onto a cell is by physically altering the chromatin state. Indeed, calcium signaling downstream of cyclic stretch triggers changes in chromatin organization, and is proposed to elicit a mechanical memory in response to environmental mechanics [36]. Of note, multiple rounds and not singular episodes of loading are required to achieve a memory in MSCs, thereby providing flexibility in the system for cells to transiently survey diverse environments without being irreversibly sent down a specific fate pathway. The observation also highlights the value of understanding the molecular mechanisms underlying mechanically-induce fate transitions and mechanical memory, in order to identify therapeutic gateways that prevent additional fibrotic accumulation.
Molecular mediators of matrix stiffness
It is now understood that a number of transcriptional factors are activated downstream of mechanosensitive focal adhesion assembly and maturation to transduce physical cues to biochemical intracellular cascades [41]. Among the growing list of mechanosensitive transcription factors and transcription factor activators are the serum response factor (SRF) [27,42], hippo signaling pathway Yes-associated protein (YAP)/transcriptional co-activator with PDZ-binding motif (TAZ) [43], c-jun N-terminal kinase (JNK) [42], members of the the mitogen activated protein kinase (MAPK) family such as extracellular signal-regulated kinase (ERK)[42], myocardin-related transcription factors (MRTFs) [44], nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) [45-48], and β-catenin [49]. Several of these proteins are activated by focal adhesion associated kinases, many of which associate with focal adhesions based on focal adhesion maturation state (small vs. large adhesions). For some transcription factors, the actin cytoskeleton takes center stage as the chief entity responsible for relaying and moderating intracellular activity in response to extracellular cues [50]. Actin dynamics are moderated by the activity of GTPase molecular switches belonging to the Rho family [51]. Notably, several of the proteins recruited to the nascent focal adhesions, like SRC [52] and FAK [53], associate with and influence the activity of Rho guanine nucleotide exchange factors (GEFs). GEFs dissociate GDP from the GTPase, thereby permitting GTP to bind and reactivate GTPase activity [23]. Rho family GTPase activity stimulates the activity of downstream effectors involved in controlling various aspects of actin assembly and disassembly [54].
One mechanism linking actin dynamics to transcriptional events occurs via the myocardin protein family (myocardin and MRTFs) of transcriptional cofactors [50]. MRTFs are maintained in the cytoplasm bound to globular actin (G-actin). Factors that drive formation of filamentous actin (F-actin) stress fibers, such as focal adhesions, encourage MRTF dissociation from G-actin, allowing them instead to bind and translocate transcription factors to the nucleus (Figure 4). By this mechanism, the transcriptional activity of serum response factor (SRF), which induces expression of genes involved in cell motility and fate regulation, is dynamically tied to cytoskeletal state by the availability of its co-factor MRTF-B (aka MAL) [55-57].
Figure 4. Actin dynamics control transcriptional activators.
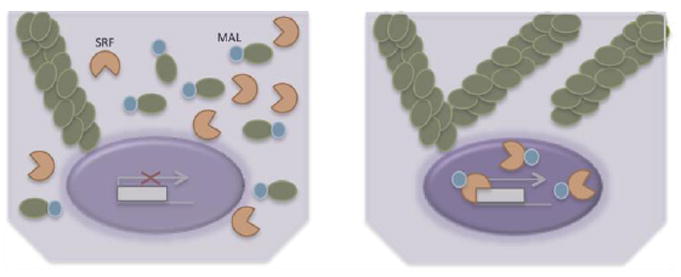
Non-contractile cells contain a pool of globular actin (g-actin) monomers in addition to fibrillar actin (f-actin) fibers. G-actin binds to MAL, a SRF transcriptional co-activator, and sequesters the factor in the cytoplasm (left). Intracellular tension is characterized by the formation of stress fibers causing the g-actin pool to diminish, and releasing MAL to bind to SRF, translocate to the nucleus, and initiate transcription (right).
Likewise, YAP/TAZ nuclear activity is mechanosensitive, but in a G-actin independent manner. The role of YAP and TAZ in mechanotransduction was first described in MSCs [43], but has since been shown to be activated downstream of elevated matrix stiffness in many cell types [58]. The YAP/TAZ complex translocates to the nucleus of MSCs cultured on stiff substrates, as well as those induced to spread on large adhesive islands of ECM [59]. YAP/TAZ activity drives MSC differentiation phenotypes associated with stiff culture substrates and by blocking YAP/TAZ activity, MSCs will instead be specified to cell types associated with culture on soft substrates. While YAP/TAZ activity is dependent on stress fiber formation, unlike SRP, YAP/TAZ activity is not dependent on G-actin, suggesting the enticing possibility that F-actin might instead sequester a YAP/TAZ antagonist.
The elucidation of mechanosensitive signal transduction pathways and transcriptional activators has lent new appreciation of functionality to these factors, and assigned molecular downstream events to extracellular changes in mechanics. Given the prevailing impact of mechanotransduction on cell fate, additional mechanosensitive mechanisms to modify gene signatures are sure to emerge.
Modeling features of in vivo mechanotransduction in 2D culture
The great majority of studies exploring mechanotransduction utilized synthetic hydrogels tuned to a variety of stiffnesses by modulating the extent of polymer cross-linking, and presenting an adhesive interface to cells with unlimited area to spread. An emerging focus in the field of mechanotransduction research is to refine culture systems in order to recapitulate additional features of the native cellular environment. For example, while synthetic hydrogels are primarily elastic, natural ECMs that a cell would interact with in the body are viscoelastic and exhibit stress relaxation, such that cellular traction forces are expected to remodel the ECM. Indeed, both computational modeling and experimental approaches confirm this hypothesis [60,61]. Hyaluronin is a natural ECM that is tightly regulated to ensure expression at times of tissue reorganization and fate specification, such as occurs during development and in adult tissue repair [62]. Though hyaluronin yields very soft substrates, untransformed muscle and non-muscle cells were observed spreading, assembling focal adhesions and stress fibers to a degree matching levels seen on much more rigid elastic hydrogels [61]. Interestingly, this study also reports that cell spreading on soft hyaluronin occurs independently of the typical traction force measurements obtained for contractile cells on synthetic materials. In another study, alginate hydrogels were covalently or ionically cross-linked to create elastic and stress-relaxing substrates respectively, but with paired biochemical properties. By decoupling physical and chemical properties the authors confirmed that cell spreading behavior and YAP/TAZ nuclear translocation on soft materials with stress relaxation properties matches those of more rigid elastic materials, but only at high ligand densities [60]. Hence, one can conclude that modeling cellular environments in culture to assess cellular mechanotransductive responses requires a careful consideration of the types of ECM and ligands that are expected to be present in the native cellular environment.
A three-dimensional (3D) matrix perfectly positions chemical cues and physical forces, but also exerts spatial constraints that will support or impede cell spreading. This concept was tested in a Watt et al report in the late 1980s, by demonstrating that restricting epidermal keratinocyte cell spreading area had the effect of forcing a rounded cell shape, cell cycle exit, and upregulation of involucrin, a marker of terminal differentiation [63]. A recent follow-up study concluded that keratinocyte terminal differentiation on small islands that limit ECM contact is mediated by SRF-MAL activity [27]. Using microcontact printing to carefully control the size of adhesive interfaces and produce ECM islands [64] led to the knowledge that cell shape also controls cell viability [65] and directs MSC lineage progression [66].
Numerous additional cell fate decisions appear to take advantage of synergies between spatial constraints and the biochemical milieu. For example, a recent study indicates that controlling cell shape in conjunction with exposure to inductive cytokines dictates macrophage phenotype and polarization [67]. Specifically, ECM patterning that encourages macrophage elongation favored polarization to the M2 fate and synergized with M2 specific cytokines to upregulate arginase-1, an M2 specific marker. Cell patterning also mitigated iNOS levels, a marker of M1 macrophages, when the elongated macrophages were exposed to M1-inducing cytokines. The concept that cell shape supports specific gene expression patterns was elegantly shown by systematically exposing murine fibroblasts to microfabricated fibronectin patterns engineered with a wide variety of aspect ratios and shapes [68]. Gene expression analysis revealed modular changes in gene expression, correlating most robustly with cell size and less so across different aspect ratios or shapes of the same area. Cell shape-related gene profiles correlated with cytoplasmic-to-nuclear translocation of histone deacetylase-3 and MRTF-A and were dependent on actin-myosin contractility.
Another commonly dismissed aspect of in vivo environments (often missed in culture studies) is the convergence of several different forms of mechanical stress. One such stress is cyclic stretch, a mechanical parameter long appreciated for its ability to impact cell fate. More recently, mechanistic insights into the downstream effects of cyclic stretch have been revealed. Mammary epithelial cell proliferation was induced in response to cyclic stretch, the proliferation being contingent on YAP activity. Surprisingly, the YAP-dependent effect on proliferation was independent of Hippo signaling since cyclic stretch induced JNK activity, which in turn silenced the Hippo pathway by increasing binding to a Hippo pathway inhibitor [69]. Similarly, a study of mouse embryonic fibroblasts exposed to stretch and relaxation cycles revealed an impact on cell cycle entry [70]. Stretching cells cultured on soft micropillars led to phenotypes matching those of a stiff culture substrate, namely, stress fiber formation, cell spreading and proliferation, demonstrating the dominance of the stretch cue over matrix stiffness. Mechanistically, cyclic stretch supported MRTF-A nuclear translocation within 2 hours, while YAP was observed to relocate from the cytoplasm to the nucleus at later time-points. However, disrupting expression of MRTF-A or YAP was sufficient to abolish the stretch-induced effect on proliferation [70] (Figure 5).
Figure 5. Stretch forces override matrix mechanics to control fibroblast identity.
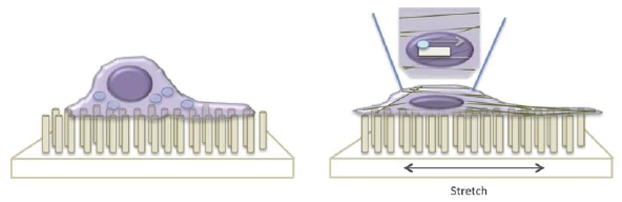
When cultured on soft micropillar arrays (left), cells maintain a rounded cell shape, small focal adhesions, and are devoid of stress fibers. However, if the soft micropillars are subjected to a cyclic stretch routine (right), cells respond by exhibiting phenotypes and gene expression patterns that are reminiscent of stiff culture substrates.
Visualizing actin cytoskeletal dynamics in response to mechanical stimulation at the plasma membrane demonstrated that actin accumulates in the perinuclear region of cells within two minutes of stimulation. This effect is dependent on bursts of intracellular Ca2+, as well as the activity of an actin polymerization activator called inverted formin-2 (INF2) that is found localized to the endoplasmic reticulum and the nuclear membrane [71]. It is unclear what role perinuclear actin assembly fulfills – be it to protect the nucleus from damage, or modify nucleoskeletal dynamics which drive gene expression or transcription factor mobility. However, what is clear is that the response is fast. Ca2+ transients occur in response to external stimulation at a timescale that precludes focal adhesion involvement, and this highlights the very rapid effect that external mechanical stimuli can exert on cell fate. Indeed, it is enticing to consider that another mechanism by which mechanical stimulation and ion channels may synchronize is to activate proteins that are both mechano- and pH-sensitive [72]. And given the potent effect of stretch activated channels on cell fate, as highlighted by the ability of the Piezo-1 channel to direct the lineage choice of neural stem cells [73], it will be important to consider how a variety of mechanical signals and chemical signals experienced by cells are integrated to produce a behavioral or fate output.
Mechanotransduction clearly plays a central role in controlling cell behavior and fate. It is perhaps not surprising that mechanosensors, focal adhesion proteins, and downstream effectors of extracellular force are commonly mutated in cancer cells [16]. As a result, the fundamental knowledge gained through mechanotransduction studies on single cells has rapidly accelerated our understanding of these key factors in malignant transformation events, as well as cancer progression in three-dimensional tissues.
Tension at the multicellular level
Epithelial tissues maintain tight contact with neighboring cells that are mediated by cadherins. Like integrins, cadherins at adherens junctions are sites of actin nucleation. However, in the case of adherens junctions, the actin cytoskeletal network not only functions to coordinate signaling transduction events, but also can propagate tension to neighboring cells as part of a mechanical relay. Cadherins lend to the cobblestone appearance of epithelial tissues like skin, lung, and mammary gland, as well as the morphologies of cultured embryonic stem cells and induced pluripotent stem cells. The regulation of tension across groups of cells is a driving force that determines how they assemble into tissues [74]. Indeed, an important emerging concept is that rather than being merely passive mediators of adhesion, cadherin junctions distribute intracellular stress to modulate epithelial sheet proliferation and organization, and are involved in mechanotransduction – serving as sites of tissue integration [75]. This is particularly vital during development and following wound repair, as discussed at length in the Yu and Fernandez-Gonzalez review found in this Special Issue. The current review will focus on key cadherin-specific concepts that lend to a greater understanding of the mechanical origins of cancer and tumor pathogenesis.
Matrix stiffness and the epithelial-to-mesenchymal transition
As with single cells, epithelial sheet homeostasis is acutely sensitive to culture substrate stiffness. Indeed, when the balance is tipped, emerging phenotypic changes are consistent with tumor initiation. Non-malignant mammary epithelial cells form two-dimensional organized acini with a hollow lumen area when cultured on soft polyacrylamide substrates (~200 pascals) and provided with a reconstituted basement membrane to ligate integrin receptors [76]. If instead the cultures are initiated on hydrogels matching the stiffness of tumors, acini morphogenesis fails to occur and instead, non-polarized structures with an invasive phenotype arise. These phenotypic changes were correlated with elevated Rho-dependent cytoskeletal tension. This then lends one to speculate that a ‘second hit’ to tumors bearing epidermal growth factor receptor mutations might arise from deregulated mechanotransduction that then elevates ERK and Rho activity – a cell extrinsic mechanism of tumor progression. This concept was supported using pharmacological compounds. Intriguingly, mammary progenitor and luminal cells take on basal-like properties with age and a recent study suggests that improper mechano-responses to matrix stiffness may underlie this observation [77].
The phenotype of mammary epithelial cells cultured within or on top of hydrogels with tumor-like stiffness was found to be reminiscent of cells undergoing epithelial to mesenchymal transition (EMT) [78]. Transitioning from the cobblestone morphology to that of a more independent and contractile cell phenotype, in which cell-cell contacts are broken and an invasive leading edge emerges, is a primary characteristic of transformed cells. EMT is induced by the activity of transcription factors including Twist [79], Snail [80,81], and the Zeb family [82]. It was recently reported that matrix stiffness pushes cells into EMT via Twist activity that occurs by release from a cytoplasmic binding partner that then enables nuclear translocation [83]. Consistently, the authors found that mutations in the Twist cytoplasmic binding partner are correlated with poor patient survival and highlight a mechanotransduction mechanism hijacked in cancer.
Tension within the epithelial sheet must be established and dynamically maintained for the tissue to respond to mechanical cues in concert. In a study evaluating development of the drosophila wing it was noted that tissue mechanics was achieved through the process of proliferation [84]. During development it was observed that differential rates of division in two regions of the wing produced two axes of tension – one along the proximal-distal axis and the other perpendicular to the axis. In positions where there is local overgrowth, neighboring cells respond by stretching and modifying orientation of cell division, and this ultimately sets up tension and defines the shape of the wing.
Cadherins as mediators of intracellular tension
Ultimately it is the cell-cell contacts at points of adhesion that maintain and relay tension to elicit collective cell responses. Cadherins link to the actin cytoskeleton via β-catenin and α-catenin in a force dependent manner [85] and it is this association with cytoskeletal elements that underlies tensional homeostasis in epithelial tissues (Figure 6). Understanding how this homeostasis is coordinated is complicated by the existence of many systems working in concert, including interactions between cell-cell adhesion proteins, intercellular forces, and the dynamics of the epithelial sheet. In one study, a systematic approach was implemented to evaluate cellular velocity and deformation rates, in addition to measuring intracellular, intercellular, and extracellular forces following downregulation of key transmembrane proteins associated with tight junctions, adherens junctions, and gap junctions [86]. In addition to defining the processes of forming a collective epithelial monolayer, it was found that cadherins take on quite distinct roles. While E-cadherin is generally considered the only cadherin involved in force generation, it was observed that the concentration of P-cadherin determines the level of intracellular force, while E-cadherin concentration predicts the rate at which the force will build. Intriguingly, although E-cadherin is generally correlated with mechanotransductive events owing to its association with α-catenin, experimental evidence suggests that when E-cadherin is absent, P-cadherin is capable of triggering mechanotransduction. Despite somewhat redundant functions, both cadherins are required to balance tissue force and when one or the other is deregulated, altered tissue dynamics or tissue transformation ensues. These findings provide overdue insight into a long noted conundrum in cancer biology. Mutations in P-cadherin are associated with enhanced metastatic potential. Indeed, mammary carcinoma collective migration was recently shown dependent on ECM identity and the presence of P-cadherin [87]. The knowledge that P-cadherin can be involved in mechanotransduction and is primarily responsible for determining the level of intracellular force offers some reconciliation to this quandary.
Figure 6. Cadherins mechanosense and transmit intracellular force.
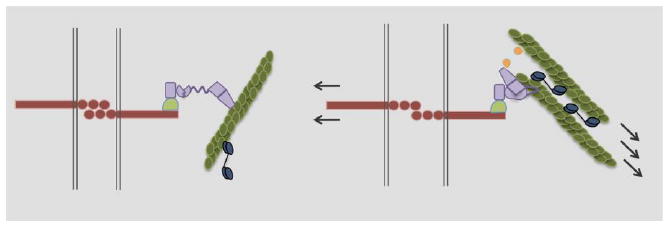
Epithelial cells maintain tight cell-cell contacts in part through the interaction of cadherin homodimers (red). In the absence of intracellular tension (left), cadherin interacts loosely and transiently with actin fibers through a β-catenin (pale green), α-catenin (purple) complex. In response to force (right), α-catenin undergoes a conformational change that allows for tight actin binding. In addition, vinculin (yellow) is recruited to α-catenin and can also bind to actin. In this way, intracellular force is transmitted between cells in an actin-myosin (blue) dependent manner.
Cellular effects of intracellular tension
Intracellular tension not only maintains the shape of a tissue, but also the behavior and fate. For example, within colonies of embryonic stem cells, despite the size, it was observed that inner and outer regions exhibit different mechanical properties. Heterogenous colony mechanical properties was correlated with attaining distinct downstream developmental fates suggesting that the transition to different mechanical states is a key feature of defining differentiation transitions [88]. Stretching or compressing epithelial sheets has the effect of modulating cell cycle progression [89,90]. Drosophila wing size can be controlled by stretching the wing, which induces cell proliferation in a force dependent manner [89]. Likewise, studies of a model mammalian epithelial monolayer studied the interplay between stretch and compression, which arise in tissues as a response to physical spatial constraints and cell crowding. Indeed, when compressed using a barrier or allowed to stretch by removing the barrier, the effect was to halt cell cycle progression or induce proliferation, respectively [90]. A recent study provided mechanistic insight into strain-mediated cell proliferation. Cell cycle entry in response to mechanical strain was shown to be associated with nuclear accumulation of YAP1 followed by β-catenin, while inhibiting either protein disrupted stretch-mediated effects on monolayer proliferation. The molecular effects of strain were contingent on extracellular E-cadherin engagement and cytoskeletal tension [91].
Collective responses to surface topography
In addition to force, surface topographies encountered by epithelial sheets serve to modify collective cell behavior. In silico combined with in vitro studies discovered that cells clear or detach from regions of local negative curvature, an effect that does not occur in regions with positive or no curvature [92]. Single cell changes in contact angle influence the collective dynamics of the epithelial sheet and are contingent on tissue contractility and adhesive forces, a feature that is lost when cancer oncogenes are induced in non-transformed cells.
Together, these studies provide new cellular and molecular insight into the behavior of epithelial cancers and highlight the importance of understanding basic mechanisms underlying developmental processes.
Tension at the tissue level
Tissues are three-dimensional entities and understanding how forces are established, maintained, and manipulated to modify behavior and fate must take into consideration dimensionality [18,93-96]. The knowledge gained from culture studies of single cells and epithelial sheets paved the way for understanding effects at the tissue level, which is appreciated by the increasing number of studies focused on validating mechanotransduction concepts at the tissue level. Indeed, many of the players remain the same – mechanotransducers, the actin-myosin network, and the ECM. The following sections will provide a selection of insights relating force to normal and malignant processes as they occur in the context of a three-dimensional tissue.
Tissue mechanics and development
Matrix stiffness and intracellular force transduction is intimately tied to normal developmental processes. Consider epithelial patterning - all epithelial tissues contain regularly organized sheets of interacting cells, but the ultimate morphology of the structure can vary tremendously. For example, intestinal crypts take on an undulating shape. How this arises is a matter of debate ranging from a proposed ‘spatial buckling’ model to a somewhat more active establishment of shape, involving defining matrix stiffness and spatial mechanics. In silico modeling that takes into account aspects of both hypotheses supports the notion that crypt formation along a basal lamina that is free-moving (as expected during development or tissue repair) would support an undulating morphology, while basal lamina attachment to an underlying matrix would in effect ‘stiffen’ the environment and support more regular patterning [97].
Tuning matrix stiffness is also central to achieving proper gastrulation ex vivo from single embryonic stem cells. Embedding single embryonic stem cells within a soft fibrin matrix supports cell proliferation and formation of large colonies, as compared to culture within stiffer fibrin gels, and highlighting the importance of stiffness to the initial phases of development [98]. Achieving formation of all three germ layers in culture from a single cell has proved a difficult challenge. However, by placing 3D colonies in contact with a soft matrix, self-organization occurred and all three-germ layers with correct positioning was attained. Disrupting induced pluripotent stem cell colony contractile machinery disrupted the process, indicating that matrix attachment elicits gastrulation-like events by a mechanism that includes discrete spatial generation of intracellular force. Indeed, the culture platform affords a unique opportunity to explore the interplay between chemical gradients and cellular forces on the process of gastrulation and explore mechanistic origins of development. For example, it was recently determined that human embryonic stem cells cultured on soft substrates enhances mesoderm specification by supporting adherens junction formation and also by sustaining Wnt levels required for mesoderm specification by supporting β-catenin nuclear translocation [99]. These stiffness-dependent cellular and molecular events, then primed the soft-cultured embryonic stem cells to be more sensitive to mesoderm inducing morphogens in the culture milieu.
Mammary branching morphogenesis during development and in puberty is another process in which force and mechanotransduction is taking a leading role. Mammary patterning and understanding the cues that dictate branch points has long captured the attention of developmental biologists. Elegant studies enlisting three-dimensional patterning provided new insight into the process and implicated the importance of stress and mechanotransduction in branch initiation. Patterned regions with sharp curvature produced sites of high mechanical stress that were anticipated by modeling and supported by 3D traction force microscopy measurements – using this information they were predicted as branch sites [100]. Indeed, branch sites initiated from positions of high mechanical stress upon exposing the 3D patterned cultures to proteins that support branching, and could be abolished by disrupting mechanotransduction.
The mammary ductal system is highly organized, with the mammary fat pad instilled with regular arrays of branches. Force generation and stiffness are vital to the integrity of duct pattern formation. In the body, branches align with axially oriented Type 1 collagen fibrils, and culture models suggest that mammary acini-generated forces produce fibril orientation, which is used as a track to position branches. When placed in 3D environments containing aligned collagen fibrils, mammary organoids will drive branch extension in line with the collagen tracks [101]. If instead the organoids are embedded within randomly oriented collagen, the mammary structures rapidly reorganize collagen at duct branch points through a process of force generation to establish axially oriented collagen fibril tracks and proceed to extend. In the setting of cancer, tumor cells adopt the ability to establish ECM architectural cues for the purpose of malignant transformation.
Disrupted tensional homeostasis potentiates breast cancer
Mammography analysis of breast cancer patients reveals dense breast tissue that is caused in part by overproduction of collagen and excessive cross-linking. Notably, collagen fibrils at the very boundary of breast tumors are aligned perpendicular to the tumor border, clearly poised to enable transformed mammary cells to escape the boundary and metastasize. While aligned fibers are indeed found to be more mechanically rigid than randomly oriented collagen matrices, this does not translate to an increased rate of cell migration as one might predict [102]. Instead, fiber alignment encourages persistence to direct the movement of tumor cells far from the lesion. Mammary structure disorganization can be accelerated by the concerted activity of two or more mammary acini with compromised organization (pre-malignant like) [103]. Specifically, radially aligned tracks of collagen connect between two compromised acini to create a super highway that increases the rate of acini disorganization in a synergistic manner, while single acini also disorganize, but at a substantially slower rate. Hence, long-distance mechanical interactions between two pre-malignant lesions accelerate transition to an invasive phenotype and stress the importance of ensuring the removal of nodules that are morphologically abnormal (Figure 7). However, it should be noted that potential effects of chemical gradients arising from acini proximity were not ruled out and may synergize with the collagen super highways or even play a dominant role.
Figure 7. Long distance mechanical interactions accelerate cancer cell invasion.
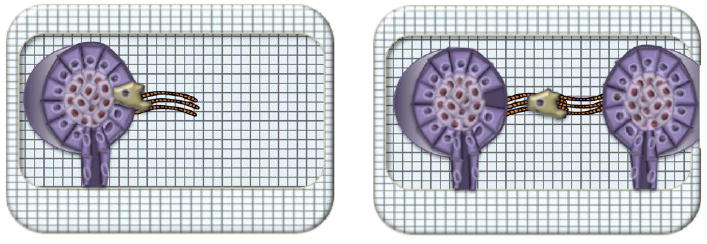
Transformed mammary acini orient collagen fibrils (orange) perpendicular to the invasive boundary, which serves as a track for metastatic cells (left). If two transformed mammary acini are within a critical distance, they will interact mechanically to produce a long, collagen fibril superhighway. Metastatic cells are observed to move at a faster rate via the superhighway than they do along a self-generated track that is not linked to a neighbor track.
Clearly tissue tension and cell-generated forces are critically important to normal development, but also serve an important role in cancer progression when deregulated. Indeed, it is often the synergy between genetic alterations and altered tissue mechanics that ultimately tip the balance towards malignancy. Chronic ECM stiffening may arise through several mechanisms including deregulated enzymes that control matrix cross-linking, or cancer-associated fibroblasts causing pathological levels of matrix remodeling w induced by mechanotransduction and YAP-dependent changes in matrix organization [104]. ECM stiffening precedes obvious signs of mammary transformation, but may in fact be responsible for early stages of tumorigenesis. For example, normal mammary epithelial cells embedded within a compliant matrix undergo normal morphogenesis producing polarized acini with hollow lumens [105]. If the matrix is then cross-linked to increase the effective stiffness and tissue density, acini contained within the newly stiffened matrix re-enter cell cycle and fill the central region of the lumen. The morphology of the resulting structures is quite similar to histological preparations of ductal carcinoma in situ. Both in silico modeling and experimental methods point towards the idea that acini with filled lumens are stiffer than those with hollow lumens and that filled lumens are also perceived by interacting cells to be stiffer. Hence, lumen filling contributes to the rapidly changing mechanical environment that characterizes tumorigenesis [106]. In addition, acquisition of a metastatic state is further promoted by the mechanics of the ECM just surrounding the transformed acini, which was shown to be stiffer than other areas. This ECM was able to potently induce focal adhesion associated vinculin activity and Akt signaling in cells at the invasive border of lesions [107]. If instead an oncogene is then activated, lesions acquire the ability to escape the basal lamina barrier and migrate deep into the matrix akin to a metastatic event [105], an effect that is further enhanced by the stiff invasive border. Beneficial results were observed in a transgenic animal model in which expression of the MMTV-Neu tumor oncogene is induced in mammary epithelial cells and then the matrix cross-linking is halted by manipulating the activity of lysyl oxidase (LOX), a collagen cross-linking enzyme.
In addition to synergizing with oncogene activity, matrix stiffening also acts to modulate tumor suppressor expression and function. Specifically, matrix stiffness was shown to elevate microRNA-18a (miR-18a) levels, which in turn directly silenced the tumor suppressor HOXA9 [108] that was further shown to be a direct transcriptional regulator of the PTEN tumor suppressor [109]. microRNAs notoriously exert multiple effects owing to the ability to target numerous genes. In the setting of a stiff mammary matrix, not only did miR-18a expression lead to tumor suppressor silencing, but also was also responsible for inducing mechanotransduction via MYC and β-catenin activity. Indeed, miR-18a expression predicted poor prognosis in patients with luminal breast cancer, and suggests the intriguing possibility that targeting miR-18a expression might delay cancer progression in a subset of patients. From these culture and in vivo studies one gains an appreciation of the stepwise nature of cancer progression and the complex interplay between oncogenes and tissue mechanics that drives malignant transformation. Indeed, not only can oncogenes tune cell tension to modulate tissue mechanics, but also matrix stiffening induced by other means offers mechanical cues that push a pre-malignant lesion down the path of metastasis.
Deregulated tensional homeostasis underlies many human conditions
Disrupted tensional homeostasis is emerging as a common theme in the pathogenesis of a diverse set of disease conditions, especially those characterized by fibrosis. Following are several recent examples. Osteoarthritis, a painful degenerative condition that afflicts joint cartilage, is most prevalent in aged individuals and can occur as a result of mechanical stress. Cartilage matrix stiffening is a clinical feature of osteoarthritis and can be explained by advanced glycation end products as well as LOX up-regulation. As a result, mechanotransduction pathways are aberrantly activated in cartilage cells leading to NFκB signaling, which have the effect of favoring catabolic processes that break proteins over anabolic pathways [110]. Accordingly, inhibiting LOX activity acted to prevent the initiation of osteoarthritic phenotypes in mice.
In culture and transgenic animal studies it was shown that chronic inflammation induces matrix stiffening resulting in mechanotransduction and activation of YAP/TAZ and β-catenin activities. As a result, epithelial differentiation occurs on the ocular surface indicating a causal link between inflammation-induced corneal disorders and tissue mechanics [111]. Yap activation was also observed to underlie mechanical-tension induced regeneration of lung pulmonary alveolar epithelium [112]. This suggests that appropriate spatio-temporal control of tissue mechanics is key to lung repair and its deregulation may be a reason for incomplete repair after injury.
As in the mammary gland, miRNA expression is induced downstream of matrix remodeling in the context of pulmonary hypertension [113]. Vascular ECM stiffening induced YAP/TAZ activity to upregulate miRNA-130/131, which led to further vascular stiffening by promoting collagen deposition and LOX-dependent remodeling. Targeting the miRNA or LOX activity pharmacologically ameliorated ECM remodeling and pulmonary hypertension in an animal model.
Although less studied, diminished tissue mechanics also impacts tissue integrity by failing to support mechanotransduction, which can promote disease. Bethlehem myopathy and Ulrich congenital muscular dystrophy are caused by mutations in the COL6 gene. Patients suffering from these disorders incur progressive muscle wasting, a clinical manifestation that is recapitulated in Collagen VI null (Col6a1−/−) mice. In normal muscle repair Collagen VI levels dramatically increase, a regenerative aspect that is lacking in Col6a1−/− mice and which results in diminished tissue mechanics [114]. Since muscle stem cell self-renewal activity is required to resolve tissue injury and is controlled by matrix stiffness [115], tissue degeneration in the context of Collagen VI loss appears to be due in part to disrupted tensional homeostasis.
Concluding remarks and future directions
To truly understand the cellular and molecular mechanisms driving development, tissue repair, and disease progression one must consider matrix mechanics and mechanotransduction. Furthermore, to treat diseases that include an element of defective mechanotransduction, putative compounds must be tested in the appropriate 3D matrix mechanics to accurately predict responses [116], and active efforts to develop compounds that selectively target key elements of the mechanotransduction pathway will be critical [117]. New mechanistic insights into how cells sense and respond to tissue mechanics will follow from the widespread implementation of in silico modeling [118] to develop complex cell-cell and cell-matrix models [92,97,106,119], and the development of 3D models to systematically tease apart the influence of diverse matrix mechanical properties, biomolecules, and genetic alterations on cell behaviors and fate in a controlled manner [120-123].
Acknowledgments
We would like to acknowledge the Canadian Institutes of Health Research (ONM-137370 to PMG), Natural Sciences and Engineering Research Council (RGPIN-4357 to PMG), National Institutes of Health (CA192914-01, CA138818-01A1, CA085492-11A1, and CA174929 to VMW), and Department of Defense (BC122990 to VMW) funding agencies for supporting the preparation of this review article. In addition, we thank Mr. James Morrissey-Scoot for providing editorial assistance and Ms. Aliyah Nissar for contributing a review figure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Handorf AM, Zhou Y, Halanski MA, Li W-J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis. 2015;11:1–15. doi: 10.1080/15476278.2015.1019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.HUXLEY AF, NIEDERGERKE R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. [November 18, 2015];Nature. 1954 173:971–3. doi: 10.1038/173971a0. http://www.ncbi.nlm.nih.gov/pubmed/13165697. [DOI] [PubMed] [Google Scholar]
- 3.HUXLEY H, HANSON J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. [October 16, 2015];Nature. 1954 173:973–6. doi: 10.1038/173973a0. http://www.ncbi.nlm.nih.gov/pubmed/13165698. [DOI] [PubMed] [Google Scholar]
- 4.Spudich JA. The myosin swinging cross-bridge model. Nat Rev Mol Cell Biol. 2001;2:387–92. doi: 10.1038/35073086. [DOI] [PubMed] [Google Scholar]
- 5.Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. [February 19, 2016];Exp Cell Res. 1971 67:359–67. doi: 10.1016/0014-4827(71)90420-4. http://www.ncbi.nlm.nih.gov/pubmed/5097522. [DOI] [PubMed] [Google Scholar]
- 6.Heath JP, Dunn GA. Cell to substratum contacts of chick fibroblasts and their relation to the microfilament system. A correlated interference-reflexion and high-voltage electron-microscope study. [February 19, 2016];J Cell Sci. 1978 29:197–212. doi: 10.1242/jcs.29.1.197. http://www.ncbi.nlm.nih.gov/pubmed/564353. [DOI] [PubMed] [Google Scholar]
- 7.Ludueña MA, Wessells NK. Cell locomotion, nerve elongation, and microfilaments. [February 19, 2016];Dev Biol. 1973 30:427–40. doi: 10.1016/0012-1606(73)90100-0. http://www.ncbi.nlm.nih.gov/pubmed/4703680. [DOI] [PubMed] [Google Scholar]
- 8.CURTIS AS. THE MECHANISM OF ADHESION OF CELLS TO GLASS. A STUDY BY INTERFERENCE REFLECTION MICROSCOPY. [February 19, 2016];J Cell Biol. 1964 20:199–215. doi: 10.1083/jcb.20.2.199. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2106393&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izzard CS, Lochner LR. Cell-to-substrate contacts in living fibroblasts: an interference reflexion study with an evaluation of the technique. [February 19, 2016];J Cell Sci. 1976 21:129–59. doi: 10.1242/jcs.21.1.129. http://www.ncbi.nlm.nih.gov/pubmed/932106. [DOI] [PubMed] [Google Scholar]
- 10.Humphrey JD, Dufresne ER, Schwartz Ma. Mechanotransduction and extracellular matrix homeostasis I E r. Nat Publ Gr. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–22. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia. 2004;9:325–42. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- 13.SCHERER WF, SYVERTON JT, GEY GO. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. [January 26, 2016];J Exp Med. 1953 97:695–710. doi: 10.1084/jem.97.5.695. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2136303&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.TEMIN HM, RUBIN H. Characteristics of an assay for Rous sarcoma virus and Rous sarcoma cells in tissue culture. [February 11, 2016];Virology. 1958 6:669–88. doi: 10.1016/0042-6822(58)90114-4. http://www.ncbi.nlm.nih.gov/pubmed/13616179. [DOI] [PubMed] [Google Scholar]
- 15.SANFORD KK, LIKELY GD, EARLE WR. The development of variations in transplantability and morphology within a clone of mouse fibroblasts transformed to sarcoma-producing cells in vitro. [February 11, 2016];J Natl Cancer Inst. 1954 15:215–37. http://www.ncbi.nlm.nih.gov/pubmed/13233880. [PubMed] [Google Scholar]
- 16.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iskratsch T, Wolfenson H, Sheetz MP. Appreciating force and shape [mdash] the rise of mechanotransduction in cell biology. Nat Rev Mol Cell Biol. 2014 doi: 10.1038/nrm3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12:308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris AK, Wild P, Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. [February 5, 2016];Science. 1980 208:177–9. doi: 10.1126/science.6987736. http://www.ncbi.nlm.nih.gov/pubmed/6987736. [DOI] [PubMed] [Google Scholar]
- 20.Hynes RO. The emergence of integrins: a personal and historical perspective. Matrix Biol. 2004;23:333–40. doi: 10.1016/j.matbio.2004.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 22.Margadant F, Chew LL, Hu X, Yu H, Bate N, Zhang X, et al. Mechanotransduction in vivo by repeated talin stretch-relaxation events depends upon vinculin. PLoS Biol. 2011;9:e1001223. doi: 10.1371/journal.pbio.1001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–19. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9391082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliver T, Dembo M, Jacobson K. Traction forces in locomoting cells. Cell Motil Cytoskeleton. 1995;31:225–40. doi: 10.1002/cm.970310306. [DOI] [PubMed] [Google Scholar]
- 26.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 27.Connelly JT, Gautrot JE, Trappmann B, Tan DW, Donati G, Huck WT, et al. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat Cell Biol. 2010;12:711–718. doi: 10.1038/ncb2074. [DOI] [PubMed] [Google Scholar]
- 28.Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science 80. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 29.Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 80. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014 doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy WL, McDevitt TC, Engler AJ. Materials as stem cell regulators. Nat Mater. 2014;13:547–557. doi: 10.1038/nmat3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 35.Dingal PCDP, Bradshaw AM, Cho S, Raab M, Buxboim A, Swift J, et al. Fractal heterogeneity in minimal matrix models of scars modulates stiff-niche stem-cell responses via nuclear exit of a mechanorepressor. Nat Mater. 2015;14:951–960. doi: 10.1038/nmat4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heo SJ, Thorpe SD, Driscoll TP, Duncan RL, Lee DA, Mauck RL. Biophysical Regulation of Chromatin Architecture Instills a Mechanical Memory in Mesenchymal Stem Cells. Sci Rep. 2015;5:16895. doi: 10.1038/srep16895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talele NP, Fradette J, Davies JE, Kapus A, Hinz B. Expression of α-Smooth Muscle Actin Determines the Fate of Mesenchymal Stromal Cells. Stem Cell Reports. 2015;4:1016–30. doi: 10.1016/j.stemcr.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater. 2014;13:645–52. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, Hinz B. The mechanical memory of lung myofibroblasts. Integr Biol. 2012;4:410. doi: 10.1039/c2ib00149g. [DOI] [PubMed] [Google Scholar]
- 40.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. [February 17, 2016];Mol Biol Cell. 2001 12:2730–41. doi: 10.1091/mbc.12.9.2730. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=59708&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janmey PA, Wells RG, Assoian RK, McCulloch CA. From tissue mechanics to transcription factors. Differentiation. 2013;86:112–120. doi: 10.1016/j.diff.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wozniak MA, Cheng CQ, Shen CJ, Gao L, Olarerin-George AO, Won KJ, et al. Adhesion regulates MAP kinase/ternary complex factor exchange to control a proliferative transcriptional switch. Curr Biol. 2012;22:2017–6. doi: 10.1016/j.cub.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 44.Connelly JT, Gautrot JE, Trappmann B, Tan DWM, Donati G, Huck WTS, et al. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat Cell Biol. 2010;12:711–718. doi: 10.1038/ncb2074. [DOI] [PubMed] [Google Scholar]
- 45.Sero JE, Sailem HZ, Ardy RC, Almuttaqi H, Zhang T, Bakal C. Cell shape and the microenvironment regulate nuclear translocation of NF-κB in breast epithelial and tumor cells. [August 24, 2016];Mol Syst Biol. 2015 11:790. doi: 10.15252/msb.20145644. http://www.ncbi.nlm.nih.gov/pubmed/25735303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen NX, Geist DJ, Genetos DC, Pavalko FM, Duncan RL. Fluid shear-induced NFkappaB translocation in osteoblasts is mediated by intracellular calcium release. [August 24, 2016];Bone. 2003 33:399–410. doi: 10.1016/s8756-3282(03)00159-5. http://www.ncbi.nlm.nih.gov/pubmed/13678782. [DOI] [PubMed] [Google Scholar]
- 47.Rosette C, Karin M. Cytoskeletal control of gene expression: depolymerization of microtubules activates NF-kappa B. [August 24, 2016];J Cell Biol. 1995 128:1111–9. doi: 10.1083/jcb.128.6.1111. http://www.ncbi.nlm.nih.gov/pubmed/7896875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Németh ZH, Deitch EA, Davidson MT, Szabó C, Vizi ES, Haskó G. Disruption of the actin cytoskeleton results in nuclear factor-kappaB activation and inflammatory mediator production in cultured human intestinal epithelial cells. J Cell Physiol. 2004;200:71–81. doi: 10.1002/jcp.10477. [DOI] [PubMed] [Google Scholar]
- 49.Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P, et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and β-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell. 2011;19:776–91. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11:353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sit S-T, Manser E. Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci. 2011;124:679–83. doi: 10.1242/jcs.064964. [DOI] [PubMed] [Google Scholar]
- 52.Del Pozo MA, Kiosses WB, Alderson NB, Meller N, Hahn KM, Schwartz MA. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat Cell Biol. 2002;4:232–9. doi: 10.1038/ncb759. [DOI] [PubMed] [Google Scholar]
- 53.Zhai J, Lin H, Nie Z, Wu J, Cañete-Soler R, Schlaepfer WW, et al. Direct interaction of focal adhesion kinase with p190RhoGEF. J Biol Chem. 2003;278:24865–73. doi: 10.1074/jbc.M302381200. [DOI] [PubMed] [Google Scholar]
- 54.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 55.Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316:1749–52. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- 56.Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. [February 14, 2016];Cell. 1999 98:159–69. doi: 10.1016/s0092-8674(00)81011-9. http://www.ncbi.nlm.nih.gov/pubmed/10428028. [DOI] [PubMed] [Google Scholar]
- 57.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 58.Low BC, Pan CQ, Shivashankar GV, Bershadsky A, Sudol M, Sheetz M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014;588:2663–70. doi: 10.1016/j.febslet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 59.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 60.Chaudhuri O, Gu L, Darnell M, Klumpers D, Bencherif SA, Weaver JC, et al. Substrate stress relaxation regulates cell spreading. Nat Commun. 2015;6:6364. doi: 10.1038/ncomms7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chopra A, Murray ME, Byfield FJ, Mendez MG, Halleluyan R, Restle DJ, et al. Augmentation of integrin-mediated mechanotransduction by hyaluronic acid. Biomaterials. 2014;35:71–82. doi: 10.1016/j.biomaterials.2013.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toole BP. Hyaluronan is not just a goo! J Clin Invest. 2000;106:335–6. doi: 10.1172/JCI10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watt FM, Jordan PW, O’Neill CH. Cell shape controls terminal differentiation of human epidermal keratinocytes. Proc Natl Acad Sci U S A. 1988;85:5576–5580. doi: 10.1073/pnas.85.15.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Albrecht DR, Underhill GH, Wassermann TB, Sah RL, Bhatia SN. Probing the role of multicellular organization in three-dimensional microenvironments. Nat Methods. 2006;3:369–75. doi: 10.1038/nmeth873. [DOI] [PubMed] [Google Scholar]
- 65.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 66.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 67.McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci U S A. 2013;110:17253–8. doi: 10.1073/pnas.1308887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jain N, Iyer KV, Kumar A, Shivashankar GV. Cell geometric constraints induce modular gene-expression patterns via redistribution of HDAC3 regulated by actomyosin contractility. Proc Natl Acad Sci. 2013;110:11349–11354. doi: 10.1073/pnas.1300801110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Codelia VA, Sun G, Irvine KD. Regulation of YAP by mechanical strain through Jnk and Hippo signaling. Curr Biol. 2014;24:2012–2017. doi: 10.1016/j.cub.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cui Y, Hameed FM, Yang B, Lee K, Pan CQ, Park S, et al. Cyclic stretching of soft substrates induces spreading and growth. Nat Commun. 2015;6:6333. doi: 10.1038/ncomms7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shao X, Li Q, Mogilner A, Bershadsky AD, Shivashankar GV. Mechanical stimulation induces formin-dependent assembly of a perinuclear actin rim. Proc Natl Acad Sci U S A. 2015;112:E2595–601. doi: 10.1073/pnas.1504837112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671–7. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 73.Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DT, et al. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci U S A. 2014;111:16148–16153. doi: 10.1073/pnas.1409802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heller E, Fuchs E. Tissue patterning and cellular mechanics. J Cell Biol. 2015;211:219–231. doi: 10.1083/jcb.201506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lecuit T, Yap AS. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat Cell Biol. 2015;17:533–539. doi: 10.1038/ncb3136. [DOI] [PubMed] [Google Scholar]
- 76.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 77.Pelissier FA, Garbe JC, Ananthanarayanan B, Miyano M, Lin C, Jokela T, et al. Age-Related Dysfunction in Mechanotransduction Impairs Differentiation of Human Mammary Epithelial Progenitors. Cell Rep. 2014;7:1926–1939. doi: 10.1016/j.celrep.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei SC, Yang J. Forcing through Tumor Metastasis: The Interplay between Tissue Rigidity and Epithelial-Mesenchymal Transition. Trends Cell Biol. 2015;26:111–120. doi: 10.1016/j.tcb.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang M-H, Hsu DS-S, Wang H-W, Wang H-J, Lan H-Y, Yang W-H, et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12:982–92. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- 80.Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–9. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 81.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 82.Sánchez-Tilló E, Lázaro A, Torrent R, Cuatrecasas M, Vaquero EC, Castells A, et al. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene. 2010;29:3490–500. doi: 10.1038/onc.2010.102. [DOI] [PubMed] [Google Scholar]
- 83.Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, et al. Matrix stiffness drives epithelial mesenchymal transition and tumour metastasis through a TWIST1 G3BP2 mechanotransduction pathway. Nat Cell Biol. 2015;17:678–688. doi: 10.1038/ncb3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mao Y, Tournier AL, Hoppe A, Kester L, Thompson BJ, Tapon N. Differential proliferation rates generate patterns of mechanical tension that orient tissue growth. EMBO J. 2013;32:2790–803. doi: 10.1038/emboj.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buckley CD, Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, et al. The minimal cadherin-catenin complex binds to actin filaments under force. 2014;346 doi: 10.1126/science.1254211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bazellières E, Conte V, Elosegui-Artola A, Serra-Picamal X, Bintanel-Morcillo M, Roca-Cusachs P, et al. Control of cell-cell forces and collective cell dynamics by the intercellular adhesome. Nat Cell Biol. 2015;17:409–420. doi: 10.1038/ncb3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nguyen-Ngoc KV, Cheung KJ, Brenot A, Shamir ER, Gray RS, Hines WC, et al. ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc Natl Acad Sci. 2012;109:E2595–E2604. doi: 10.1073/pnas.1212834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rosowski KA, Mertz AF, Norcross S, Dufresne ER, Horsley V. Edges of human embryonic stem cell colonies display distinct mechanical properties and differentiation potential. Sci Rep. 2015;5:14218. doi: 10.1038/srep14218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schluck T, Nienhaus U, Aegerter-Wilmsen T, Aegerter CM. Mechanical Control of Organ Size in the Development of the Drosophila Wing Disc. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Streichan SJ, Hoerner CR, Schneidt T, Holzer D, Hufnagel L. Spatial constraints control cell proliferation in tissues. Proc Natl Acad Sci U S A. 2014;111:5586–91. doi: 10.1073/pnas.1323016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Benham-Pyle BW, Pruitt BL, Nelson WJ. Mechanical strain induces E-cadherin-dependent Yap1 and -catenin activation to drive cell cycle entry. Science 80. 2015;348:1024–1027. doi: 10.1126/science.aaa4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Broaders KE, Cerchiari AE, Gartner ZJ. Coupling between apical tension and basal adhesion allow epithelia to collectively sense and respond to substrate topography over long distances. Integr Biol. 2015;7:1611–1621. doi: 10.1039/C5IB00240K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Case LB, Waterman CM. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat Cell Biol. 2015;17:955–963. doi: 10.1038/ncb3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shao Y, Sang J, Fu J. On human pluripotent stem cell control: The rise of 3D bioengineering and mechanobiology. Biomaterials. 2015;52:26–43. doi: 10.1016/j.biomaterials.2015.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chanet S, Martin AC. Mechanical Force Sensing in Tissues. 2014 doi: 10.1016/B978-0-12-394624-9.00013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Davidson LA. Epithelial machines that shape the embryo. Trends Cell Biol. 2012;22:82–87. doi: 10.1016/j.tcb.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ovadia J, Nie Q. Stem cell niche structure as an inherent cause of undulating epithelial morphologies. Biophys J. 2013;104:237–246. doi: 10.1016/j.bpj.2012.11.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Poh YC, Chen J, Hong Y, Yi H, Zhang S, Chen J, et al. Generation of organized germ layers from a single mouse embryonic stem cell. Nat Commun. 2014;5:4000. doi: 10.1038/ncomms5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Przybyla L, Lakins JN, Weaver VM. Tissue Mechanics Orchestrate Wnt-Dependent Human Embryonic Stem Cell Differentiation. Cell Stem Cell. 2016 doi: 10.1016/j.stem.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gjorevski N, Nelson CM. Endogenous patterns of mechanical stress are required for branching morphogenesis. Integr Biol (Camb) 2010;2:424–34. doi: 10.1039/c0ib00040j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brownfield DG, Venugopalan G, Lo A, Mori H, Tanner K, Fletcher Da, et al. Patterned collagen fibers orient branching mammary epithelium through distinct signaling modules. Curr Biol. 2013;23:703–709. doi: 10.1016/j.cub.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Riching KM, Cox BL, Salick MR, Pehlke C, Riching AS, Ponik SM, et al. Article Persistence. Biophysj. 2014;107:2546–2558. doi: 10.1016/j.bpj.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shi Q, Ghosh RP, Engelke H, Rycroft CH, Cassereau L, Sethian Ja, et al. Rapid disorganization of mechanically interacting systems of mammary acini. Proc Natl Acad Sci U S A. 2014;111:658–63. doi: 10.1073/pnas.1311312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Venugopalan G, Camarillo DB, Webster KD, Reber CD, Sethian Ja, Weaver VM, et al. Multicellular architecture of malignant breast epithelia influences mechanics. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rubashkin MG, Cassereau L, Bainer R, DuFort CC, Yui Y, Ou G, et al. Force engages vinculin and promotes tumor progression by enhancing PI3K activation of phosphatidylinositol (3,4,5)-triphosphate. Cancer Res. 2014;74:4597–611. doi: 10.1158/0008-5472.CAN-13-3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gilbert PM, Mouw JK, Unger Ma, Lakins JN, Gbegnon MK, Clemmer VB, et al. HOXA9 regulates BRCA1 expression to modulate human breast tumor phenotype. J Clin Invest. 2010;120:1535–1550. doi: 10.1172/JCI39534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mouw JK, Yui Y, Damiano L, Bainer RO, Lakins JN, Acerbi I, et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat Med. 2014;20:360–7. doi: 10.1038/nm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim J-H, Lee G, Won Y, Lee M, Kwak J-S, Chun C-H, et al. Matrix cross-linking-mediated mechanotransduction promotes posttraumatic osteoarthritis. Proc Natl Acad Sci U S A. 2015;112:9424–9429. doi: 10.1073/pnas.1505700112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nowell CS, Odermatt PD, Azzolin L, Hohnel S, Wagner EF, Fantner GE, et al. Chronic inflammation imposes aberrant cell fate in regenerating epithelia through mechanotransduction. Nat Cell Biol. 2015 doi: 10.1038/ncb3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu Z, Wu H, Jiang K, Wang Y, Zhang W, Chu Q, et al. MAPK-Mediated YAP Activation Controls Mechanical-Tension-Induced Pulmonary Alveolar Regeneration. Cell Rep. 2016;16:1810–9. doi: 10.1016/j.celrep.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 113.Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, Annis S, et al. Matrix Remodeling Promotes Pulmonary Hypertension through Feedback Mechanoactivation of the YAP/TAZ-miR-130/301 Circuit. Cell Rep. 2015;13:1016–1032. doi: 10.1016/j.celrep.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun. 2013;4:1964. doi: 10.1038/ncomms2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 80. n.d;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin C-H, Pelissier FA, Zhang H, Lakins J, Weaver VM, Park C, et al. Microenvironment rigidity modulates responses to the HER2 receptor tyrosine kinase inhibitor lapatinib via YAP and TAZ transcription factors. Mol Biol Cell. 2015;26:3946–3953. doi: 10.1091/mbc.E15-07-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Surcel A, Ng WP, West-Foyle H, Zhu Q, Ren Y, Avery LB, et al. Pharmacological activation of myosin II paralogs to correct cell mechanics defects. Proc Natl Acad Sci U S A. 2015;112:1428–33. doi: 10.1073/pnas.1412592112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mak M, Kim T, Zaman MH, Kamm RD. Multiscale mechanobiology: computational models for integrating molecules to multicellular systems. Integr Biol. 2015;7:1093–1108. doi: 10.1039/C5IB00043B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paszek MJ, Boettiger D, Weaver VM, Hammer DA. Integrin clustering is driven by mechanical resistance from the glycocalyx and the substrate. PLoS Comput Biol. 2009;5:e1000604. doi: 10.1371/journal.pcbi.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chaudhuri O, Koshy ST, Branco da Cunha C, Shin J-W, Verbeke CS, Allison KH, et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat Mater. 2014;13:1–35. doi: 10.1038/nmat4009. [DOI] [PubMed] [Google Scholar]
- 121.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2015 doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cassereau L, Miroshnikova Y, Ou G, Lakins J, Weaver VM. A 3D tension bioreactor platform to study the interplay between ECM stiffness and tumor phenotype. J Biotechnol. 2014;193:66–69. doi: 10.1016/j.jbiotec.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mabry KM, Lawrence RL, Anseth KS. Dynamic stiffening of poly(ethylene glycol)-based hydrogels to direct valvular interstitial cell phenotype in a three-dimensional environment. Biomaterials. 2015;49:47–56. doi: 10.1016/j.biomaterials.2015.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]


