Abstract
Background
Respiratory weakness and spinal deformity are common in patients with Spinal Muscular Atrophy (SMA). Posterior (distraction type) growing rods have recently gained favor as a treatment option in this population, due to their ability to prevent spinal deformity progression and their potential to allow lung volumes to increase over time. The objective of this study was to determine the impact of posterior growing rods on the spinal alignment and respiratory function in children with SMA with intermediate term follow-up.
Methods
A single center, retrospective review was performed on SMA patients treated with growing rods, inserted between 2004 and 2010, with a minimum of two-year follow-up. SMA type, changes in the route of bi-level positive airway pressure respiratory support and the amount of time receiving respiratory support are reported. Pulmonary function tests (PFTs) and radiographs were reviewed and data evaluated pre-insertion, post insertion, and at latest follow-up.
Results
Sixteen children with SMA (five Type I, eleven Type II) met inclusion criteria. The average age of insertion was 5.8 (+/−1.5) years, the median number of lengthenings was 4 (range 3-5), and the median time between insertion and last clinical review was 4.7 (range 2.7-9.5) years. Radiographic review demonstrated significant (p<0.05) improvements in the following: Spinal curve magnitude, pelvic obliquity, space available for the lung, rib vertebral angle difference, and thoracic kyphosis following growing rod implantation. Thoracic and lumbar height and chest width and depth increased significantly (p<0.05) over the lengthening process. None of the patients initially required more than non-invasive positive pressure ventilation (NIPPV) support. Fifteen of the sixteen experienced no changes in their NIPPV support needs throughout the study duration, requiring support only at night and naps. Serial PFTs were available for six children with SMA Type II. PFTs demonstrated significant improvements in absolute forced vital capacity (FVC), minimal changes in the maximal inspiratory and expiratory pressures, and a gradual worsening of percent predicted FVC.
Conclusions
Clinical respiratory support requirements appear to stabilize following the insertion and lengthening of posterior based growing rods in the SMA population. Similar to previous studies, increased spinal height and thoracic cavity size were noted throughout the process. Despite an increasing absolute FVC, the percent predicted FVC diminished over time.
Level of Evidence
Therapeutic Level IV
Introduction
Spinal Muscular Atrophy (SMA) is a rare disease that has devastating effects on the neuromuscular system. The disease generally results from homozygous absence of the SMN1 gene, affecting creation of the SMN protein needed for lower motor neuron survival.[1-3] The consequences of this genetic mutation includes progressive weakness and in most children, especially those with the more common severe and intermediate subtypes (Type I and II)[4], results in a shortened lifespan due to progressive respiratory failure.[5, 6]
In addition to respiratory complications, children with SMA Types I and II almost universally develop spinal deformity, often at a very young age.[7] Despite being difficult to treat historically, surgical management with posterior (distraction type) growing rods has emerged as safe and effective for curve correction.[8, 9] Treatment has been advocated as it is believed that these procedures allow increases in lung volume over time as the thorax is lengthened while preventing curve progression. Maximizing lung growth and development is especially appealing in this population due to their fragile respiratory state. However, it has previously been shown that when compared to those with idiopathic early-onset scoliosis, patients with SMA have much smaller yearly improvements in spinal height and more rib collapse after growing rods treatment.[9] Thus, while assumed, the benefits of growing rod treatment on the pulmonary function in children with SMA has not been clearly demonstrated.
The objective of this study was to determine the impact of posterior growing rods on the spinal alignment and respiratory function of children with types I and II spinal muscular atrophy at intermediate term follow-up. Our hypothesis was that growing rods would improve and maintain spinal alignment and stabilize pulmonary function.
Methods
This retrospective longitudinal analysis was completed with approval from the University of Wisconsin-Madison Health Sciences Institutional Review Board. Medical records were reviewed for children with SMA who had received dual posterior (distraction type) growing rods at our institution between 2004 and 2010 that had a minimum of two-year follow-up. The child's SMA subtype, age at growing rod insertion, dates of lengthenings, and pulmonary support and pulmonary function test (PFT) results were recorded. Radiographic measurements were made using digital radiographs and measurement tools available through our clinical picture archiving and communicating system (PACS) (Mckesson, San Francisco, CA).
Radiographic Outcomes
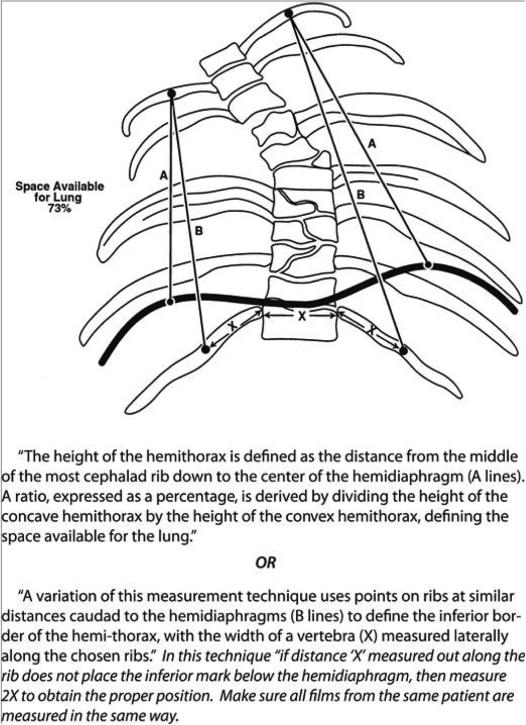
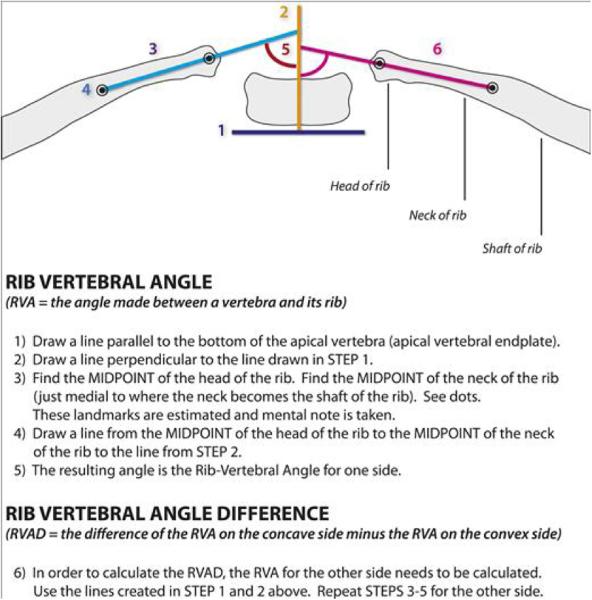
Spinal radiographs were reviewed for Cobb angle (of the major curve), pelvic obliquity, thoracic and lumbar heights, the space available for the lung (SAL), rib vertebral angle (RVA), rib vertebral angle difference (RVAD), thoracic kyphosis, and chest width and depth. These measurements were repeated on pre-operative, post-operative, and most recent radiographs available. Space available for the lung (SAL) is a measurement described by Campbell et al[10] and is done on standard x-rays. The value is a ratio of the heights of the two hemithoraces. The value suggests asymmetry in the thorax and inhibition of growth of one hemithorax. This value allows for comparison across multiple sizes of children and is insensitive to growth, so can be used to follow the severity of impairment even as a child grows. An example of these measurements can be found in Appendix I.
Pulmonary Function
The medical record was reviewed for available pulmonary function testing. When available, forced vital capacity (FVC), percent predicted FVC (%FVC), maximal inspiratory pressure (MIP), and maximal expiratory pressure (MEP) were recorded before lengthening, after lengthening, and at most recent follow-up; (N=6). Percent predicted FVC was calculated using Zaplatel reference equations for all subjects under 8 years of age. For subjects 8 year of age and older the Zaplatel reference equation was used until 2011 when the percent predicted reference equation was changed to NHANES (National Health and Nutrition Examination Survey). All reference equations are determined based on height, age, and gender.[11] FVC and FVC percent predicted is a reliable measure in children with SMA.[12, 13] However, due to reduced strength, not all patients (especially those with the more severe Type I SMA) were capable of completing pulmonary function testing. Therefore, the charts of all patients were also reviewed to determine their baseline need for respiratory support. Both the type of support (e.g. non-invasive positive pressure ventilation (NIPPV) with bi-level positive airway pressure (BiPap) and the number of hours per day were recorded at similar time periods as pulmonary function testing (i.e. before surgery, after surgery, and most recent).
Statistical Analysis
To analyze the radiographic variables paired t-tests with Holm adjustment for 2 tests were used to determine if patient data was different at pre-surgery versus post-surgery and post-surgery versus most recent follow-up. Significance was set at p ≤ 0.05. Patients were only included in a particular statistical test if values existed for both time points being compared in each particular test. Step-wise comparisons of pulmonary function test variables were compared between time points, using a paired student T-test.
Results
Demographics
Sixteen children with SMA met inclusion criteria. Five of the children had SMA Type I, while eleven had SMA Type II. The average age of insertion was 5.8 (+/−1.5) years, the median number of lengthenings was 4 (range 3-5), and the median time between insertion and last clinical review was 4.7 (range 2.7-9.5) years. Eight of the sixteen children had completed the lengthening process. Only one of the eight underwent definitive fusion.
Radiographic Analysis
Radiographic review demonstrated significant (p<0.05) improvements in the following: major curve magnitude as measured by Cobb angle, pelvic obliquity, thoracic and lumbar heights, the space available for the lung (SAL), rib vertebral angle difference (RVAD), and thoracic kyphosis following growing rod implantation. Thoracic and lumbar height continued to increase significantly (p<0.05) over the lengthening process. While chest width and depth increased, only chest width changes reached statistical significance. Finally rib collapse, as measured by the RVA, did not change significantly throughout the process. These findings are summarized in Table 1, and radiographs of a patient during the lengthening process are demonstrated in Figure 1.
Table 1.
Radiographic measures for patients pre-surgery, post-surgery, and at last follow up.
| Variable | Preop | Postop | Latest | Pre vs Post* | Post vs Latest* |
|---|---|---|---|---|---|
| Cobb Angle | 70.7 (24.6) | 27.2 (8.9) | 23.4 (11.9) | < 0.001 | 0.134 |
| Pelvic Obliquity | 23.1 (22) | 5.8 (4.9) | 4.8 (3.1) | 0.021 | 0.367 |
| Thoracic Height | 179.9 (14.9) | 195.1 (13.7) | 229.7 (24.9) | < 0.001 | < 0.001 |
| Lumbar Height | 109.6 (11.6) | 118.2 (11.9) | 134.6 (16) | 0.007 | < 0.001 |
| SAL | 0.77 (0.17) | 0.90 (0.08) | 0.93 (0.08) | 0.013 | 0.104 |
| Acute RVA | 26 (20.3) | 27.7 (14.2) | 28.1 (18) | 1 | 1 |
| RVAD | 47.8 (24.2) | 36.1 (17.6) | 33.1 (23.9) | 0.075 | 0.712 |
| Thoracic Kyphosis | 53.1 (32.2) | 34.7 (19.8) | 30.3 (17.8) | 0.084 | 0.242 |
| Chest Width | 160.5 (27.2) | 165.2 (28.9) | 182.2 (35.9) | 0.211 | 0.044 |
| Chest Depth | 148.7 (30.1) | 147.2 (24.8) | 170.8 (32.1) | 0.159 | 0.088 |
| Reported as Mean (SD) | |||||
p-values reported as Holm adjusted p-values from paired t-tests
Figure 1.
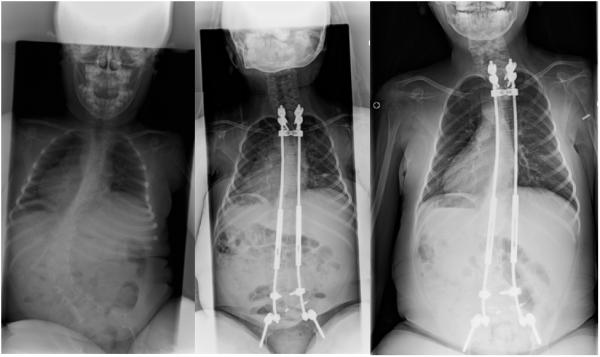
Radiographs of a patient throughout the lengthening process: pre-operatively, post-surgery, and at follow-up.
Pulmonary Function
None of the 16 patients required more than NIPPV support at baseline and only one patient had a an increase of their respiratory support. This child required the addition of NIPPV use at night. Except for this one patient, none of the sixteen patients experienced significant changes in their positive pressure respiratory support needs. All patients required support only at night and naps. One child with SMA Type I recently died, cause of death was determined to not be respiratory in nature.
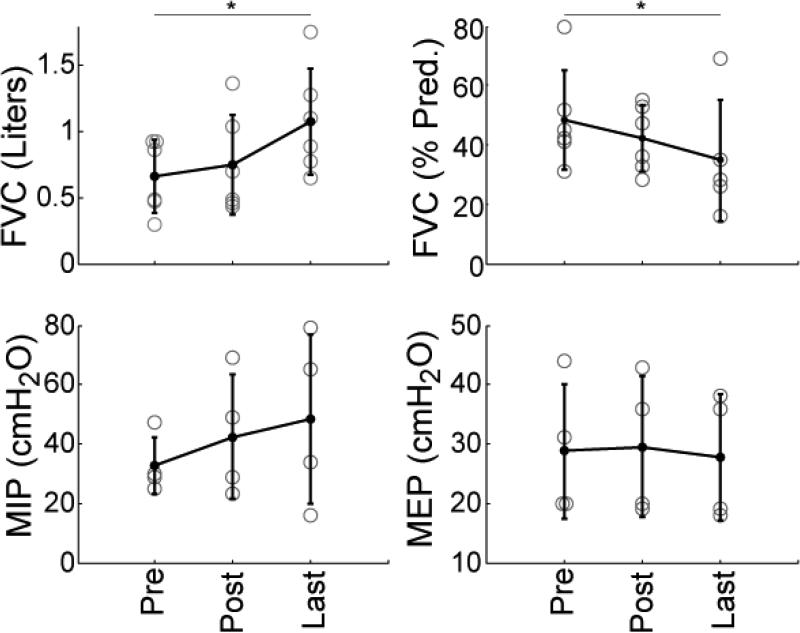
Serial pulmonary function tests (PFTs) were available for six children with SMA Type II. PFTs demonstrated significant improvements in absolute FVC. On average, children improved 0.41 L from initial testing to final follow-up (standard deviation +/− 0.25 L) n=6, p<0.05). Percent predicted FVC (%FVC) showed an average decline over time. Patients (n=5) began with an average of 48% of expected FVC and declined to 35% at latest follow-up (p<0.05). Inspiratory and Expiratory respiratory muscle force pressure as a group appeared to stabilize over the testing, with some patients showing improvement and others showing decline. Average maximum inspiratory pressure increased from a pre-operative value of 33 to 43 cm H2O post-operatively and 49 cm H2O at latest follow-up (n=4), but none of these changes were statistically significant (p=0.22, p=0.24 respectively). Maximum expiratory pressures stayed nearly constant with average values of 29, 30, and 28 cm H2O at pre-operative, post-operative, and latest evaluations respectively. Figure 2 demonstrates these PFT findings.
Figure 2.
PFTs for subjects pre-surgery (Pre), post-surgery (Post), and at last follow-up (Last). Means are indicated by the filled black dots, and error bars represent +/− 1 standard deviation. Gray circles represent individual patients. * = p<0.05 (FVC = Forced Vital Capacity, MIP = Maximal Inspiratory Pressure, MEP = Maximum Expiratory Pressure).
Discussion
The purpose of this study was to determine the effects of posterior growing rods on the spinal alignment and pulmonary function of children with SMA. Due to the relatively rare occurrence of this disease, this is the first single center study, using a uniform spinal treatment, to evaluate the pulmonary outcomes that this surgery has on a population of children with SMA. Our results indicate that spinal deformity is improved and maintained over the long term. Further, forced vital capacity was found to increase after surgery. However, percent of predicted forced vital capacity has the opposite trend, actually reducing over time.
Traditionally, scoliosis in SMA had been treated with bracing and/or early fusion. However, bracing was found minimally effective and lead to problems with respiration.[14, 15] Similarly early fusion didn't always stop curve progression[16] and is thought to reduce maximal lung volumes.[14, 17, 18] Because of these consequences, other treatments approaches have been sought in this population. Surgical placement of growing rods as treatment gained favor after Chandran et al. showed much improved spinal curvature with very few complications (none surgical), and increased FVC at 9 months of follow-up (0.53 to 0.67 L).[8] Another group, demonstrated improvement even when curves began much larger (89 to 55 degrees at 55 months of follow-up).[9] Despite these gains, this study showed that rib collapse continued in the children with SMA. However, neither of these studies determined whether pulmonary benefits would persist over time following growing rod insertion. In our study, the average most acute rib vertebral angle did not change, over the study period, the average chest width and average trunk heights (sum of thoracic and lumbar heights) increased during the lengthening process. All of these radiographic findings likely contributed to our generally favorable pulmonary results.
Our pulmonary function test results mirror other studies evaluating the surgical treatment of early-onset scoliosis associated with different devices or a variety of diagnosis. Dede et al. reported that the use of VEPTR in children with early-onset scoliosis due to a variety of conditions led to large improvements in FVC (a 45% improvement (0.31 L) at 72 months).[19] However, similar to our results, these children had reduced percent predicted FVC (77% to 58%).[19] Other work however, has shown increases in percent predicted FVC. A study of six children with neuromuscular disease with magnetic growing rods showed an improvement of 14% percent predicted FVC at an average of 30 months of follow-up.[20] Comparing our results with recently reported PFTs for children with type II SMA, it does appear we are improving FVCs over the natural history and perhaps slowing the rate of percent predicted FVC decline.[21]
Pulmonary function is a major issue in children with SMA. A majority of children die to pulmonary compromise, due to restrictive lung disease with progression to respiratory failure. Therefore, sources of gain or stabilization in pulmonary function are often sought. Given that scoliosis is known to limit space available for the lungs and hinder lung development,[22] treatment of scoliosis at an early age is hypothesized to improve pulmonary function. From our results, we are encouraged by the increases in forced vital capacity seen over time and the stabilization of the inspiratory and expiratory respiratory muscle pressures. The reduced percent predicted FVC could be in part due to continued change in rib shape or further reduction in chest wall and lung growth of the child relative to their length compared to normal (altering the effects of normalization over time). Time between lengthenings being less than ideal could also contribute to reduced %FVC, though risks of surgery must be weighed before increasing frequency.
While we feel this study presents valuable new information, we should acknowledge the following limitations. First the study lacks a control group. It is unclear whether children with SMA not receiving growing rods would show similar changes in their lung volumes over time. Another limitation is the small sample size, especially of children actually receiving full pulmonary function testing. Furthermore, our study included patients who were too young or too weak to complete standard pulmonary function testing. Inclusion of this group is important to characterize the change in pulmonary function in this severely affected group. Despite the disease severity of this group (many SMA type 1), we surprisingly found that only one patient had an increase in respiratory support during this period and most needed NIPPV only during sleep. Another potential shortcoming of this study was the inability of to capture or quantify adverse respiratory events in the perioperative period. The authors did not identify these events in the study design and while doing so may have strengthened this manuscript such data would have required a prospective design to capture. Also using three-dimensional imaging may have provided more accurate measurements of changes in thoracic volume, but this imaging is not routinely obtained serially. Due to the rare nature of the disease, we feel that the new information provided on these patients can give insight not provided to date.
In conclusion, in children with SMA, posterior growing rods can stabilize spinal deformity over time. Further, after implantation, vital capacity improves, but the percentage of predicted FVC tends to decrease. The need for pulmonary supportive therapy remains relatively stable. From these findings, we conclude that growing rods may help to stabilize pulmonary function and slow decline, though this cannot be stated with certainty due to a lack of a comparison group. Because of the fragile pulmonary status of children with SMA, growing rods are a good option for patients to help achieve spinal and pulmonary stabilization.
Acknowledgments
Disclosure: The authors would like to acknowledge support from the Cure SMA, NIH (F30 AR065838), and the University of Wisconsin-Madison Medical Scientist Training Program (T32 GM008692).
Appendix I
Radiographic measurements used in SMA study. (Upright films used if available).
Anterior Posterior Radiographs
-
1)
Max Coronal Cobb – Largest Cobb angle of largest curve
-
2)
S1-L1 vertical distance – Measure distance (mm) from mid-point of S1 endplate to the midpoint of the superior endplate of L1
-
3)
T12-T1- vertical distance from midpoint of inferior endplate of T12 to the midpoint of the superior endplate of T1
-
4)Space Available for Lung – See Figure Below from Campbell et al JBJS 2003.[10]
- 5)
-
7)
Width Chest Cavity -Inner rib to inner rib (measured in (mm) at the widest spot)
-
8)
Pelvic Obliquity – Iliac crest from horizontal (or clavicles if supine).
Lateral Radiograph
-
1)
Maximum Chest Depth – distance from posterior aspect of sternum/or rib (anteriorly) with the anterior edge of posterior rib. (widest measurement)
-
2)
Thoracic Kyphosis – T2-T12, the angle formed between the superior endplate of T2 and the inferior endplate of T12.
References
- 1.Battaglia G, Princivalle A, Forti F, et al. Expression of the SMN gene, the spinal muscular atrophy determining gene, in the mammalian central nervous system. Human molecular genetics. 1997;6:1961–1971. doi: 10.1093/hmg/6.11.1961. [DOI] [PubMed] [Google Scholar]
- 2.Parker GC, Li X, Anguelov RA, et al. Survival motor neuron protein regulates apoptosis in an in vitro model of spinal muscular atrophy. Neurotoxicity research. 2008;13:39–48. doi: 10.1007/BF03033366. [DOI] [PubMed] [Google Scholar]
- 3.Wirth B. An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA). Human mutation. 2000;15:228–237. doi: 10.1002/(SICI)1098-1004(200003)15:3<228::AID-HUMU3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Munsat TL, Davies KE. International SMA consortium meeting. (26-28 June 1992, Bonn, Germany). Neuromuscular disorders : NMD. 1992;2:423–428. doi: 10.1016/s0960-8966(06)80015-5. [DOI] [PubMed] [Google Scholar]
- 5.Chung BH, Wong VC, Ip P. Spinal muscular atrophy: survival pattern and functional status. Pediatrics. 2004;114:e548–553. doi: 10.1542/peds.2004-0668. [DOI] [PubMed] [Google Scholar]
- 6.Wang CH, Finkel RS, Bertini ES, et al. Consensus statement for standard of care in spinal muscular atrophy. Journal of child neurology. 2007;22:1027–1049. doi: 10.1177/0883073807305788. [DOI] [PubMed] [Google Scholar]
- 7.Granata C, Merlini L, Magni E, et al. Spinal muscular atrophy: natural history and orthopaedic treatment of scoliosis. Spine. 1989;14:760–762. doi: 10.1097/00007632-198907000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Chandran S, McCarthy J, Noonan K, et al. Early treatment of scoliosis with growing rods in children with severe spinal muscular atrophy: a preliminary report. Journal of pediatric orthopedics. 2011;31:450–454. doi: 10.1097/BPO.0b013e31821722b1. [DOI] [PubMed] [Google Scholar]
- 9.McElroy MJ, Shaner AC, Crawford TO, et al. Growing rods for scoliosis in spinal muscular atrophy: structural effects, complications, and hospital stays. Spine. 2011;36:1305–1311. doi: 10.1097/BRS.0b013e3182194937. [DOI] [PubMed] [Google Scholar]
- 10.Campbell RM, Jr., Smith MD, Mayes TC, et al. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. The Journal of bone and joint surgery American volume. 2003;85-A:399–408. doi: 10.2106/00004623-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. The European respiratory journal. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 12.Iannaccone ST, Hynan LS, American Spinal Muscular Atrophy Randomized Trials G Reliability of 4 outcome measures in pediatric spinal muscular atrophy. Archives of neurology. 2003;60:1130–1136. doi: 10.1001/archneur.60.8.1130. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann P, McDermott MP, Darras BT, et al. Prospective cohort study of spinal muscular atrophy types 2 and 3. Neurology. 2012;79:1889–1897. doi: 10.1212/WNL.0b013e318271f7e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aprin H, Bowen JR, MacEwen GD, et al. Spine fusion in patients with spinal muscular atrophy. The Journal of bone and joint surgery American volume. 1982;64:1179–1187. [PubMed] [Google Scholar]
- 15.Tangsrud SE, Carlsen KC, Lund-Petersen I, et al. Lung function measurements in young children with spinal muscle atrophy; a cross sectional survey on the effect of position and bracing. Archives of disease in childhood. 2001;84:521–524. doi: 10.1136/adc.84.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zebala LP, Bridwell KH, Baldus C, et al. Minimum 5-year radiographic results of long scoliosis fusion in juvenile spinal muscular atrophy patients: major curve progression after instrumented fusion. Journal of pediatric orthopedics. 2011;31:480–488. doi: 10.1097/BPO.0b013e318220ba33. [DOI] [PubMed] [Google Scholar]
- 17.Brown JC, Zeller JL, Swank SM, et al. Surgical and functional results of spine fusion in spinal muscular atrophy. Spine. 1989;14:763–770. doi: 10.1097/00007632-198907000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Vitale MG, Matsumoto H, Bye MR, et al. A retrospective cohort study of pulmonary function, radiographic measures, and quality of life in children with congenital scoliosis: an evaluation of patient outcomes after early spinal fusion. Spine. 2008;33:1242–1249. doi: 10.1097/BRS.0b013e3181714536. [DOI] [PubMed] [Google Scholar]
- 19.Dede O, Motoyama EK, Yang CI, et al. Pulmonary and Radiographic Outcomes of VEPTR (Vertical Expandable Prosthetic Titanium Rib) Treatment in Early-Onset Scoliosis. The Journal of bone and joint surgery American volume. 2014;96:1295–1302. doi: 10.2106/JBJS.M.01218. [DOI] [PubMed] [Google Scholar]
- 20.Yoon WW, Sedra F, Shah S, et al. Improvement of pulmonary function in children with early-onset scoliosis using magnetic growth rods. Spine. 2014;39:1196–1202. doi: 10.1097/BRS.0000000000000383. [DOI] [PubMed] [Google Scholar]
- 21.Khirani S, Colella M, Caldarelli V, et al. Longitudinal course of lung function and respiratory muscle strength in spinal muscular atrophy type 2 and 3. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2013;17:552–560. doi: 10.1016/j.ejpn.2013.04.004. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 22.Davies G, Reid L. Effect of scoliosis on growth of alveoli and pulmonary arteries and on right ventricle. Archives of disease in childhood. 1971;46:623–632. doi: 10.1136/adc.46.249.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corona J, Sanders JO, Luhmann SJ, et al. Reliability of radiographic measures for infantile idiopathic scoliosis. The Journal of bone and joint surgery American volume. 2012;94:e86. doi: 10.2106/JBJS.K.00311. [DOI] [PubMed] [Google Scholar]
- 24.Mehta MH. The rib-vertebra angle in the early diagnosis between resolving and progressive infantile scoliosis. The Journal of bone and joint surgery British volume. 1972;54:230–243. [PubMed] [Google Scholar]