Abstract
Recent studies have suggested a close association between prenatal maternal distress and allergic diseases in the offspring. We selected relevant birth-cohort or national registry studies using a keyword search of the PubMed database and summarized current evidence on the impact of prenatal maternal distress on the development of offspring's allergic diseases. Moreover, we postulated possible pathways linking prenatal distress and allergic diseases based on relevant human and animal studies. Both dysregulated hypothalamic-pituitary-adrenal axis and increased oxidative stress may cause structural (altered brain/lung development) and functional (skewed immune development) changes, which may predispose the fetus to developing allergic diseases during childhood. Although many facts are yet to be discovered, changes in the placental response and epigenetic modification are presumed to mediate the whole process from maternal distress to allergic diseases. Maternal prenatal distress can also interact with other physical or environmental factors, including familial or physical factors, indoor and outdoor pollutants, and early childhood psychological distress. The gut-microbiome-brain axis and the role of the microbiome as an immune modulator should be considered when investigating the stress-allergy relationship and exploring potential intervention modalities. Further research is needed, and particular attention should be given to defining the most vulnerable subjects and critical time periods. To this end, studies exploring relevant biomarkers are warranted, which can enable us to explore adequate intervention strategies.
Keywords: Allergic diseases, asthma, child and fetal development, developmental programming, placenta, prenatal stress
INTRODUCTION
Allergic diseases, hypersensitivity disorders of the immune system, are a major global health concern. Pediatric allergic diseases comprise a large component of general pediatric care and are increasing in prevalence.1 The phenotype of allergic diseases tends to transform from atopic dermatitis to allergic rhinitis and asthma, and threaten the quality of life of affected children and their parents.
During pregnancy, women are more vulnerable to stress and at risk of distress due to profound hormonal and physiologic changes. Prenatal maternal distress is thought to influence various fetal and neonatal outcomes: prematurity and intrauterine growth restriction, psychiatric diseases, and the metabolic syndrome.2 Given the high prevalence of prenatal maternal distress and substantial medical burden of allergic diseases, investigation of the association between prenatal maternal distress and allergic diseases in offspring, and the possible underlying mechanisms, warrants attention.
In this review, we aim to summarize current evidence on the impact of prenatal maternal psychological distress on the development of allergic diseases and to discuss possible pathways linking prenatal stress and allergic diseases. Through this, we will present a viewpoint of selecting vulnerable subgroups and choosing intervention strategies.
EVIDENCE OF THE IMPACT OF PRENATAL MATERNAL DISTRESS ON ALLERGIC DISEASES IN THEIR OFFSPRING
To locate current evidence on the topic, we conducted a keyword search through the PubMed databases (see Supplementary Table available from http://www.e-aair.org). From the 238 results, we selected cohort or registry studies published between January 1, 2000 and June 30, 2016 that examined the association between prenatal maternal distress and allergic diseases in offspring. We chose original articles where the primary exposure involved prenatal maternal psychological stress, and the main outcome covered any allergic disorder (wheezing, asthma, allergic rhinitis, atopic dermatitis, atopic eczema, allergic conjunctivitis, or food allergy).
Table summarizes the selected published studies. A total of 20 studies were chosen:3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22 17 prospective cohort studies 3,4,5,6,8,9,10,11,12,13,14,15,18,19,20,21,22 and 3 national registry studies.7,16,17 Most studies used general population samples, although not all were nationally representative. Prenatal maternal distress had a close association with wheezing/asthma in 17 studies3,4,6,7,8,9,10,11,12,13,14,15,16,17,18,19,21,22 and atopic dermatitis in 5 studies.5,11,12,20,22 Two studies reported good relationships with both asthma and atopic dermatitis.11,22 Only 1 cohort study assessed the possible relationship between maternal distress and allergic rhinitis.11 The earliest report on the effect of prenatal maternal distress on allergic diseases was published only in 2009,21,22 which may be related to the fact that the fetal origins hypothesis began gaining substantial attention during the late 1990s and the International Society for Developmental Origins of Health and Disease was established in 2003. Although we have not proceeded a meta-analysis, the attributable risk of prenatal maternal stress seems to be present but limited. Although these are lists of studies that presented only positive results, 16 out of 20 (80%) studies presented odds, relative risk, or hazard ratio of less than 2. Moreover, no study has found a significant association between prenatal maternal stress and allergic rhinitis development.
Table. Published cohort or registry studies examining the association between prenatal maternal distress and allergic diseases in offspring.
| First author (yr) | Country | Design | Sample | Prenatal maternal distress assessment | Outcome assessment | Effect size (adjusted) (95% CI) | Main conclusion |
|---|---|---|---|---|---|---|---|
| Trump (2016)3 | Germany | Prospective | 443 | Maternal response to PSQ | Wheezing: maternally reported | OR 2.73 (1.13–6.55) | Prenatal maternal distress increased the risk of persistent childhood wheeze |
| Rosa (2016)4 | Mexico | Prospective | 417 | Maternal negative life events | Wheezing: caregiver reported at 48 months | RR 1.12 (1.00–1.26) | Prenatal distress in mothers was associated with wheeze in preschool-aged children |
| Chang (2016)5 | Korea | Prospective | 973 (COCOA) 1,531 (PSKC) |
Maternal response to questionnaires: for COCOA, CESD-10 and STAI-T; for PSKC, K6 | Atopic dermatitis: physician-diagnosed (COCOA) or caregiver-reported (PSKC) | [COCOA] HR CESD-10 1.31 (1.02–1.69) HR STAI-T 1.41 (1.06–1.89) [PSKC] OR 1.85 (1.06-3.25) |
Prenatal maternal distress increased the risk of AD in off-spring in both cohorts independently |
| Lee (2016)6 | USA | Prospective | 765 | Maternal negative life events | Asthma: maternally reported plus clinician-diagnosed | OR 1.38 (1.06–1.79) | Higher stress in the prenatal period was associated with increased odds of asthma diagnosis in girls, but not boys |
| Liu (2015)7 | Denmark | Retrospective (national registry) | 750,058 | Maternal bereave- ment: losing a child, partner/spouse, a parent or a sibling 1 year prior to or during pregnancy | Asthma event based on the ICD-10 codes and the ATC codes | HR 1.04 (1.00–1.07) | Prenatal distress following maternal bereavement was associated with a marginally increased risk of asthma events in children aged 0-3 years, but not in children aged 4-15 years |
| Bandoli (2016)8 | USA | Prospective | 2,543 | Pregnancy anxiety (evaluated by a questionnaire), chronic stress (by PSS), acute stress (by negative life events) | Asthma: maternally reported | RR 1.40 (1.07–1.83) | Multiple maternal prenatal stressors are associated with increased risk of lifetime wheeze in young offspring, with slight effect modification by Latina ethnicity |
| Hovland (2015)9 | Norway | Prospective | 550 | Familial prenatal distress by summing visual analogue scores | Asthma: 2 or more of the doctor's diagnosis, asthma symptoms or use of asth- ma medication | OR 1.04 (1.00+1.09) | Perinatal familial distress was one of the important early risk factors for pubertal asthma |
| Chiu (2014)10 | USA | Prospective | 708 | Maternal response on the exposure to community violence prenatally | Repeated wheeze: maternal self-report | OR 1.95 (1.13–3.36) | Exposure to community violence was associated with wheeze independently from the black carbon exposure |
| Hartwig (2014)11 | Australia | Prospective | 1,587 | Prenatal negative life events | Current asthma, atopy, eczema, and hay fever: physician diagnosed | OR 2.08 (1.22–3.54) | The likelihood of asthma and eczema at age 14 years was significantly increased in children of mothers who had experienced adverse life events during the second half of gestation |
| Larsen (2014)12 | Denmark | Prospective | 32,104 | Telephone interview: maternal self-reported job strain during pregnancy | Asthma and atopic dermatitis: maternal self-report to ISAAC questionnaire | OR 1.15 (1.02–1.31) | Maternal exposure to high job strain during pregnancy elevated the odds for atopic dermatitis among 7-year-old children. For asthma, an association was found only with a subset of groups |
| Turcotte-Tremblay (2014)13 | Canada | Prospective | 68 | Maternal responses to IES-R and LES | Asthma: maternal self-report at the age of 12 | OR 1.09 (1.00–1.19) | In girls only, higher levels of subjective maternal distress in pregnancy were associated with increased lifetime risk for wheezing, doctor-diagnosed asthma, and inhaled corticosteroid usage |
| Guxens (2014)14 | Netherland | Prospective | 4,848 | Maternal response to Brief Symptom Inventory at 20 weeks of gestation | Wheezing: maternally reported annually; Asthma: physician-diagnosed at 6 years | OR 1.60 (1.32–1.93) | Prenatal maternal distress was positively associated with wheezing in offspring |
| Chiu (2012)15 | USA | Prospective | 653 | Maternal negative life events during pregnancy | Wheezing: maternally reported at 3-month interval | OR 3.04 (1.67–5.53) | Exposure-response relationship between prenatal distress and child wheeze |
| Khashan (2012)16 | Sweden | Retrospective (national register) | 3,200,000 | Maternal bereavement: death of their spouse or child up to 6 months before or during pregnancy | Asthma: hospitalization due to ICD codes of asthma | RR 1.20 (1.03–1.39) | Children of exposed mothers were at increased risk of being hospitalized for asthma |
| Fang (2011)17 | Sweden | Retrospective (national register) | 426,334 (1 to 4 years old) 493,813 (7 to 12 years old) |
Maternal bereavement shortly before and during pregnancy | Asthma: hospital contact for asthma or at least 2 dispenses of inhaled corticosteroids or montelukast |
(in boys) HR 1.55 (1.19–2.02) |
Exposed boys, especially those exposed during their second trimester, were at increased risk of asthma |
| Reyes (2011)18 | USA | Prospective | 279 | Maternal demoralization: PERI-D scale at 3rd trimester | Wheezing: maternally reported during birth to 5 years | OR 1.66 (1.29–2.14) | Prenatal maternal demoralization was associated with overall, transient, and persistent wheeze |
| Wood (2011)19 | USA | Prospective | 560 | Maternal distress: 9-distress related questionnaire including PAS, EPDS, and PSS | Number of wheeze assessed every 3 months by questionnaire | OR EPDS 1.37 (P value < 0.01) OR PSS 1.59 (P value<0.01) |
Positive associations were detected between multiple wheeze and cotinine, maternal stress, and maternal depression |
| Wen (2011)20 | Taiwan | Prospective | 730 | Maternal self-reported mental status | Maternally reported atopic dermatitis via telephone survey | OR 2.3 (1.1–5.3) | Maternal distress during pregnancy was associated with ever having physician-diagnosed AD in 2 years old children |
| Cookson (2009)21 | UK | Prospective | 5,810 | Anxiety score on the self-reported Crown-Crisp Experiential Index at 18 and 32 weeks of gestation | Asthma: maternal report of doctor-diagnosed asthma at 7½ years | OR 1.64 (1.25–2.17) | Children of mothers in the higher anxiety scores were more likely to have asthma, with evidence for a dose-response |
| Sausenthaler (2009)22 | Germany | Prospective | 3,004 | The presence of 2 or more stress factors of the 12 factors | Maternally reported physician-diagnosed atopic dermatitis during birth to 6 years | OR 1.48 (0.95–2.30) | Maternal factors during pregnancy were positively associated with childhood eczema in terms of cumulative prevalence up to the age of 2 years, but not beyond |
AD, atopic dermatitis; ATC, Anatomical Therapeutic Chemical Classification; CESD-10, the 10 item Center for Epidemiologic Studies Depression Scale; CI, confidence interval; COCOA, the COhort for Childhood Origin of Asthma and allergic diseases; EPDS, Edinburgh Postnatal Depression Scale; HR, hazard ratio; ICD-10, International Classification of Diseases, Tenth Edition; IES-R, Impact of Event Scale-Revised; ISAAC, International Study of Asthma and Allergies in Childhood; LES, Life Experiences Survey; OR, odds ratio; PAS, Pregnancy Anxiety Scale; PERI-D, psychiatric epidemiology research interview-demoralization, PSKC, Panel Study on Korean Children; PSQ, Perceived Stress Questionnaire; PSS, Perceived Stress Scale; RR, relative risk; STAI-T, State Trait Anxiety Inventory-Trait scale.
Prenatal maternal distress was ascertained by various methods during different time periods. Regarding assessment points, studies using negative life events encompassed the whole pregnancy period,4,6,7,8,11,15,16,17 while those using self-reported questionnaires on depression or anxiety were conducted during the second or third trimester. All but one study assessed maternal distress at just 1-time point, thus making it difficult to ascertain the critical period for the effect of prenatal maternal distress on fetal and neonatal immune development. A study conducted in the UK assessed prenatal anxiety at 2 time points, 18 and 32 weeks of gestation, and found similar trends of a positive association between prenatal anxiety and subsequent childhood asthma at 7½ years, though the estimated parameter was larger for anxiety during the third trimester.21
In some studies, negative or stressful life events were used as a proxy for prenatal maternal distress.4,6,8,11,15 Maternal bereavement involving a spouse or child, one of the most extreme traumas a person may face, was found to be significantly associated with later allergic diseases,7,16,17 and the number of stressful life events during pregnancy was also reported to show a dose-response relationship.6 Recently, studies using validated self-reported questionnaires have also been published.3,5,8,13,14,18,19,21 However, there are as yet no studies that applied observational or objective measures to assess maternal psychological status. While there is evidence that maternal distress during pregnancy is associated with cortisol disruption,23 the relationship between cortisol dysregulation and its effect on the fetus is yet to be examined.
Regarding the outcomes, most studies used maternal reports on their child's allergic symptoms. However, it is possible that mother's recognition and reporting of the child's symptoms may be affected by their psychological status, since depression or anxiety is known to color perception.24 Two studies in Sweden utilized hospital records to assess the presence of asthma in a large representative general population sample.16,17 While hospital records provide comparatively more objective information on the medical status of subjects, registry studies are limited by potential residual confounding due to factors not recorded in the register. One study used physician's diagnosis of allergic diseases based on symptoms reported by mothers and children to ascertain asthma,14 with no objective measurement, such as pulmonary function or bronchial provocation testing.
In summary, there are several points to note in designing or interpreting studies involving the relationship between prenatal distress and allergy diseases. First, prospective data is crucial to examineing the association between more delicate psychological status and later development of illness, since symptoms like depression or anxiety are more prone to become biased. For fear of a recall bias, the retrospective approach should be confined to certain life events, such as the death of a spouse. Secondly, ascertainment of allergic diseases based on the maternal reports may be easily biased since mood states are known to influence the perception of current allergic illnesses.24 Mothers who are depressed or anxious may be more vigilant to minimal changes in the child, which may result in the overestimation of outcome diseases. Thirdly, the definitions of prenatal distress are variable: some studies measured 1 or 2 major life events, such as bereavements or the number of stressful life events, while others used validated questionnaires. Finally, most of the studies did not adjust for potential confounders, such as other risk factors for allergic diseases and postnatal maternal distress.
These studies demonstrate that there is sufficient evidence to conclude that prenatal maternal distress is associated with later development of offspring's allergic diseases. Most studies are, however, focused on asthma/wheezing; hence, there is a need for publications concerning other allergic diseases, such as allergic rhinitis or food allergy.
POSSIBLE PATHWAYS LINKING PRENATAL MATERNAL DISTRESS AND ALLERGIC DISEASES
Psychological stress is known to exacerbate allergic diseases via endocrine or immune responses.25 Considering that atopy is related to dysregulated immunity and that exposure to any risk factors during the critical period of development can affect the immune system's maturation, psychological stress during the fetal period may play a role in the onset of allergic diseases. However, few reports have postulated mechanisms by which prenatal maternal distress affects allergic diseases in offspring.
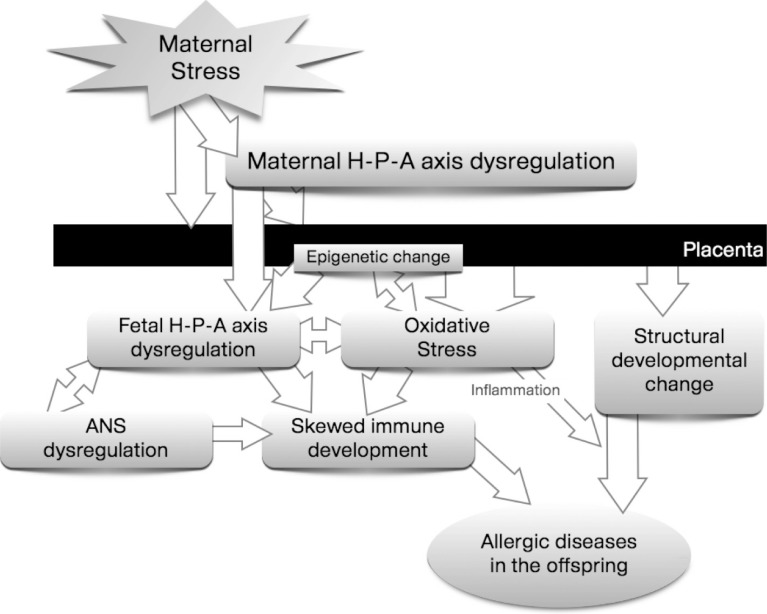
Since fetal and perinatal periods are the most critical phases of development, the risk of atopic disorders may mainly be ascribed to perinatal programming, and contributed to by non-genetic and environmental factors.26 Psychological stress is one of the suspected key factors playing a role in programming.2 In response to distress, the homeostatic regulatory system acts to maintain the body's equilibrium. This is necessary as a short-term response to stress, but may produce damage in the long-term.27 If not checked and eventually terminated, the stress response might permanently alter the responsiveness of major regulatory systems with serious impacts on the developing immune system and, consequently, may enhance the risk of diseases. It will be helpful to review the possible mechanism in terms of 5 aspects, in order to understand the whole process linking prenatal distress and allergic diseases in offspring collectively (Figure), as discussed below.
Figure. Possible mechanisms linking prenatal distress to the allergic diseases in offspring.
Psycho-neuro-endocrine dysregulation
The hypothalamic-pituitary-adrenal (HPA) axis and autonomic nervous system (ANS) are key regulatory systems activated in neuroendocrine pathways in response to environmental stress. The offspring's HPA axis or ANS, when persistently activated, can push the offspring's immune development toward being allergy-prone. This is a suspected mechanism of the maternal stress-induced development of asthma and allergic diseases in offspring.28,29 Since psycho-neuro-endocrine systems are interrelated, it may be difficult to separate the effect of each pathway, and altered neuroendocrine or autonomic systems can induce dysregulation of other systems. Any physical or psychological stress that can affect fetal psycho-neuro-endocrine systems could cause offspring's allergic diseases.26,28
A dysregulated HPA axis is one of the representative pathophysiologies in this relationship.
Chronic stress may alter the maternal HPA axis.2,30 Pregnant women experiencing much distress have persistently high levels of cortisol.31 Elevated levels of maternal serum cortisol result in more cortisol passing through the placental barrier, thereby increasing the cortisol level in the fetus.30 Maternal cortisol can also stimulate production of placental cortisol-releasing hormone (CRH) leading to elevated fetal CRH levels.2,28 Moreover, maternal distress itself reduces uteroplacental blood flow initiating a fetal stress response with subsequent fetal HPA axis activation.32 These all may eventually alter the responsiveness of the fetal HPA axis. Persistently elevated fetal CRH and glucocorticoid levels can influence brain and central nervous system (CNS) development, leading to long-term changes in HPA axis regulation, which in turn predisposes to later expression of asthma and allergic diseases.25
Alterations in the ANS, along with a dysregulated HPA axis, may polarize the fetal immune system toward allergic diseases. Maternal psychological stress activates the fetal sympathetic adrenomedullary system as well as the HPA axis.28 Furthermore, catecholamines increase the level of glucocorticoids, which in turn affects the HPA axis. ANS activation affects the immunologic system, possibly in a bidirectional way. This is called the neural-immune response.29 Vagal output regulates peripheral immune responses by suppressing the innate immune defense to pathogens and by altering the balance of pro-inflammatory cytokines.28,33 On the other hand, local immune activation elevates pro-inflammatory molecular mediators, which not only influence cells of the innate/adaptive immune system in the periphery but also activate sensory pathways that relay information to the CNS.29 In asthma, cholinergic vagal systems are deeply engaged in the pathophysiology. Inflammation damages the m2-autoreceptors which in health downregulate cholinergic transmission at the level of the postganglionic nerve terminal, thereby limiting the constriction of airway smooth muscles.34 Animal studies have demonstrated that the neural control of airway smooth muscle function and irritant receptor systems are established during the perinatal period.
The ANS can also influence atopic sensitization and the expression of allergic disorders via neurotransmitters. Some neurotrophins are thought to be mediators or moderators of allergic diseases, and their expression and signaling may be influenced by stress: brain-derived neurotrophic factor, tumor necrosis factor-alpha, and tachykinin-like substance P were suggested in an animal and human study.28
Concerning the programming of the ANS response, the role of catecholamines circulating at a high level in maternal blood is also worth focusing on. High levels of circulating catecholamines can alter placental or fetal 11β-hydroxysteroid dehydrogenase type II (HSD2) levels and activity, nutrient transport, and glucocorticoid receptor (GR) density, which may link maternal distress and fetal ANS alteration.28,29
Altered placental function
The placenta is a major organ; it delivers maternal oxygen and nutrition to the fetus. In a stressed mother, blood flow to the placenta commonly decreases. Insufficient oxygen and nutrition can activate the fetal hormonal system and ANS, which may increase production of reactive oxygen species (ROS).35,36 The placenta also moderates fetal exposure to maternal factors. 30 In a healthy pregnancy, glucocorticoids can pass freely across the placenta, but placental HSD2 provides a functional protective barrier between the fetus and mother. By converting active cortisol to inactive cortisone, HSD2 shields the glucocorticoid-sensitive tissues in the fetus from exposure to excessive glucocorticoid levels during development. Maternal distress, on the other hand, can down-regulate HSD2 activity. As maternal glucocorticoid levels are significantly higher than those of the fetus, subtle changes in HSD2 activity, including ones induced by maternal distress, can allow significant fetal exposure to excessive concentrations of glucocorticoids.25,30
Maternal and fetal distress also stimulate CRH secretion in the placenta, which results in increased levels of CRH in the fetal circulation. This excessive fetal CRH can overstimulate the fetal HPA axis and amplify the state of fetal glucocorticoid excess and activating additional elements of the fetal stress response, influencing the ANS and immune system development.28,30
Oxidative stress and epigenetic changes
ROS denotes a number of reactive molecules and free radicals derived from molecular oxygen, such as hydrogen peroxide, superoxide ions, hydroxide radicals, and nitric oxide. During normal cellular activities, various processes within the cells produce ROS. ROS take part in host cell defense mechanisms via radical formation and also play a role in cell signaling, including apoptosis, gene expression, and the activation of cell signaling cascades.37
Oxidative stress, on the other hand, refers to an imbalance favoring the pro-oxidative state, i.e., production of ROS exceeds the removal of ROS.36 Pregnancy is one of the typical physiological conditions associated with oxidative stress. A high metabolic turnover and elevated tissue oxygen requirements increase oxidative stress. Markers of oxidative stress rise in the serum even during a normal pregnancy.36 Newborns exhibit an accelerated production of free radicals and have limited antioxidant protection, making them highly vulnerable to oxidative stress. Therefore, any maternal condition that may induce oxygen-radical overproduction, such as hypoxia, inflammation, or infection, 37 can result in a state in which the capacity of defensive mechanisms to neutralize them may be exceeded and may augment the detrimental impacts of oxidative stress on the fetus.
Maternal psychological stress can increase oxygen requirements and may place the fetus under more oxidative stress during pregnancy. The higher levels of glucocorticoid induced by distress can inhibit glucose utilization and compromise the activity of energy-dependent excitatory amino acid transporters, which may increase intracellular Ca2+ concentrations and activate Ca2+-dependent enzymes. As a result, these processes can activate production of ROS. In addition, compromised mitochondrial respiration contributes further to ROS production.37
Recently, for the first time, researchers using a Korean birth cohort study have succeeded in demonstrating clues for a series of pathways involving prenatal psychological distress, HPA axis dysregulation, and elevated oxidative stress.5 They measured placental HSD2 levels directly from a subgroup of the cohort sample and found that children without either allergic diseases or prenatally distressed mothers had significantly higher placental HSD2 levels than those with prenatally distressed mothers, regardless of their later allergic disease development. Moreover, they assessed the glutathione to glutathione disulfide (GSH/GSSG) ratio—a marker for oxidative stress—directly from the placenta and found that the GSH/GSSG ratio was lower in mothers who were distressed than in those who were not.
Findings suggest that oxidative stress can induce an atopic condition which is perpetuated by the activation of oxidative stress-related genes.36 Oxidative stress could influence T-cell signal transduction and gene expression, and modulate T-cell polarization toward a T helper 2 (Th2) cellular subset, which might, in turn, be a further source of ROS. Furthermore, oxidative stress can alter the physiologic functioning of the HPA axis, induce damage in keratinocytes, and facilitate the release of cytokines in the airway. These observations collectively suggest that oxidative stress induced by prenatal distress may play some role in the pathogenesis of offspring's allergic diseases.
On the other hand, epigenetic dysregulation of gene expression has recently drawn attention as a potential mechanism for developmental programming.2,36 Not only the genetic code but also the epigenetic modification (i.e., DNA methylation, histone modification, regulatory RNA molecules) can affect gene expression and regulation.38 Since plasticity is the main feature of fetal and early infant development, long-lasting changes in gene expression, i.e., transcriptional repression during a critical period, may cause adverse outcomes in the offspring.27 DNA hypermethylation and specific histone residue hypoacetylation are reported to be involved in the pathogenesis of asthma and other allergic diseases. The methylation of anti-inflammatory genes (i.e., the runt-related transcription factor 3 [Runx3]) and the methylation of CpG islands covering the promoter and exon 1 region of the HSD11B2 gene raise the risk for allergy development. Also, exposures that inhibit histone deacetylases (i.e., oxidative stress) enhance NFkB driven transcription of inflammatory genes, and up-regulate Th2 cytokine (interleukin [IL]-13, IL-5) and GATA3-mediated T cell responses, which may induce characteristic features of asthma and allergic diseases.38,39
Several studies have reported the association between prenatal maternal distress and epigenetic changes in the region that increases allergy risk. Increased methylation of the NR3C1 promoter40 and decreased methylation of the SLC6A4 promoter41 are found in the cord blood of newborns born to stressful mothers. Prenatal distress also influences methylation in the corticotrophin releasing factor promoter and GR promoter regions of the hypothalamus as well as in the HSD11B2 promoter region within the placenta, controlling excessive glucocorticoid exposure to the fetus.42 Due to the rapid development of laboratory technology, a recent study has succeeded in demonstrating direct links between maternal distress, epigenetic changes, and the development of allergic diseases. They studied differential DNA methylation at the time of birth by whole genome bisulfite sequencing of 10 mother-child-pairs, which is complemented by longitudinal targeted methylation and transcriptional analysis in over 300 children registered in a birth cohort.3
Skewed immune development
Programming of the immune system, a skewed immune development prone to allergic disorders, comprises the later part of the mechanism by which maternal distress affects offspring's allergic sensitization and risk of allergic diseases. Evidence shows that maternal distress can affect offspring's immune system in various ways. First, maternal distress directly changes the immune system. Innate immunity is compromised by maternal distress.29 Maternal distress limits macrophage and neutrophil function, and attenuates natural killer cell cytotoxicity.43 By resetting homeostatic set points and the responsiveness of stress pathways, maternal distress can change the pattern of macrophage-derived cytokine release and, consequently, may adjust the regulatory mechanisms related to spreading and phagocytosis. Acquired immunity can also be affected by maternal distress.29 The size, morphology, and function of the thymus may already be altered at birth.43 In an animal study, the number of total peripheral blood lymphocytes and the numbers of CD4+ and CD8+ lymphocytes decreased in offspring whose mothers were stressed. Lymphocyte responses to mitogens decreased, but to varying degrees depending on the age and sex of the offspring and the specific mitogen. Maternal distress can also affect passive immunity29,43 by modifying the ability to transfer maternal immunoglobulin G (IgG) to the offspring in utero. Reduced circulating serum IgG levels have been observed in offspring after psychological stress during the third trimester of pregnancy, the time of active transplacental transfer of maternal IgG.
Maternal distress modulates physiological systems that regulate the immune system.28,29 It stimulates the fetal HPA axis to amplify fetal glucocorticoid production and activates additional elements of the fetal stress response, i.e., catecholamine release. Glucocorticoid levels are elevated by stress trigger apoptosis of all thymocyte subpopulations, especially immature CD8+ cells that are highly sensitive to glucocorticoids. Moreover, glucocorticoids inhibit the production of IL-12 and interferon-γ by antigen presenting cells and Th1 cells, upregulate the production of IL-4, IL-10, and IL-13 by Th2 cells, and prevent the development of regulatory T (Treg) cells.28,29,38 These all may induce selective suppression of Th1-mediated cellular immunity and trigger a shift toward Th2-mediated humoral immunity.
Finally, prenatal maternal distress can also affect central autonomic sites with or without altering the innervation of target tissues that are essential for immune modulation.28 The sympathetic nervous system regulates the balance of Th1 and Th2 in the uterus to influence the implantation and development of embryos during early pregnancy. Based on animal study results, sympathetic denervation is thought to up-regulate T-lymphocyte numbers and change cytokine levels in the uterus, altering its immune environment.
Structural developmental changes
Structural changes other than inflammation have also been noticed in asthma and allergic diseases. In asthma, structural alterations in small airways drive the airway physiology so as to promote airway narrowing, hyperresponsiveness, and inflammation.42 In atopic dermatitis, dysfunction in the epidermis, especially in the stratum corneum, results in defective epidermal permeability and a propensity to secondary infection.36
Concerning asthma, glucocorticoids play a significant role in regulating fetal and postnatal lung development.42 Fetal and early postnatal exposure to endogenous or exogenous corticosteroids attenuates lung development, and these structural alterations persist. Moreover, a high level of fetal glucocorticoids is itself related to preterm delivery or low birth weight, which may potentiate the later development of asthma and allergic diseases. In subjects from stressed mothers, oxidants can react with all cellular macromolecules, particularly polyunsaturated fatty acids on the cell membrane. Thereafter, a chain reaction is started, proceeding to cell injury and, ultimately, cell death. Oxidative stress also promotes tissue inflammation through up-regulation of genes that encode proinflammatory cytokines.37 All these processes may damage keratinocytes and increase the risk of atopic dermatitis.44
Interaction with other potential confounders
Fetal exposure to various environmental factors is also thought to influence the development of allergic diseases later in life,26 which can explain why we fail to estimate an individual's risk for developing later allergic diseases merely based on maternal distress exposure. These factors are thought to modulate the allergic manifestations independently of, or via indirect interaction with, prenatal distress.
First, familial factors, such as parental history of atopic diseases, are associated with an increased risk of atopic diseases in offspring.45 Although no reports are available on whether parental atopy plays a synergistic or antagonistic role with prenatal distress in developing allergic diseases, the effect of prenatal distress is enhanced in the absence of a maternal atopic predisposition. 11 Sex difference can also modulate the risk of allergy development in offspring. In general, childhood asthma is more common in boys. Sex-specific differences have also been reported for bronchial hyperresponsiveness, allergic sensitization, serum immunoglobulin E (IgE) levels, and the developmental cytokine response profiles.46 In a study using data from the Lifestyle and environmental factors and their Influence on Newborns Allergy risk cohort, the risk for allergic diseases at 1 year increased in boys and was associated with fewer Treg cells in their cord blood specimens.47 Sex differences can modulate the impact of other factors on asthma risk via the genotype-by-sex interaction. Moreover, the effect of prenatal distress on HPA function is substantially more marked in girls than in boys.48 Secondly, physical factors, such as intrauterine growth restriction, are also closely associated with prenatal distress,49 possibly through uteroplacental insufficiency and maternal unhealthy habits, including poor sleep pattern, sedentary lifestyle, or a greater propensity for alcohol abuse and cigarette smoking. Moreover, intrauterine growth restriction in itself can affect the development of asthma.26 Thirdly, in utero exposure to indoor pollutants, such as environmental tobacco smoke (ETS), independently influences fetal lung development and uteroplacental flow,50 thus increasing the risk of asthma in offspring. Moreover, in a study, fetal exposure to ETS decreased the number of Treg cells, which was associated with elevated risk for offspring's allergic diseases.51 Both ETS and prenatal maternal distress can augment oxidative toxicity and airway inflammation in offspring,52,53 which may predispose offspring to an allergic airway disease. Fourthly, exposure to outdoor pollutants is itself closely associated with the intermediate phenotype of asthma. In children, air pollution interacts with past episodes of bronchiolitis in the development of asthma.54 Moreover, exposure to traffic exhaust particles increases oxidative stress and has epigenetic effects during pregnancy.55 In a study, exposure to particulate matter with a diameter of less than 2.5 µm (PM2.5) during pregnancy was associated with increases in wheezing during early life. Moreover, when combined with exposure to community violence during fetal periods, the risk of wheezing is greater in infants with exposure to PM2.5 or black carbon.10
Finally, postnatal maternal distress may impact the early childhood environment and caregiving experience, which may change the development of stress reactivity in children and, in turn, can influence the occurrence and persistence of allergic diseases.56 According to a birth cohort study, parental distress is a predictor of wheezing in infancy and chronic caregiver stress is associated with dysregulation of immune function.57 On the other hand, a child's psychosocial stress may also operate as a moderating factor. Allergic and psychological problems have a tendency to co-occur, and, according to a meta-analysis, a bidirectional relationship is found between mental health symptoms and atopic disorders.58 Moreover, children with depressive or anxious temperaments are at risk of asthma and other allergic diseases.59
We conclude that prenatal maternal distress may affect fetal programming to allergic diseases directly or indirectly. Both dysregulation of the HPA axis and increased oxidative stress may cause structural (altered brain/lung development) or functional (skewed immune system development) changes, which may predispose the fetus to develop allergic diseases during childhood. Although many facts are yet to be discovered, changes in placental responses and epigenetic modifications are presumed to mediate the process from maternal distress to allergic diseases.
FUTURE PERSPECTIVES
Challenging role of gut microbiome: the most potent modulator in the prenatal distress-allergic disease association
The gut-brain axis links emotional and cognitive centers of the brain with peripheral intestinal functions. Since the central and enteric nervous systems communicate bidirectionally, psychological problems or stress can affect functional bowel disorders, and vice versa. Neuro-immuno-endocrine mechanisms mediate the bidirectional communication network that includes the CNS (both brain and spinal cord), ANS, enteric nervous system, and HPA axis.60 Recent advances in research have demonstrated that the gut microbiota plays an important role in modulating these interactions. This interaction between microbiota and gut-brain axis appears to be bidirectional, namely through signaling from gut microbiota to the brain and from the brain to gut microbiota, which is called the Microbiota-Gut-Brain (MGB) axis.61 In germ-free animals, introduction of and changes in microbiota are shown to modulate an anxiety-related behavior against stress testing.62 Moreover, fecal microbiota transplantation is now used to treat autism, attention-deficit hyperactivity disorder, multiple sclerosis, and chronic fatigue syndrome61 as well as inflammatory bowel diseases, metabolic syndrome, and obesity. Possible mechanisms include Treg cell induction through probiotic-related innate immunity activation. 63 Moreover, the MGB axis involves neural, endocrine, immune, and humoral mediators; hence, it may be inferred that the MGB axis can modulate the pathogenesis of allergic diseases. 63 In an experimental food allergy model, microbial signatures determined allergic sensitization and anaphylaxis.64 At a human level, the hygiene hypothesis initially introduced by Strachan to explain the allergic epidemic, is now re-interpreted as changes to the gut microbiome that are crucial to normal immune development or the pathogenesis of allergy.65 Even probiotics are now appraised for their role in preventing or modulating allergic diseases.63 Furthermore, there is indirect evidence of the involvement of microbiota in the pathogenesis of the prenatal distress-induced offspring's allergy. The microbiome affects psycho-neuro-endocrine dysregulation, oxidative stress and epigenetic regulation, skewed immune development concerning the increased inflammation and lack of tolerance induction as well as the allergy-prone deviation in T-cell response, physical growth, and development, moderating responses to pollutants, and psychological problems in early childhood.63 Nevertheless, few studies have clearly demonstrated the role of microbiota in modulating the association between prenatal distress and the development of allergic diseases. This should be explored as it offers a promising target for secondary intervention.
Possible biomarkers to identify highly susceptible subgroups
A few years ago, when the epidemiologic association was not verified, researchers focused only on building a prospective birth cohort. Hence, they did not gather sufficient prenatal or perinatal biologic samples related to prenatal exposure or immunologic changes. Moreover, the lack of information on potential confounders still limits the interpretation of marginal associations. As a result, despite the clear association between prenatal distress and offspring's allergic diseases, no single biomarker was identified that could specify vulnerable subjects regarding the prenatal distress-offspring's allergic diseases relationship. Some markers are currently known to be elevated in subjects who are prenatally distressed or who are at risk of developing a later allergy. First, genetic polymorphisms in offspring's blood can explain the different effect size in response to a specific exposure.66,67 These can synergistically or antagonistically interact with environmental risk factors. Secondly, placental biomarkers deserve attention. Since the mother and fetus are connected through the placenta, the possible pathway through which the maternal distress influences offspring's allergic diseases may involve changes in the placenta. Based on the suggested mechanism, placental HSD2 or oxidative markers in the placenta may reflect in-utero exposures and can quantify the risk for later disease development.5 Furthermore, in some studies, immune markers in cord blood have been regarded as surrogate markers for later allergy development.19,20
Other than allergic diseases, epigenetic changes, including methylation status in placenta or cord blood, are now actively being investigated as markers for later disease development.68 In some cohorts, infant stool samples are actively gathered and metagenomically analyzed to explain individual differences in outcomes despite very similar exposures. From offspring's side, markers for stress-reactivity, such as cortisol levels in saliva, are good candidate biomarkers for prenatal distress and later disease development.69 Moreover, as another marker of early exposure and later disease potential, telomere shortening is noticeable for predicting development of noncommunicable disease. 70 The role of these biomarkers, however, has not been verified in the development allergic diseases and now warrants attention.
CONCLUSION
Current evidence had succeeded in demonstrating a close association between prenatal maternal distress and the development of allergic diseases in offspring. However, recall bias must be avoided to ensure a causal relationship by gathering information on maternal distress prenatally. Furthermore, environmental modifying factors, including air pollution, diet, or postnatal distress, should be adjusted for during the analysis. Considering the complex and ambiguous relationship between stress, allergic diseases, and other potentially modifying factors, a population-based prospective birth cohort study could verify a causal relationship between prenatal distress and allergic diseases in offspring; this should also be replicated in another cohort study. In spite of this epidemiologic evidence, researchers have not demonstrated direct evidence that intervention strategies that restore altered stress response systems during the maternal or early infant period can reverse the risk of allergy development. Further studies are needed to clarify the efficacy of intervention strategies.
ACKNOWLEDGMENTS
We appreciate the unremitting endeavors of members of the Korea Centers for Disease Control & Prevention and COhort for Childhood Origin of Asthma and allergic Diseases teams. The authors especially thank So-Yeon Lee and Yee-Jin Shin. This research was supported by funding (2014-E51004-02) from the Research of Korea Centers for Disease Control and Prevention.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIAL
Searching strategies and outcomes in the PubMed database
References
- 1.Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 2.Padmanabhan V, Cardoso RC, Puttabyatappa M. Developmental programming, a pathway to disease. Endocrinology. 2016;157:1328–1340. doi: 10.1210/en.2016-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trump S, Bieg M, Gu Z, Thürmann L, Bauer T, Bauer M, et al. Prenatal maternal stress and wheeze in children: novel insights into epigenetic regulation. Sci Rep. 2016;6:28616. doi: 10.1038/srep28616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosa MJ, Just AC, Tamayo Y, Schnaas L, Svensson K, Wright RO, et al. Prenatal and postnatal stress and wheeze in Mexican children: sex-specific differences. Ann Allergy Asthma Immunol. 2016;116:306–312.e1. doi: 10.1016/j.anai.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang HY, Suh DI, Yang SI, Kang MJ, Lee SY, Lee E, et al. Prenatal maternal distress affects atopic dermatitis in offspring mediated by oxidative stress. J Allergy Clin Immunol. 2016;138:468–475.e5. doi: 10.1016/j.jaci.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Lee A, Mathilda Chiu YH, Rosa MJ, Jara C, Wright RO, Coull BA, et al. Prenatal and postnatal stress and asthma in children: temporal- and sex-specific associations. J Allergy Clin Immunol. 2016;138:740–747.e3. doi: 10.1016/j.jaci.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Olsen J, Agerbo E, Yuan W, Sigsgaard T, Li J. Prenatal stress and childhood asthma in the offspring: role of age at onset. Eur J Public Health. 2015;25:1042–1046. doi: 10.1093/eurpub/ckv129. [DOI] [PubMed] [Google Scholar]
- 8.Bandoli G, von Ehrenstein O, Ghosh JK, Flores ME, Dunkel Schetter C, Ritz B. Prenatal maternal stress and the risk of lifetime wheeze in young offspring: an examination by stressor and maternal ethnicity. J Immigr Minor Health. 2016;18:987–995. doi: 10.1007/s10903-015-0269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hovland V, Riiser A, Mowinckel P, Carlsen KH, Lødrup Carlsen KC. Early risk factors for pubertal asthma. Clin Exp Allergy. 2015;45:164–176. doi: 10.1111/cea.12409. [DOI] [PubMed] [Google Scholar]
- 10.Chiu YH, Coull BA, Sternthal MJ, Kloog I, Schwartz J, Cohen S, et al. Effects of prenatal community violence and ambient air pollution on childhood wheeze in an urban population. J Allergy Clin Immunol. 2014;133:713–722.e4. doi: 10.1016/j.jaci.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartwig IR, Sly PD, Schmidt LA, van Lieshout RJ, Bienenstock J, Holt PG, et al. Prenatal adverse life events increase the risk for atopic diseases in children, which is enhanced in the absence of a maternal atopic predisposition. J Allergy Clin Immunol. 2014;134:160–169. doi: 10.1016/j.jaci.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 12.Larsen AD, Schlünssen V, Christensen BH, Bonde JP, Obel C, Thulstrup AM, et al. Exposure to psychosocial job strain during pregnancy and odds of asthma and atopic dermatitis among 7-year old children - a prospective cohort study. Scand J Work Environ Health. 2014;40:639–648. doi: 10.5271/sjweh.3452. [DOI] [PubMed] [Google Scholar]
- 13.Turcotte-Tremblay AM, Lim R, Laplante DP, Kobzik L, Brunet A, King S. Prenatal maternal stress predicts childhood asthma in girls: project ice storm. Biomed Res Int. 2014;2014:201717. doi: 10.1155/2014/201717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guxens M, Sonnenschein-van der Voort AM, Tiemeier H, Hofman A, Sunyer J, de Jongste JC, et al. Parental psychological distress during pregnancy and wheezing in preschool children: the Generation R Study. J Allergy Clin Immunol. 2014;133:59–67. 67.e1–67.e12. doi: 10.1016/j.jaci.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 15.Chiu YH, Coull BA, Cohen S, Wooley A, Wright RJ. Prenatal and postnatal maternal stress and wheeze in urban children: effect of maternal sensitization. Am J Respir Crit Care Med. 2012;186:147–154. doi: 10.1164/rccm.201201-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khashan AS, Wicks S, Dalman C, Henriksen TB, Li J, Mortensen PB, et al. Prenatal stress and risk of asthma hospitalization in the offspring: a Swedish population-based study. Psychosom Med. 2012;74:635–641. doi: 10.1097/PSY.0b013e31825ac5e7. [DOI] [PubMed] [Google Scholar]
- 17.Fang F, Höglund CO, Arck P, Lundholm C, Långström N, Lichtenstein P, et al. Maternal bereavement and childhood asthma-analyses in two large samples of Swedish children. PLoS One. 2011;6:e27202. doi: 10.1371/journal.pone.0027202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reyes M, Perzanowski MS, Whyatt RM, Kelvin EA, Rundle AG, Diaz DM, et al. Relationship between maternal demoralization, wheeze, and immunoglobulin E among inner-city children. Ann Allergy Asthma Immunol. 2011;107:42–49.e1. doi: 10.1016/j.anai.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood RA, Bloomberg GR, Kattan M, Conroy K, Sandel MT, Dresen A, et al. Relationships among environmental exposures, cord blood cytokine responses, allergy, and wheeze at 1 year of age in an inner-city birth cohort (Urban Environment and Childhood Asthma study) J Allergy Clin Immunol. 2011;127:913–919. 919.e1–919.e6. doi: 10.1016/j.jaci.2010.12.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen HJ, Wang YJ, Lin YC, Chang CC, Shieh CC, Lung FW, et al. Prediction of atopic dermatitis in 2-yr-old children by cord blood IgE, genetic polymorphisms in cytokine genes, and maternal mentality during pregnancy. Pediatr Allergy Immunol. 2011;22:695–703. doi: 10.1111/j.1399-3038.2011.01177.x. [DOI] [PubMed] [Google Scholar]
- 21.Cookson H, Granell R, Joinson C, Ben-Shlomo Y, Henderson AJ. Mothers' anxiety during pregnancy is associated with asthma in their children. J Allergy Clin Immunol. 2009;123:847–853.e11. doi: 10.1016/j.jaci.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sausenthaler S, Rzehak P, Chen CM, Arck P, Bockelbrink A, Schäfer T, et al. Stress-related maternal factors during pregnancy in relation to childhood eczema: results from the LISA Study. J Investig Allergol Clin Immunol. 2009;19:481–487. [PubMed] [Google Scholar]
- 23.Suglia SF, Staudenmayer J, Cohen S, Enlow MB, Rich-Edwards JW, Wright RJ. Cumulative stress and cortisol disruption among Black and Hispanic pregnant women in an urban cohort. Psychol Trauma. 2010;2:326–334. doi: 10.1037/a0018953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braido F, Baiardini I, Scichilone N, Musarra A, Menoni S, Ridolo E, et al. Illness perception, mood and coping strategies in allergic rhinitis: are there differences among ARIA classes of severity? Rhinology. 2014;52:66–71. doi: 10.4193/Rhino13.040. [DOI] [PubMed] [Google Scholar]
- 25.Wright RJ, Cohen RT, Cohen S. The impact of stress on the development and expression of atopy. Curr Opin Allergy Clin Immunol. 2005;5:23–29. doi: 10.1097/00130832-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Grieger JA, Clifton VL, Tuck AR, Wooldridge AL, Robertson SA, Gatford KL. In utero programming of allergic susceptibility. Int Arch Allergy Immunol. 2016;169:80–92. doi: 10.1159/000443961. [DOI] [PubMed] [Google Scholar]
- 27.McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright RJ. Stress-related programming of autonomic imbalance: role in allergy and asthma. Chem Immunol Allergy. 2012;98:32–47. doi: 10.1159/000336496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellinger DL, Lubahn C, Lorton D. Maternal and early life stress effects on immune function: relevance to immunotoxicology. J Immunotoxicol. 2008;5:419–444. doi: 10.1080/15476910802483415. [DOI] [PubMed] [Google Scholar]
- 30.O'Donnell K, O'Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Dev Neurosci. 2009;31:285–292. doi: 10.1159/000216539. [DOI] [PubMed] [Google Scholar]
- 31.Corwin EJ, Guo Y, Pajer K, Lowe N, McCarthy D, Schmiege S, et al. Immune dysregulation and glucocorticoid resistance in minority and low income pregnant women. Psychoneuroendocrinology. 2013;38:1786–1796. doi: 10.1016/j.psyneuen.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teixeira JM, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. BMJ. 1999;318:153–157. doi: 10.1136/bmj.318.7177.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fryer AD, Jacoby DB. Muscarinic receptors and control of airway smooth muscle. Am J Respir Crit Care Med. 1998;158:S154–S160. doi: 10.1164/ajrccm.158.supplement_2.13tac120. [DOI] [PubMed] [Google Scholar]
- 35.Entringer S, Buss C, Swanson JM, Cooper DM, Wing DA, Waffarn F, Wadhwa PD. Fetal programming of body composition, obesity, and metabolic function: the role of intrauterine stress and stress biology. J Nutr Metab. 2012;2012:632548. doi: 10.1155/2012/632548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manti S, Marseglia L, D'Angelo G, Cuppari C, Cusumano E, Arrigo T, et al. “Cumulative Stress”: the effects of maternal and neonatal oxidative stress and oxidative stress-inducible genes on programming of atopy. Oxid Med Cell Longev. 2016;2016:8651820. doi: 10.1155/2016/8651820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat Rev Immunol. 2013;13:349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martino D, Prescott S. Epigenetics and prenatal influences on asthma and allergic airways disease. Chest. 2011;139:640–647. doi: 10.1378/chest.10-1800. [DOI] [PubMed] [Google Scholar]
- 39.Su RC, Becker AB, Kozyrskyj AL, Hayglass KT. Epigenetic regulation of established human type 1 versus type 2 cytokine responses. J Allergy Clin Immunol. 2008;121:57–63.e3. doi: 10.1016/j.jaci.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 41.Devlin AM, Brain U, Austin J, Oberlander TF. Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth. PLoS One. 2010;5:e12201. doi: 10.1371/journal.pone.0012201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright RJ. Perinatal stress and early life programming of lung structure and function. Biol Psychol. 2010;84:46–56. doi: 10.1016/j.biopsycho.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merlot E, Couret D, Otten W. Prenatal stress, fetal imprinting and immunity. Brain Behav Immun. 2008;22:42–51. doi: 10.1016/j.bbi.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Bito T, Nishigori C. Impact of reactive oxygen species on keratinocyte signaling pathways. J Dermatol Sci. 2012;68:3–8. doi: 10.1016/j.jdermsci.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 45.Alford SH, Zoratti E, Peterson EL, Maliarik M, Ownby DR, Johnson CC. Parental history of atopic disease: disease pattern and risk of pediatric atopy in offspring. J Allergy Clin Immunol. 2004;114:1046–1050. doi: 10.1016/j.jaci.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 46.Xuan W, Marks GB, Toelle BG, Belousova E, Peat JK, Berry G, et al. Risk factors for onset and remission of atopy, wheeze, and airway hyperresponsiveness. Thorax. 2002;57:104–109. doi: 10.1136/thorax.57.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinz D, Bauer M, Röder S, Olek S, Huehn J, Sack U, et al. Cord blood Tregs with stable FOXP3 expression are influenced by prenatal environment and associated with atopic dermatitis at the age of one year. Allergy. 2012;67:380–389. doi: 10.1111/j.1398-9995.2011.02767.x. [DOI] [PubMed] [Google Scholar]
- 48.McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- 49.Wainstock T, Anteby E, Glasser S, Shoham-Vardi I, Lerner-Geva L. The association between prenatal maternal objective stress, perceived stress, preterm birth and low birthweight. J Matern Fetal Neonatal Med. 2013;26:973–977. doi: 10.3109/14767058.2013.766696. [DOI] [PubMed] [Google Scholar]
- 50.Lødrup Carlsen KC, Jaakkola JJ, Nafstad P, Carlsen KH. In utero exposure to cigarette smoking influences lung function at birth. Eur Respir J. 1997;10:1774–1779. doi: 10.1183/09031936.97.10081774. [DOI] [PubMed] [Google Scholar]
- 51.Herberth G, Bauer M, Gasch M, Hinz D, Röder S, Olek S, et al. Maternal and cord blood miR-223 expression associates with prenatal tobacco smoke exposure and low regulatory T-cell numbers. J Allergy Clin Immunol. 2014;133:543–550. doi: 10.1016/j.jaci.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 52.Zhu Z, Li X, Chen W, Zhao Y, Li H, Qing C, et al. Prenatal stress causes gender-dependent neuronal loss and oxidative stress in rat hippocampus. J Neurosci Res. 2004;78:837–844. doi: 10.1002/jnr.20338. [DOI] [PubMed] [Google Scholar]
- 53.Noakes PS, Thomas R, Lane C, Mori TA, Barden AE, Devadason SG, et al. Association of maternal smoking with increased infant oxidative stress at 3 months of age. Thorax. 2007;62:714–717. doi: 10.1136/thx.2006.061630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim BJ, Seo JH, Jung YH, Kim HY, Kwon JW, Kim HB, et al. Air pollution interacts with past episodes of bronchiolitis in the development of asthma. Allergy. 2013;68:517–523. doi: 10.1111/all.12104. [DOI] [PubMed] [Google Scholar]
- 55.Perera F, Tang WY, Herbstman J, Tang D, Levin L, Miller R, et al. Relation of DNA methylation of 5′-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS One. 2009;4:e4488. doi: 10.1371/journal.pone.0004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- 57.Wright RJ, Finn P, Contreras JP, Cohen S, Wright RO, Staudenmayer J, et al. Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. J Allergy Clin Immunol. 2004;113:1051–1057. doi: 10.1016/j.jaci.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 58.Chida Y, Hamer M, Steptoe A. A bidirectional relationship between psychosocial factors and atopic disorders: a systematic review and meta-analysis. Psychosom Med. 2008;70:102–116. doi: 10.1097/PSY.0b013e31815c1b71. [DOI] [PubMed] [Google Scholar]
- 59.Alati R, O'Callaghan M, Najman JM, Williams GM, Bor W, Lawlor DA. Asthma and internalizing behavior problems in adolescence: a longitudinal study. Psychosom Med. 2005;67:462–470. doi: 10.1097/01.psy.0000161524.37575.42. [DOI] [PubMed] [Google Scholar]
- 60.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 61.Evrensel A, Ceylan ME. Fecal microbiota transplantation and its usage in neuropsychiatric disorders. Clin Psychopharmacol Neurosci. 2016;14:231–237. doi: 10.9758/cpn.2016.14.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis DJ, Bryda EC, Gillespie CH, Ericsson AC. Microbial modulation of behavior and stress responses in zebrafish larvae. Behav Brain Res. 2016;311:219–227. doi: 10.1016/j.bbr.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Azad MB, Kozyrskyj AL. Perinatal programming of asthma: the role of gut microbiota. Clin Dev Immunol. 2012;2012:932072. doi: 10.1155/2012/932072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noval Rivas M, Burton OT, Wise P, Zhang YQ, Hobson SA, Garcia Lloret M, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol. 2013;131:201–212. doi: 10.1016/j.jaci.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu AH. Revisiting the hygiene hypothesis for allergy and asthma. J Allergy Clin Immunol. 2015;136:860–865. doi: 10.1016/j.jaci.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 66.Seo JH, Kim HY, Jung YH, Lee E, Yang SI, Yu HS, et al. Interactions between innate immunity genes and early-life risk factors in allergic rhinitis. Allergy Asthma Immunol Res. 2015;7:241–248. doi: 10.4168/aair.2015.7.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu J. Gene-environment interactions should be considered in future studies to understand the association between prenatal folate supplementation and asthma development. Allergy Asthma Immunol Res. 2015;7:523–524. doi: 10.4168/aair.2015.7.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gheorghe CP, Goyal R, Mittal A, Longo LD. Gene expression in the placenta: maternal stress and epigenetic responses. Int J Dev Biol. 2010;54:507–523. doi: 10.1387/ijdb.082770cg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolf JM, Nicholls E, Chen E. Chronic stress, salivary cortisol, and alpha-amylase in children with asthma and healthy children. Biol Psychol. 2008;78:20–28. doi: 10.1016/j.biopsycho.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 70.Entringer S, Buss C, Wadhwa PD. Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings. Curr Opin Endocrinol Diabetes Obes. 2010;17:507–516. doi: 10.1097/MED.0b013e3283405921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Searching strategies and outcomes in the PubMed database