Abstract
Critically ill populations incur high levels of oxidative stress and commonly present with vitamin D deficiency. This study aimed to investigate the relationship between vitamin D status and plasma markers of glutathione (GSH) and cysteine (Cys) redox and immunity in critically ill children. This was a cross-sectional study of n = 50 PICU patients. Subjects were categorized according to their plasma 25-hydroxyvitamin D [25(OH)D] concentrations: (<20, 20–30, and ≥30 ng/dL). Plasma GSH, glutathione disulfide (GSSG), Cys, and cystine (CySS) were measured with high-performance liquid chromatography, and their associated redox potentials determined (EhGSSG and Eh CySS, respectively). Plasma LL-37, an indicator of innate immune function, was assayed with ELISA. Data were analyzed using general linear regression before and after adjustment for age, sex, and race. Results showed that EhCySS was more reduced in subjects with plasma 25(OH)D concentrations ≥30 ng/mL compared to those with 25(OH)D concentrations <20 ng/mL (P=0.009). Plasma GSH, GSSG, and total GSH decreased with increasing 25(OH)D category (P=0.06, 0.03, and 0.01, respectively), and plasma glutamine levels were lowest in subjects with plasma 25(OH)D concentrations ≥30 ng/mL (P=0.004). Plasma LL-37 concentrations did not significantly differ by vitamin D status (P=0.08). In conclusion, vitamin D sufficiency was associated with more reduced plasma EhCySS, indicative of lower oxidative stress in critically ill children. Plasma GSH, GSSG, and glutamine, however, were lower in the vitamin D sufficient group. The role of vitamin D in maintaining redox status during pediatric critical illness requires further study.
Keywords: calcidiol, oxidative stress, cathelicidin, LL-37, critical care, pediatrics
1. Introduction
The widespread prevalence of low circulating concentrations of 25-hydroxyvitamin D (25(OH)D), an indicator of vitamin D status, is well-documented in both pediatric1–3 and adult4 critically-ill populations. Less is known about the implications of concurrent vitamin D deficiency and critical illness. The ubiquitous presence of vitamin D receptors and vitamin D activating enzyme, 1α-hydroxylase, throughout tissues and cells5, as well as several studies reporting an association between vitamin D status and clinical outcomes3, 6, 7, provide support for a role for vitamin D in the pathophysiology of critical illness. To date, however, there are no published studies evaluating the effects of vitamin D repletion in critically-ill children, and limited available data in adults are suggestive that vitamin D may have benefit, but are not conclusive8, 9.
The systemic response to critical illness involves complex shifts in circulating markers of oxidative stress, antioxidant status and function, inflammation, immunity, and substrate metabolism. Research in critical illness has focused on the role of vitamin D on immunity and inflammation5. Indeed, experimental studies show that vitamin D in its active form, calcitriol, downregulates pro-inflammatory cytokines10 and upregulates expression of the antimicrobial peptide LL-37 of the cathelicidin gene11. In studies of critically ill adults with sepsis, circulating plasma LL-37 concentrations were shown to positively correlate with plasma 25(OH)D concentrations4, and supplementation with vitamin D increased both plasma LL-37 and select proinflammatory cytokines12. The relationship between vitamin D status and inflammation and immunity in critically ill children is not clear. An understanding of the role of vitamin D across the spectrum of the physiological changes that occur during critical illness is needed to determine optimal recommendations for vitamin D supplementation.
Oxidative stress has been implicated in the progression of organ dysfunction and failure in critical illness13, 14. In addition to measurement of byproducts of oxidative damage or specific antioxidants, oxidative stress can be assessed in terms of reduction-oxidation (redox) balance15,16. Glutathione (GSH) and cysteine (Cys) and their respective disulfides [glutathione disulfide (GSSG) and cystine (CySS)] represent major intracellular and extracellular redox systems, respectively16. Plasma measures of these low molecular weight thiol redox systems were recently shown to differ in critically ill children compared to healthy controls17. In a separate study, plasma glutamine concentrations, a precursor of GSH, were depleted in pediatric intensive care unit (PICU) patients18. In vitro, calcitriol directly upregulates key enzymes involved in GSH redox homeostasis, glutathione cysteine ligase and glutathione reductase19. Furthermore, in an ambulatory adult population, vitamin D status was significantly associated with biomarkers of plasma GSH/GSSG and Cys/CySS redox20. Whether vitamin D plays a role in maintaining redox balance in critical illness is not known.
In this study, we aimed to investigate the relationship between vitamin D status and biomarkers of key players in the stress response to critical illness, specifically redox and immunity, in children admitted to a pediatric intensive care unit (PICU). We hypothesized that patients with adequate vitamin D status would have more negative (i.e, less oxidized) plasma redox potentials for the GSH/GSSG and Cys/CySS systems and higher plasma LL-37 compared to vitamin D insufficient patients.
2. Materials and Methods
2.1 Subjects
This was a cross-sectional study of n = 50 PICU patients presenting to Children’s Healthcare of Atlanta at Egleston between January 2010 and April 2012. Patients ages 0–18 years and weighing ≥6 kg were eligible for study inclusion. Patients were excluded if they had the following: chronic renal disease, gastrointestinal malabsorption conditions, post-operative state following an elective surgery, trauma as related to possible abuse, or weight < 6kg. Blood was drawn within 24 hours of admission to PICU. Disease severity was assessed using the Pediatric Risk of Mortality III (PRISM III) score21 and the Pediatric Logistic Organ Dysfunction (PELOD) score22. The study was approved by both the Emory University and Children’s Healthcare of Atlanta Institutional Review Boards, and informed consent was obtained from patients’ guardians prior to any study procedures. In this sub-set of a larger cohort study2, 17 (clinicaltrials.gov identifier NCT01052207), we only included PICU subjects with available plasma redox and 25(OH)D data.
2.2 Measurement of Redox States, Plasma Glutamine, and Plasma LL-37
Quantitation of GSH, GSSG, Cys, and CySS was performed with high-performance liquid chromatography (HPLC) with fluorescence detection. Details for sample collection and processing and subsequent thiol/disulfide measurement and determination of redox states have been previously described23. In brief, whole blood was collected and transferred to microcentrifuge tubes with a preservative solution containing iodoacetic acid to minimize autooxidation. Samples were centrifuged and the supernatant mixed with a solution containing perchloric acid, boric acid, and γ-L-glutamyl-L-glutamate as an internal standard and frozen at −80°C until ready for analysis. Thawed samples were treated with a dansyl chloride solution for derivatization prior to HPLC analysis. Quantification of analytes occurred by integration relative to the internal standard. Total Cys was calculated as [Cys] + 2[CySS], and total GSH as [GSH] + 2[GSSG]. The ratios of the reduced and oxidized aminothiols (Cys:CySS and GSH:GSSG) were determined. The redox potentials, EhGSSG and EhCySS were calculated using the Nernst equation with Eο values of –264 mV and –250 mV, respectively, at a pH of 7.423. Higher (less negative) redox potentials, expressed in mV, are indicative greater levels of oxidative stress. For measurement of plasma glutamine, EDTA plasma (100 ul) was first treated with glutaminase to convert the glutamine to ammonia and then treated with glutamate dehydrogenase, α-ketoglutamate and NADH to generate L-glutamate and NAD. The remaining NADPH concentration is inversely proportional to the glutamine concentration as determined on an Analox GM7 MicroStat analyzer (London, UK). Plasma LL-37 concentrations were determined with ELISA (Hycult Biotech, Uden, Netherlands).
2.3 Determination of Vitamin D Status
Blood was collected in EDTA vacutainer tubes, separated, and stored at –80°C until ready for analysis. Plasma 25-hydroxyvitamin D [25(OH)D] was measured using a chemiluminescent automated technique (iSYS analyzer, Immunodiagnostic Systems, Inc., Fountain Hills, AZ) in a laboratory certified by the Vitamin D External Quality Assessment Scheme (DEQAS). The laboratory inter- and intra-assay CVs for measurement of 25(OH)D are 10.1–13.0% and 1.8–4.0%, respectively. As vitamin D adequacy levels have not been specifically defined for PICU patients and cut-off values for adequate vitamin D status have been debated to be ≥20 ng/mL or ≥30 ng/mL24, 25, study participants were categorized based on plasma 25(OH)D concentrations <20 ng/mL, 20–30 ng/mL, and ≥30 ng/mL2, 3. Classification of plasma 25(OH)D levels using <12 ng/mL as an indicator of severe vitamin D deficiency was also explored.
2.4 Statistical Analyses
Data analyses were performed using SAS 9.3 (Cary, NC), and statistical significance was evaluated at the 0.05 level. Demographics and clinical summaries were calculated using counts and frequencies, medians and ranges, or means and standard deviations where appropriate. Plasma biomarkers were assessed by vitamin D category (< 20, 20 – 30, and ≥30 ng/dL) using general linear regression. Residual errors for each modeled biomarker outcome were gauged for normality via histograms, boxplots, and quantile-quantile probability plots. With the exception of the redox potentials, the circulating biomarkers were not normally distributed, and were thus transformed using the natural log. Analyses were conducted both unadjusted and adjusted for patient age, gender and race. Back-transformed least-squares means and 95% confidence intervals were reported. When overall significant differences were found, pairwise comparisons were considered using the Tukey-Kramer method.
3. Results
Demographic and clinical characteristics are presented in Table 1. The mean age was approximately 12 years, and over half of the patients were male. The majority of the cohort was African American. Over 60% of subjects required mechanical ventilation. The median plasma 25(OH)D was in the deficient range (< 20 ng/mL); 58% of subjects presented to the PICU with plasma 25(OH)D < 20 ng/mL, and 74% of patients had plasma 25(OH)D < 30 ng/mL. There were no statistically significant differences in PRISM III or PELOD scores between the vitamin D status groups (P = 0.50 and 0.44, respectively). Use of advanced technologies (extracorporeal membrane oxygenation, continuous venovenous hemodialysis, and plasma exchange) also did not significantly differ between the vitamin D status groups (P = 0.33, 0.23, and 0.54, respectively).
Table 1.
Demographic and clinical characteristics of PICU patients (n = 50).
| Characteristic | Mean ± SD or n (%) |
|---|---|
| Age (years) | 11.8 ± 5.2 |
| Weight (kg) | 46.8 ± 25.2 |
| Gender | |
| Male | 28 (56%) |
| Female | 22 (44%) |
| Hispanic | 2 (4%) |
| Race | |
| African American | 34 (68%) |
| Caucasian | 11 (22%) |
| Other | 5 (10%) |
| Primary Diagnosis | |
| Shock | 24 (48%) |
| Sepsis | 22 (44%) |
| Septic Shock | 18 (36%) |
| Asthma | 11 (22%) |
| Other | 32 (64%) |
| PRISM III Score | 11.2 ± 5.8 |
| PELOD Score | 16.9 ± 14.2 |
| Hospital length of stay (days)a | 9 (5–19) |
| PICU length of stay (days)a | 6.5 (4–9) |
| Mechanical ventilation | 31 (62%) |
| 28-Day Mortality | 3 (6%) |
| Use of advanced technologies | |
| Extracorporeal membrane oxygenation | 45 (90%) |
| Continuous venovenous hemodialysis | 42 (84%) |
| Plasma exchange | 47 (94%) |
| Plasma albumin (g/dL)a,b | 3.2 (2.4–3.5) |
| Plasma 25(OH)D (ng/mL)a | 18.5 (11.3–32.9) |
| Plasma 25(OH)D < 20 ng/mL | 29 (58%) |
| Plasma 25(OH)D < 30 ng/mL | 37 (74%) |
Abbreviations: PICU, pediatric intensive care unit; 25(OH)D, 25-hydroxyvitamin D; PRISM III, Pediatric Risk of Mortality version 3; PELOD, Pediatric Logistic Organ Dysfunction.
Reported as median (IQR).
N = 40.
3.1 Relationship between vitamin D status and plasma aminothiol redox
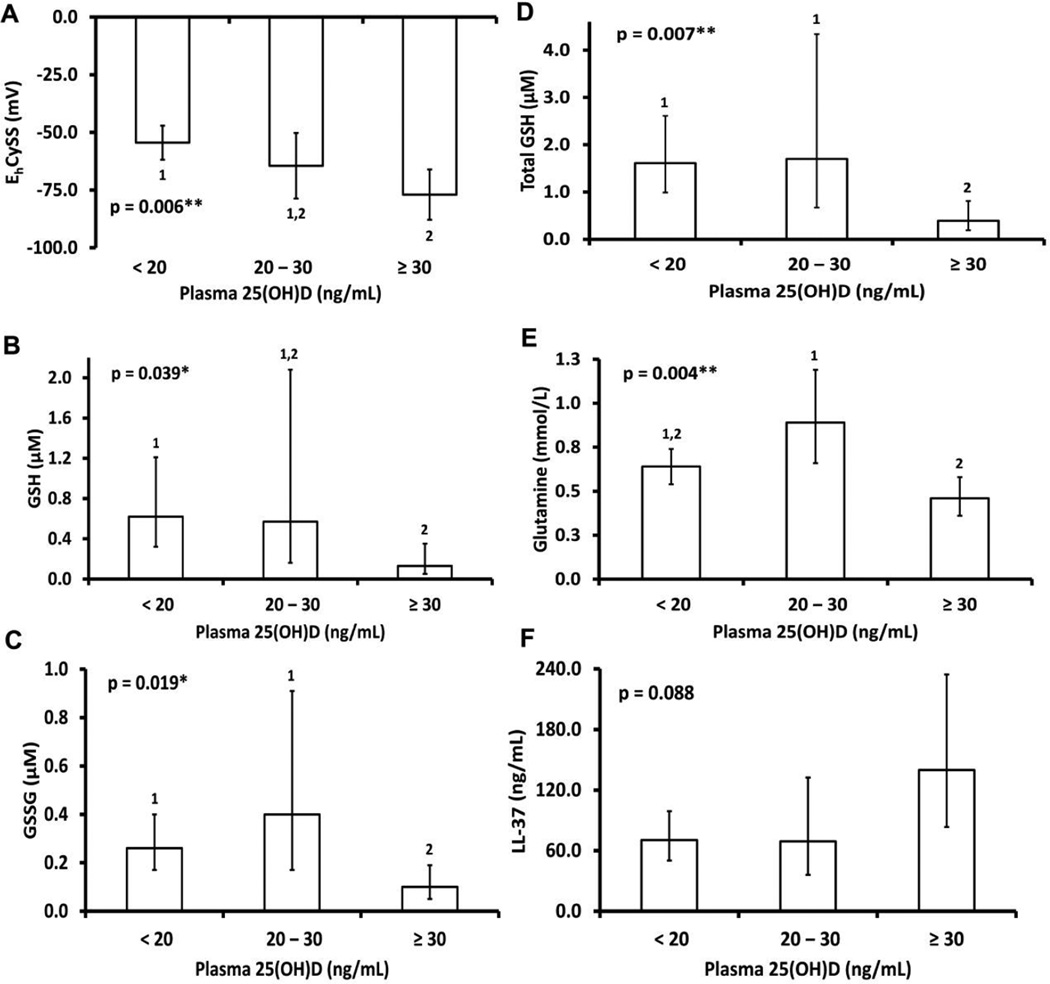
Differences in plasma GSH/GSSH and Cys/CySS redox data and glutamine based on plasma 25(OH)D concentrations are shown in Table 2. Plasma Cys, CySS, and total Cys did not significantly differ by vitamin D status (P > 0.05 for all). The ratio Cys:CySS was higher and EhCySS was significantly lower (more reduced) in subjects with plasma 25(OH)D concentrations ≥30 ng/mL compared to those with 25(OH)D concentrations <20 ng/mL. As shown in Fig. 1A, the group difference in EhCySS remained statistically significant after adjustment for age, sex, and race. Plasma GSH, GSSG, and total GSH decreased with increasing 25(OH)D category (Table 2; P = 0.06, 0.03, and 0.01, respectively); differences remained or were more pronounced after adjustment for age, sex, and race (Fig. 1B–D). Plasma glutamine levels were lowest in subjects with plasma 25(OH)D concentrations ≥30 ng/mL before and after adjustment for covariates (Table 2, Fig. 1E, P = 0.004 for both). Results were similar if a lower threshold for severe vitamin D deficiency (25(OH)D < 12 ng/mL) was compared to milder vitamin D deficiency (25(OH)D 12–30 ng/mL, Supplemental Table 1).
Table 2.
Plasma biomarkers of aminothiol redox and immunity by plasma 25(OH)D cut-off levels.
| Characteristic | N | 25(OH)D < 20 ng/mL |
N | 25(OH)D 20–30 ng/mL |
N | 25(OH)D ≥ 30 ng/mL |
P |
|---|---|---|---|---|---|---|---|
| Cys (µM) | 29 | 0.42 (0.28 – 0.65) | 8 | 0.67 (0.29 – 1.50) | 13 | 0.89 (0.47 – 1.68) | 0.160 |
| CySS (µM) | 29 | 0.58 (0.34 – 0.99) | 8 | 0.68 (0.24 – 1.87) | 13 | 0.47 (0.21 – 1.05) | 0.855 |
| Total Cys (µM) | 29 | 1.75 (1.11 – 2.76) | 8 | 2.34 (0.99 – 5.55) | 13 | 1.92 (0.98 – 3.78) | 0.845 |
| Cys:CySS | 29 | 0.73 (0.51 – 1.04) 1 | 8 | 0.98 (0.50 – 1.92) 1,2 | 13 | 1.87 (1.10 – 3.16) 2 | 0.020 |
| EhCySS (mV) | 29 | −54.64 (−62.11 – (−47.17))1 | 8 | −64.49 (−78.71 – (−50.26))1,2 | 13 | −76.54 (−87.70 – (−65.38))2 | 0.009 |
| GSH (µM) | 29 | 0.63 (0.32 – 1.25) | 8 | 0.51 (0.14 – 1.89) | 13 | 0.13 (0.05 – 0.37) | 0.055 |
| GSSG (µM) | 29 | 0.27 (0.17 – 0.42)1 | 8 | 0.35 (0.15 – 0.81)1,2 | 13 | 0.10 (0.05 – 0.19)2 | 0.034 |
| GSH:GSSG | 29 | 2.34 (1.27 – 4.32) | 8 | 1.47 (0.46 – 4.70) | 13 | 1.34 (0.54 – 3.35) | 0.557 |
| Total GSH (µM) | 29 | 1.65 (0.99 – 2.75)1 | 8 | 1.48 (0.56 – 3.91)1,2 | 13 | 0.40 (0.19 – 0.86)2 | 0.013 |
| EhGSSG (mV) | 29 | −89.03 (−104.96 – (−73.11)) | 8 | −80.23 (−110.55 – (−49.92)) | 13 | −61.68 (−85.46 – (−37.90)) | 0.183 |
| Glutamine (mmol/L) | 25 | 0.64 (0.55 – 0.75)1 | 7 | 0.87 (0.65 – 1.17)1 | 11 | 0.45 (0.36 – 0.57)2 | 0.004 |
| LL-37 (ng/mL) | 28 | 70.51 (50.27 – 98.90) | 8 | 68.10 (36.16 – 128.25) | 12 | 140.85 (84.00 – 236.18) | 0.081 |
Data are presented as back-transformed, model-based mean estimates and 95% CI (redox potential data were not transformed).
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; Cys, cysteine; CySS, cystine; Eh, redox potential; GSH, glutathione; GSSG, glutathione disulfide. Total Cys was calculated as [Cys] + 2[CySS], and total GSH as [GSH] + 2[GSSG]. Bold values indicate statistical significance; values not connected by same superscript are significantly different.
Fig. 1.
Plasma redox and LL-37 concentrations by vitamin D status, after adjustment for age, sex, and race. Data are shown as back-transformed, model-based mean estimates and 95% CI (redox potential data were not transformed). Abbreviations: 25(OH)D, 25-hydroxyvitamin D; Cys, cysteine; CySS, cystine; Eh, redox potential; GSH, glutathione; GSSG, glutathione disulfide. Total Cys was calculated as [Cys] + 2[CySS], and total GSH as [GSH] + 2[GSSG]. Bold values indicate statistical significance; values not connected by same superscript are significantly different. *P < 0.05, **P <0.01, ***P < 0.001
3.2 Relationship between vitamin D status and plasma LL-37
Group differences in plasma LL-37 based on vitamin D status were not statistically significant (Table 2, P = 0.08), although plasma LL-37 was quantitatively higher in subjects with plasma 25(OH)D ≥30 ng/mL. Findings were similar after adjustment for age, sex, and race (Fig. 1F), or if different thresholds for vitamin D status were compared (25(OH)D < 12 ng/mL vs 12–30 ng/mL vs 30 ng/mL, Supplemental Table 1).
4. Discussion
Vitamin D has been previously suggested to play a role in regulation of oxidative stress, inflammation, and the immune response. The purpose of this study was to examine the associations between vitamin D status and plasma markers of redox and immunity in PICU patients. Our data showed variable relationships between vitamin status and redox biomarkers, depending on whether Cys/CySS or GSH/GSSG was assessed.
Given their abundance and central roles in redox signaling pathways, quantification of the major plasma intracellular (Cys/CySS) and extracellular (GSH/GSSG) thiol pools and their respective redox potentials (EhCySS and EhGSSG) can be useful clinical indicators of systemic oxidative stress16, 23. In this cohort of critically-ill children and compared to healthy controls, Grunwell et al.17 reported a more oxidized plasma EhGSSG, higher plasma GSSG, and lower plasma Cys and CySS, with no difference in plasma EhCySS. These data suggested a role of the plasma GSH redox system in maintaining the extracellular redox balance of the Cys thiol system in critically-ill children. In our study, low plasma 25(OH)D concentrations, which have been previously reported in this cohort2 and other pediatric ICU cohorts1, 3, was associated with more oxidized plasma EhCySS, thus suggesting a role for vitamin D in control of oxidative stress in pediatric critical illness. Furthermore, subjects with higher 25(OH)D levels presented with lower plasma GSH, GSSG, total GSH and glutamine concentrations. Although mechanisms remain to be elucidated, it is possible that vitamin D amplifies the plasma GSH-mediated effects on extracellular Cys redox during critical illness. Lower GSH and glutamine concentrations in vitamin D sufficient patients may reflect enhanced plasma substrate utilization for the maintenance of the plasma Cys redox potential during critical illness.
This is the first study, to our knowledge, to investigate a relationship between vitamin D status and oxidative stress in a critically-ill cohort. The associations differ from those of a previous study of generally healthy, ambulatory adults in which we described an independent, inverse relationship of plasma 25(OH)D with plasma EhGSSG (but not EhCySS) and a positive relationship with GSH20. Clinical trials in chronic diseases, such as diabetes26, have shown vitamin D to increase circulating GSH concentrations. In vitro and animal studies show vitamin D, as calcitriol (1,25(OH)2D) or vitamin D3, to alter the expression or action of redox regulating proteins, such as glutathione peroxidase, glutamate cysteine ligase, glutathione reductase, and thioredoxin binding protein-2 (also known as vitamin D3 upregulating protein 1)19, 27, 28, although results have not been consistent. For example, George et al.28 reported an increase in hepatic glutathione peroxidase gene expression in streptozotocin-induced diabetic rats. In contrast, Bhat et al.29 reported a decrease in the activity of GSH-dependent enzymes glutathione peroxidase and glutathione reductase in rat muscle following vitamin D repletion. The influence of disease state (e.g, acute, chronic, healthy) and tissue system (plasma, muscle, liver) on the relationship between vitamin D status and the GSH/GSSG and Cys/CySS redox pairs will require additional investigation to establish whether vitamin D may be a useful adjunctive therapy to reduce oxidative stress in pediatric critical illness.
In this cohort, subjects with plasma 25(OH)D concentrations ≥30 ng/mL had the highest plasma LL-37 concentrations, although the relationship was not statistically significant. Our small sample size likely limited the power to produce a statistically significant result; nevertheless, the biologic plausibility for a role of vitamin D on this marker of innate immunity is strong. LL-37 is an anti-microbial peptide of the human cathelicidin gene, hCAP18, which contains a vitamin D response element on its promoter region30, and calcitriol directly upregulates the expression of cathelicidin11. Although we do not have plasma calcitriol available in our subjects for comparison, it is possible that plasma LL-37 is more influenced by plasma calcitriol. On the other hand, in vitro studies suggest upregulation of cathelicidin only occurs in the setting of adequate serum 25(OH)D concentrations11. In critically ill adults with sepsis, higher plasma 25(OH)D concentrations correlated with higher plasma LL-374. Furthermore, in a recent randomized, placebo-controlled trial in adult ICU patients with sepsis, high-dose supplementation with cholecalciferol (400,000 IU) resulted in a 30% increase in plasma LL-37 concentrations12. Randomized, controlled trials in critically ill children are needed to verify an effect of vitamin D on plasma LL-37. Importantly, whether an increase in plasma LL-37 will translate to improved clinical outcomes in critical illness, in either adults or children, will need to be determined.
There is on-going debate regarding the specific circulating 25(OH)D concentrations that define an “adequate” or “deficient” vitamin D status24, 25. Optimal concentrations for PICU populations are not known. Our data show complex relationships between vitamin D status and the major constituents of intra- and extracellular aminothiol redox and immunity. Additional studies are clearly required to describe the clinical and metabolic effects of vitamin D supplementation in pediatric critical illness in order to determine the optimal level of supplementation needed. It is also possible that repletion of vitamin D following the initiation of critical illness may not prove to be efficacious, rather adequate stores of vitamin D may be necessary prior to the onset of critical illness to reap the benefits of reduced oxidative stress and increased levels of LL-37.
A major strength of this study was the novel investigation of the major intracellular and extracellular redox systems in association with vitamin D status during critical illness in children. However, given the limitations in sample size, this can only be considered a pilot study. As this is a sub-study based off of a previous study2, 17, sample size calculations were not conducted specifically to assess the relationship between vitamin D status and plasma redox. We were limited in power to adjust for numerous covariates, such as severity or duration of illness, underlying diagnosis, diagnosis of an acute event vs. exacerbation of chronic disease, or medications. It is possible that the heterogeneity of the cohort in terms of diagnosis and severity further, as well as medication use, influenced statistical power and the ability to make clinical inferences. As this was a cross-sectional study, one cannot infer cause-effect relationships, although the data are hypothesis-generating. We do not have available measurements of plasma calcitriol or free or bioavailable 25(OH)D. The predictive value of bioavailable 25(OH)D may be greater than total circulating 25(OH)D12, particularly in critically ill patients, in whom the major 25(OH)D carriers (vitamin binding protein and albumin) are acute phase reactants31. Finally, we do not have measurements of vitamin D, redox, or nutrition status prior to reporting to the ICU, or measurements of other plasma oxidants and antioxidants, for comparison.
4.1 Conclusions
In critically ill children, vitamin D sufficiency was associated with less oxidized plasma EhCySS, the redox state of the primary extracellular redox couple (Cys and CySS). In contrast, vitamin D sufficiency was associated with lower plasma GSH, GSSG, and glutamine, suggesting complex relationships between vitamin D and aminothiol redox during critical illness in children. Limitations of this pilot study elicit a need for additional detailed investigation to verify a specific role of vitamin D in aminothiol redox balance. Clinical trials of vitamin D supplementation will be required to elucidate the various ways vitamin D may be impacting the stress response to critical illness.
Supplementary Material
HIGHLIGHTS.
We examined the link between vitamin D, oxidative stress, and immunity in the PICU.
Vitamin D deficiency was associated with a more oxidized plasma cysteine redox.
Plasma glutathione was higher in vitamin D deficient subjects.
The role of vitamin D in regulating plasma redox in the PICU needs further study.
Acknowledgments
This study was supported by the Emory+Children’s Statistical Core and the Emory+Children’s Biomarkers Core. Funding was provided by the Children’s Healthcare of Atlanta Friends (grant number 38234), the National Institutes of Health (UL1 TR000454 and K01 DK102851). Funding sources had no involvement in the study design; the collection, analysis or interpretation of the data; the writing of the report; or the decision to submit the article for publication.
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- CySS
cystine
- Cys
cysteine
- EhCySS
redox potential for the Cys/CySS couple
- EhGSSG
redox potential for the GSH/GSSG couple
- GSH
glutathione
- GSSG
glutathione disulfide
- HPLC
high-performance liquid chromatography
- PICU
pediatric intensive care unit
- PELOD
Pediatric Logistic Organ Dysfunction
- PRISM III
Pediatric Risk of Mortality III
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors report no conflict of interest.
Authorship: Author contributions were as follows: conception and design of the study (JAA, KBH, VT); generation, collection assembly, analysis and/or interpretation of data (JAA, KBH, JRG, SEG); drafting or revision of manuscript (JAA, KBH, VT, JRG, SEG); and approval of final version of the manuscript (JAA, KBH, VT, JRG, SEG).
References
- 1.Abou-Zahr R, Kandil SB. A pediatric critical care perspective on vitamin D. Pediatr Res. 2015;77:164–167. doi: 10.1038/pr.2014.167. [DOI] [PubMed] [Google Scholar]
- 2.Hebbar KB, Wittkamp M, Alvarez JA, et al. Vitamin D Deficiency in Pediatric Critical Illness. J Clin Transl Endocrinol. 2014;1:170–175. doi: 10.1016/j.jcte.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madden K, Feldman HA, Smith EM, et al. Vitamin D deficiency in critically ill children. Pediatrics. 2012;130:421–428. doi: 10.1542/peds.2011-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeng L, Yamshchikov AV, Judd SE, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kempker JA, Tangpricha V, Ziegler TR, et al. Vitamin D in sepsis: from basic science to clinical impact. Crit Care. 2012;16:316. doi: 10.1186/cc11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun AB, Gibbons FK, Litonjua AA, et al. Low serum 25-hydroxyvitamin D at critical care initiation is associated with increased mortality. Crit Care Med. 2012;40:63–72. doi: 10.1097/CCM.0b013e31822d74f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen HB, Eshete B, Lau KH, et al. Serum 1,25-dihydroxyvitamin D: an outcome prognosticator in human sepsis. PLoS One. 2013;8:e64348. doi: 10.1371/journal.pone.0064348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amrein K, Schnedl C, Holl A, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA. 2014;312:1520–1530. doi: 10.1001/jama.2014.13204. [DOI] [PubMed] [Google Scholar]
- 9.Leaf DE, Raed A, Donnino MW, et al. Randomized controlled trial of calcitriol in severe sepsis. Am J Respir Crit Care Med. 2014;190:533–541. doi: 10.1164/rccm.201405-0988OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarez JA, Zughaier SM, Law J, et al. Effects of high-dose cholecalciferol on serum markers of inflammation and immunity in patients with early chronic kidney disease. Eur J Clin Nutr. 2013;67:264–269. doi: 10.1038/ejcn.2012.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 12.Quraishi SA, De Pascale G, Needleman JS, et al. Effect of Cholecalciferol Supplementation on Vitamin D Status and Cathelicidin Levels in Sepsis: A Randomized, Placebo-Controlled Trial. Crit Care Med. 2015;43:1928–1937. doi: 10.1097/CCM.0000000000001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowley HC, Bacon PJ, Goode HF, et al. Plasma antioxidant potential in severe sepsis: a comparison of survivors and nonsurvivors. Crit Care Med. 1996;24:1179–1183. doi: 10.1097/00003246-199607000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Crimi E, Sica V, Williams-Ignarro S, et al. The role of oxidative stress in adult critical care. Free Radic Biol Med. 2006;40:398–406. doi: 10.1016/j.freeradbiomed.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 15.Bar-Or D, Bar-Or R, Rael LT, et al. Oxidative stress in severe acute illness. Redox Biol. 2015;4:340–345. doi: 10.1016/j.redox.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 17.Grunwell JR, Gillespie SE, Ward JM, et al. Comparison of Glutathione, Cysteine, and Their Redox Potentials in the Plasma of Critically Ill and Healthy Children. Front Pediatr. 2015;3:46. doi: 10.3389/fped.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekmark L, Rooyackers O, Wernerman J, et al. Plasma glutamine deficiency is associated with multiple organ failure in critically ill children. Amino Acids. 2015;47:535–542. doi: 10.1007/s00726-014-1885-x. [DOI] [PubMed] [Google Scholar]
- 19.Jain SK, Micinski D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem Biophys Res Commun. 2013;437:7–11. doi: 10.1016/j.bbrc.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez JA, Chowdhury R, Jones DP, et al. Vitamin D status is independently associated with plasma glutathione and cysteine thiol/disulphide redox status in adults. Clinical endocrinology. 2014;81:458–466. doi: 10.1111/cen.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362:192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 23.Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med. 2009;47:1329–1338. doi: 10.1016/j.freeradbiomed.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 25.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 26.Shab-Bidar S, Neyestani TR, Djazayery A. The interactive effect of improvement of vitamin D status and VDR FokI variants on oxidative stress in type 2 diabetic subjects: a randomized controlled trial. Eur J Clin Nutr. 2015;69:216–222. doi: 10.1038/ejcn.2014.240. [DOI] [PubMed] [Google Scholar]
- 27.Chung JW, Jeon JH, Yoon SR, et al. Vitamin D3 upregulated protein 1 (VDUP1) is a regulator for redox signaling and stress-mediated diseases. J Dermatol. 2006;33:662–669. doi: 10.1111/j.1346-8138.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 28.George N, Kumar TP, Antony S, et al. Effect of vitamin D3 in reducing metabolic and oxidative stress in the liver of streptozotocin-induced diabetic rats. Br J Nutr. 2012;108:1410–1418. doi: 10.1017/S0007114511006830. [DOI] [PubMed] [Google Scholar]
- 29.Bhat M, Ismail A. Vitamin D treatment protects against and reverses oxidative stress induced muscle proteolysis. J Steroid Biochem Mol Biol. 2015;152:171–179. doi: 10.1016/j.jsbmb.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 31.Madden K, Feldman HA, Chun RF, et al. Critically Ill Children Have Low Vitamin D-Binding Protein, Influencing Bioavailability of Vitamin D. Ann Am Thorac Soc. 2015;12:1654–1661. doi: 10.1513/AnnalsATS.201503-160OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.