Abstract
Age-related changes in cardiac homeostasis can be observed at the cellular, extracellular, and tissue levels. Progressive cardiomyocyte hypertrophy, inflammation, and the gradual development of cardiac fibrosis are hallmarks of cardiac aging. In the absence of a secondary insult such as hypertension, these changes are subtle and result in slight to moderate impaired myocardial function, particularly diastolic function. While collagen deposition and cross-linking increase during aging, extracellular matrix (ECM) degradation capacity also increases due to increased expression of matrix metalloproteinases (MMPs). Of the MMPs elevated with cardiac aging, MMP-9 has been extensively evaluated and its roles are reviewed here. In addition to proteolytic activity on ECM components, MMPs oversee cell signaling during the aging process by modulating cytokine, chemokine, growth factor, hormone, and angiogenic factor expression and activity. In association with elevated MMP-9, macrophage numbers increase in an age-dependent manner to regulate the ECM and angiogenic responses. Understanding the complexity of the molecular interactions between MMPs and the ECM in the context of aging may provide novel diagnostic indicators for the early detection of age-related fibrosis and cardiac dysfunction.
Keywords: Review, Matrix metalloproteinases, Cardiac aging, Collagen, Inflammation, Macrophage, Proteomics
Introduction
The myocardium undergoes a number of structural and functional responses to aging. Across a broad range of species, one consistent hallmark of cardiac aging is a decrease in myocardial reserve capacity (Bokov et al. 2009; Lakatta 1994). In the absence of pathology or stressors, cardiac performance is often maintained. When superimposed on an increased workload, however, a diminished reserve becomes apparent.
Physiological changes in humans with age include decreased sympathetic signaling (Strait and Lakatta 2012) and decreased heart rate variability (Parati et al. 1997). Rats and mice also demonstrate reduced heart rate variability with age (Lin et al. 2008; Rossi et al. 2014). Increases in LV mass, due to increased wall thickness and volumes, and prolonged systolic contraction and diastolic relaxation occur first, before there is an appreciable decline in myocardial performance (Lindsey et al. 2005). Cardiac aging by itself results in a slight but significant decline in LV function. For example, ejection fraction in mice declines from about 70% in young 7.5-month-old mice to about 60% in 38.1-month-old mice (Lin et al. 2008).
Aging-associated physiological changes in the human and rat cardiovascular system increase afterload and impair vasodilation, which increases wall stress in the left ventricle (LV) and leads to cardiomyocyte hypertrophy (Strait and Lakatta 2012). In mice, pressure overload does not naturally occur with aging, as mice are resistant to vascular adaptations, yet cardiomyocyte hypertrophy occurs, indicating that intrinsic myocardial changes are directly responsible for the shift in myocyte phenotype (Yabluchanskiy et al. 2014). Increased oxygen and energy demand by hypertrophic cardiomyocytes creates a low-grade oxygen environment, where free radical production is unbalanced and may damage cellular components (Toprak et al. 2009; Wohlgemuth et al. 2014).
In response to the hypoxic environment, cardiomyocytes release pro-inflammatory cytokines and chemokines that stimulate an immune response to increase macrophage numbers in the LV (Chiao et al. 2011). Macrophages are a rich source of matrix metalloproteinases (MMPs), and an unbalanced MMP activity profile has been linked to myocardial aging status in humans with no evidence of cardiovascular disease (Bonnema et al. 2007) and across a variety of animal models (Jugdutt et al. 2010; Lindsey et al. 2005). In aging mice, increased MMP activity has been connected to increased inflammation, extracellular matrix (ECM) deposition, and attenuated angiogenesis capacity (Yabluchanskiy et al. 2014).
Indices of aging can include cellular DNA damage and changes in protein structure and organelle function; in particular, mitochondrial dysfunction is well-studied in the context of aging (Sun et al. 2016). Molecular changes translate to cellular function impairment, including upregulation of apoptosis or necrosis pathways to enhance progressive cardiomyocyte loss (Kajstura et al. 1996). In the extracellular environment of the aged myocardium, a deregulated ECM leads to fibrosis which results in dysfunction at the cellular, extracellular, and whole organ levels. Aging-related myocardial fibrosis has been observed in mice (Bradshaw et al. 2010; Chiao et al. 2012), rats (Annoni et al. 1998; Eghbali et al. 1989b), dogs (Liu et al. 2003), sheep (Horn et al. 2012), and humans (Burkauskiene 2005; Gazoti Debessa et al. 2001).
In this review, we evaluate the aging effects on cardiac ECM and the cell types that regulate or are regulated by ECM. We discuss the relationship between aging and two cardiovascular pathologies (hypertension and myocardial infarction (MI)) and the role of MMPs in these pathologies within the context of aging.
Aging effects on cardiac structure and function
Aging effects on collagen
Myocardial ECM accumulation depends on the balance between synthesis and degradation. Cardiac ECM proteins that accrue with age include glycoproteins, proteoglycans, glycosaminoglycans, matricellular proteins, and integrins (Nguyen et al. 2014); effects of aging on these proteins are summarized in Table 1. A major component of the myocardial ECM is collagen. Total collagen content includes the summation of all collagen types (e.g., I, III, IV, V, VI) and includes all forms of collagen proteins (e.g., full length, fragmented, post-translationally modified) that reflect ECM quality. Each collagen subtype has a unique tridimensional structure, physicochemical properties, and biological function. Collagens I and III are the most abundant in the myocardium, and collagen I represents 85 ± 5% while collagen III comprises 11 ± 4% of total collagen content in young adult non-human primates (Weber et al. 1988).
Table 1.
Summary of aging impacts on ECM molecules in the left ventricle
| ECM component | Changes | Type | Species | Reference | |
|---|---|---|---|---|---|
| Collagen | Type I | ↓ | mRNA | C57BL/6C57BL/6 | (Toba et al. 2016) |
| Type III | ↓ | mRNA | Wistar rat; C57BL/6 | (Mamuya et al. 1992; Toba et al. 2016) | |
| ↓ | Protein | C57BL6/J | (Padmanabhan Iyer et al. 2016) | ||
| Type IV | ↓ | mRNA | C57BL/6 | (Toba et al. 2016) | |
| Type V | ↓ | mRNA | C57BL/6 | (Toba et al. 2016) | |
| Type XV | ↓ | Protein | C57BL6/J | (Padmanabhan Iyer et al. 2016) | |
| Procollagen | Type I | ↓ | mRNA | Fischer 344 rat | (Thomas et al. 2000) |
| Type III | ↓ | mRNA | Fischer 344 rat | (Thomas et al. 2000) | |
| Glycosaminoglycan | Hyaluronan | ↓ | Protein | Sprague-Dawley rat | (Hellstrom et al. 2006) |
| Glycoproteins | Fibronectin | ↑ | Protein | Balb-c mice | (Burgess et al. 2001) |
| ↓ | mRNA | Wistar rat; C57BL/6 | (Mamuya et al. 1992; Toba et al. 2016) | ||
| Laminin-α2 | ↑ | mRNA | C57BL/6 | (Toba et al. 2016) | |
| Laminin-γ1 | ↓ | mRNA | C57BL/6 | (Toba et al. 2016) | |
| Periostin | ↑ | mRNA | C57BL6/J | (Ma et al. 2012) | |
| ↑ | mRNA | C57BL6/J | (Chiao et al. 2012) | ||
| Integrin | α1 | ↑ | Protein | Balb-c mice | (Burgess et al. 2001) |
| α3 | ↑ | mRNA | C57BL/6 | (Toba et al. 2016) | |
| α5 | ↑ | Protein | Balb-c mice | (Burgess et al. 2001) | |
| αE | ↑ | mRNA | C57BL/6 | (Toba et al. 2016) | |
| β1 | ↓ | Protein | Balb-c mice | (Burgess et al. 2001) | |
| ↓ | mRNA | Wistar rat; C57BL/6 | (Mamuya et al. 1992; Toba et al. 2016) | ||
| Matricellular | SPARC | ↑ | Protein | C57Bl6/SV129 mice | (Bradshaw et al. 2010) |
| proteins | Thrombospondin-2 | ↑ | mRNA | C57BL/6J | (Ma et al. 2012) |
| ↑ | Protein | C57Bl6/129SvJ/EMS + Ter mice | (Swinnen et al. 2009) | ||
| Osteopontin | ↓ | mRNA | C57BL/6 | (Chiao et al. 2012) | |
| ↓ | mRNA | Sprague-Dawley rat | (Graf et al. 1997) |
↑ increased, ↓ decreased. ECM extracellular matrix, SPARC secreted protein acidic and rich in cysteine
With age, the increase in collagen content in the mouse model is relatively modest (e.g., increases from 1–2 to 2–4% of total LV area) (Chiao et al. 2012; Lin et al. 2008) compared to what is seen after a MI, where the collagen content in the scar region increases to 65% at 4 weeks post-MI (Voorhees et al. 2015). Collagen represents 6% of total LV protein content in 1-month-old rats and doubles to 12% by 22–26 months of age (Eghbali et al. 1989b). Therefore, both mice and rats have a doubling in collagen from young to old age, with the difference being the collagen concentration at baseline. While collagen fibril numbers increase, collagen fibril diameter is also larger in old rat hearts (Gazoti Debessa et al. 2001).
While total amounts vary across species including humans, there is a consistent increase in collagen with age. From autopsies of humans without cardiovascular disease history, myocardial collagen content increased from 3.9 ± 0.8% in 20–25-year-old individuals to 5.9 ± 0.8% in 67–87-year-old individuals (Gazoti Debessa et al. 2001). Collagen I increased and collagen III decreased in the hearts from autopsies of 80-year-old subjects in comparison to younger subjects (Mendes et al. 2012). This shift from collagen III to collagen I would provide a cardiac ECM mechanism that is independent of vascular changes, as the increased ratio of collagens I to III can contribute to LV stiffness (Gazoti Debessa et al. 2001; Mendes et al. 2012). Collagen I has high tensile strength, while type III collagen is more distensible; therefore, an increased ratio of types I to III can impair cardiac biomechanics (Nguyen et al. 2014). In contrast to protein levels, transcription of collagens I and III, fibronectin, and β1 integrin messenger RNAs (mRNAs) are decreased in aging LV (Chiao et al. 2012; Horn et al. 2012; Mamuya et al. 1992). The increased collagen with age, therefore, is due to post-transcriptional regulation rather than increased transcription (Nguyen et al. 2014).
Collagen cross-linking can increase LV stiffness without altering total collagen content (Horn and Trafford 2016). Collagen cross-linking, measured by hydroxylysyl pyridinoline concentration, is increased in the LVs of 23-month-old rats (Thomas et al. 1992). Fibroblasts secrete collagen into the extracellular space in the procollagen form, where it undergoes further processing to become a mature collagen fibril (Prockop and Kivirikko 1995). Secreted protein acidic and rich in cysteine (SPARC) belongs to the matricellular protein family, and SPARC is involved in cross-linked collagen fibril formation (Bradshaw 2009). SPARC is predominantly expressed in cardiac fibroblasts, although cardiomyocytes, endothelial cells, and macrophages also exhibit low SPARC expression (Toba et al. 2015). SPARC increases in the LV of 18- to 29-month-old mice and has been linked to age-related increases in myocardial diastolic stiffness as well as fibrillar and insoluble collagen content. These changes are all blunted by SPARC deletion (Bradshaw et al. 2010). Aging SPARC-null mice (18–29 months old) also have decreased collagen type III and IV expression and macrophage infiltration compared to aging wild-type control mice (de Castro Bras et al. 2014; Toba et al. 2015). The decrease in collagen III may result in an increased collagen I to collagen III ratio, which would also explain the increase in myocardial diastolic stiffness. Lysyl oxidase (LOX) activity produces a covalent cross-linking of collagen fibrils, which increases collagen tensile strength and prevent them from degradation by proteases (Biernacka and Frangogiannis 2011). Increased collagen content and increased LOX cross-linking collagen products have been observed in myocardium of old rats (Thomas et al. 2001).
Advanced glycation end-products (AGEs) are produced by a non-enzymatic reaction between proteins and sugar residues and can covalently bind other AGEs to form protein-protein cross-links among a variety of ECM components, including collagen, laminin, and elastin (Hartog et al. 2007). AGEs have been measured in the plasma and associate with the extent of diastolic dysfunction in elderly humans (Campbell et al. 2012). Age-related diastolic dysfunction can be ameliorated by AGE cross-link breaker treatment in dogs (Asif et al. 2000). Besides collagen changes, aging-related modifications in fibronectin folding have been reported (Antia et al. 2008). Increased stretching of fibronectin fibrils can lead to partial unfolding of the secondary structure. This change in protein structure may shift cell and enzyme recognition sites on ECM proteins by physically modifying binding site availability (Antia et al. 2008).
Aging effects on MMPs
MMPs are defined by their ability to proteolytically process ECM components and as such are key regulators of ECM turnover. MMPs are secreted in their pre-activated zymogen form, in which an inhibitory pro-peptide domain is bonded by a cysteine residue to the Zn2+ ion present on the catalytic domain. Classical MMP activation removes the inhibitory pro-peptide domain to disrupt the cysteine switch. MMP activity, however, does not solely rely on activation, as MMPs can have activity in the presence of substrate that is not dependent on pro-domain cleavage (Kandasamy et al. 2010). MMPs are endogenously inhibited in the tissue by interaction with the tissue inhibitors of metalloproteinases (TIMPs), of which four have been identified.
Aging effects on MMPs levels are summarized in Table 2. MMP-2, MMP-7, TIMP-1, TIMP-2, and TIMP-4 increase in the plasma of elderly human subjects, and MMP-7, TIMP-1 and -4 correlate with diastolic dysfunction variables (Bonnema et al. 2007). In mice, plasma MMP-9 concentration positively correlates with age and with monocyte chemotactic protein-1 (Yabluchanskiy et al. 2015). MMP-1, MMP-2, MMP-3, and MMP-14 increase in the LV of 31-month-old rats that underwent exercise training vs. an age-matched sedentary group (Kwak et al. 2011). MMP-3, MMP-12, MMP-13, and MMP-14 decrease in the soluble fraction of 23-month-old CB6F1 LV compared to young controls, while MMP-3, MMP-8, and MMP-14 increase in the insoluble ECM-bound fraction (Lindsey et al. 2005). MMP-28 is the newest member of the MMP family, and MMP-28 increases in the LV of 20-month-old mice (Ma et al. 2012).
Table 2.
Summary of aging impacts on MMPs in plasma and left ventricle
| Changes | Sample type | Location | Species | Reference | |
|---|---|---|---|---|---|
| MMP-1 | ↓ | Protein | LV | C57BL/6 mice | (Huet et al. 2015) |
| MMP-2 | ↑ | Protein | Plasma | Human | (Bonnema et al. 2007) |
| ↑ | mRNA | LV | C57BL/6 mice | (Toba et al. 2015) | |
| ↓ | Protein | LV | C57Bl6 mice | (Huet et al. 2015) | |
| MMP-3 | ↑ | mRNA | LV | C57BL/6 mice | (Toba et al. 2015) |
| ↓ | Soluble protein | LV | CB6F1 mice | (Lindsey et al. 2005) | |
| ↑ | Insoluble protein | LV | CB6F1 mice | (Lindsey et al. 2005) | |
| MMP-7 | ↑ | Protein | Plasma | Human | (Bonnema et al. 2007) |
| MMP-8 | ↑ | Insoluble protein | LV | CB6F1 mice | (Bonnema et al. 2007) |
| MMP-9 | ↓ | Protein | Plasma | Human | (Bonnema et al. 2007) |
| ↑ | Protein | Plasma | C57BL/6J mice | (Chiao et al. 2011) | |
| ↑ | Protein | Plasma | C57BL/6J mice | (Yabluchanskiy et al. 2015) | |
| ↑ | Soluble protein | LV | C57BL/6J mice | (Chiao et al. 2011) | |
| ↑ | mRNA | LV | C57BL/6 mice | (Toba et al. 2015) | |
| ↑ | mRNA | LV | C57BL/6J mice | (Chiao et al. 2012) | |
| ↑ | Soluble protein | LV | C57BL/6J mice | (Chiao et al. 2012) | |
| ↑ | mRNA | LV | C57BL/6J mice | (Yabluchanskiy et al. 2014) | |
| MMP-12 | ↓ | Soluble protein | LV | CB6F1 mice | (Lindsey et al. 2005) |
| MMP-13 | ↓ | mRNA | LV | C57BL/6 | (Toba et al. 2015) |
| ↓ | Soluble protein | LV | CB6F1 mice | (Lindsey et al. 2005) | |
| MMP-14 | ↓ | Soluble protein | LV | CB6F1 mice | (Lindsey et al. 2005) |
| ↑ | Insoluble protein | LV | CB6F1 mice | (Lindsey et al. 2005) | |
| ↓ | Protein | LV | C57Bl6 mice | (Huet et al. 2015) | |
| MMP-15 | ↓ | mRNA | LV | C57BL/6 | (Toba et al. 2015) |
| MMP-28 | ↑ | Protein | LV | C57BL/6J | (Ma et al. 2012) |
| TIMP-1 | ↑ | Soluble protein | Plasma | Human | (Bonnema et al. 2007) |
| ↑ | Soluble protein | Plasma | C57BL/6J mice | (Chiao et al. 2011) | |
| TIMP-2 | ↑ | Protein | Plasma | Human | (Bonnema et al. 2007) |
| TIMP-3 | ↓ | Insoluble protein | LV | CB6F1 mice | (Bonnema et al. 2007) |
| TIMP-4 | ↑ | Protein | Plasma | Human | (Bonnema et al. 2007) |
↑ increased, ↓ decreased. MMP matrix metalloproteinase
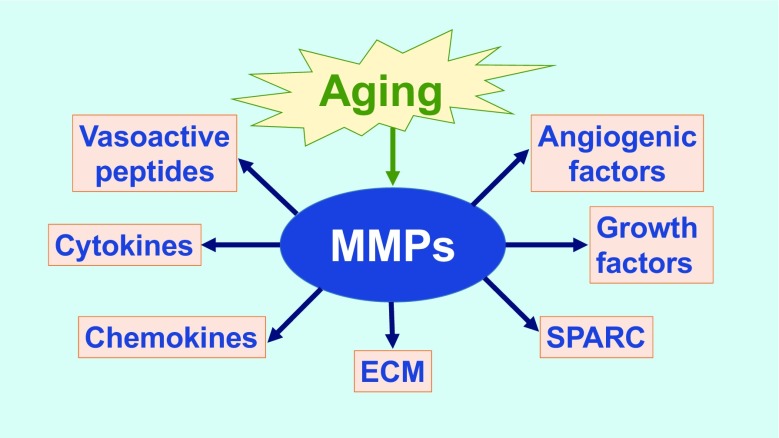
MMP functions go beyond cleaving ECM substrates. For example, MMP-9 can process a number of cytokines, growth factors, and other MMPs, including interleukin (IL)-1β, IL-6, and IL-8, tumor necrosis factor α (TNF-α), endothelin-1, transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), osteopontin, and MMP-2, MMP-9, and MMP-13 (Cauwe et al. 2007; Egeblad and Werb 2002; Lindsey et al. 2016; Sternlicht and Werb 2001). A number of intracellular substrates have been identified (Cauwe and Opdenakker 2010). Figure 1 illustrates how aging can induce MMPs to modulate a number of biological functions.
Fig. 1.
Aging modulates cell signaling and extracellular matrix (ECM) remodeling through matrix metalloproteinase (MMP) actions. SPARC secreted protein acidic and rich in cysteine
Of the MMPs evaluated to date, a number of studies have assessed MMP-9 in cardiac aging. There is strong evidence that MMP-9 is a major mediator for increased stiffness in the aging LV (Iyer et al. 2016). MMP-9 is predominantly expressed in leukocytes, with low expression in cardiomyocytes (Huet et al. 2015). Macrophage-derived MMP-9 has been implicated in cardiac aging (Chiao et al. 2011). MMP-9 expression increases twofold in the LV of aged mice. Plasma MMP-9 positively correlates with LV end-diastolic dimension (Chiao et al. 2011). Moreover, aging MMP-9-null mice have reduced expression of cadherin 1, integrin αV, and TIMP-3 (Yabluchanskiy et al. 2014). Together, MMP-9 deletion-associated changes result in increased angiogenesis and decreased cardiomyocyte hypertrophy during aging (Yabluchanskiy et al. 2014). In contrast, MMP-28 deletion amplifies inflammation and of note, MMP-9 is elevated in the absence of MMP-28, suggesting cross-talk among MMPs (Ma et al. 2015; Ma et al. 2012; Iyer et al. 2016).
Aging effects on cardiac fibroblast cell physiology
Cardiac fibroblasts are the major producer of ECM, including collagen. In this review, we classify all fibroblast subtypes (fibrocytes, fibroblasts, and myofibroblasts) under the fibroblast term and do not discuss fibroblast sources, which is currently an area of active investigation. Cardiac fibroblasts contain mRNA transcripts for collagens I, III, and IV (Eghbali et al. 1989a; Luther et al. 2012). In addition to collagen, other ECM proteins produced by cardiac fibroblasts include fibronectin, α1- , α2-, and α5-integrins (Burgess et al. 2001), MMPs, and TIMPs (Flack et al. 2006; Horn et al. 2012; Vanhoutte and Heymans 2010). While ECM expression has been evaluated, little is known about how gene or protein expression in cardiac fibroblasts changes their cell physiology.
Cardiac fibroblast senescence is affected by metabolic levels. For example, increased metabolic supply, such as increased extracellular pyruvate concentration, impairs fibroblast growth and causes mitochondrial dysfunction (Xu and Finkel 2002). Low nutrient levels can feedback to increase sirtuin-1 and sirtuin-3 activities, which are associated with increased mitochondrial biogenesis, mitochondrial protein synthesis, antioxidant defense, and life span (Sack and Finkel 2012). Little is currently known about the effect of fibroblast cell metabolism on cell physiology, particularly in the context of aging.
Aging effects on cardiomyocyte cell physiology
In the aging LV, there are quantitative and qualitative changes in the cardiomyocyte population, with hypertrophy characterizing the early phase of response. Myocyte cell volumes, cross-sectional areas, and cell length all increase with aging, resulting in reduced inter-cardiomyocyte space (Anversa et al. 2005; Yabluchanskiy et al. 2014). Together with cardiomyocyte hypertrophy, the number of multi-nucleated cardiomyocytes increases (Anversa et al. 1990; Olivetti et al. 1987). Age-induced cardiomyocyte hypertrophy can be accompanied by a deficiency in oxygen supply (Khan et al. 2002). While some reports indicate that hypertrophy is less efficient and uses more oxygen, other reports indicate that hypertrophy is more efficient and uses less oxygen (Gunning and Coleman 1973). The setting of hypertrophy (physiological vs. pathological) may explain the differences observed, and further studies are warranted. Hypoxia, in part through upregulation of hypoxia inducible factor 1, is a powerful stimulus for the expression of angiogenic signaling factors. Reactive oxygen species (ROS) production resulting from impaired mitochondrial function stimulates hypoxia inducible factor 1 activation (Liu and Finkel 2014). Cardiomyocytes contribute to ECM remodeling by expressing collagen type IV (Eghbali et al. 1989a), MMP-2, MMP-9, MMP-14, and all TIMP subtypes (Bildyug et al. 2015; Riches et al. 2009; Vanhoutte and Heymans 2010).
Cardiomyocyte aging is accompanied by organelle changes. Myocyte aging is associated with the accumulation of mitochondrial DNA mutations, protein oxidation, and altered biogenesis, which leads to impaired bioenergetic efficiency and increased ROS levels, which enhance myocyte apoptosis rates and induce an inflammatory reaction (Martin-Fernandez and Gredilla 2016). Mitochondrial function and life span are improved by the genetic inhibition of the mammalian target of rapamycin (mTOR), a serine-threonine kinase that functions as an intracellular energy sensor (Finkel 2015; Wu et al. 2013).
Sarcoplasmic reticulum function and calcium signaling are impaired with age. The sarcoplasmic reticulum Ca2+ pump (SERCA2) regulates cardiomyocyte contraction and relaxation by handling intracellular Ca2+ stores; SERCA2 activity is decreased in aging hearts (Kaplan et al. 2007). The overall result of cardiomyocyte aging is a decrease in cardiomyocyte numbers, which have been observed in both animal experiments and human clinical studies (Anversa et al. 1986; Olivetti et al. 1991). Regression analysis suggests that cardiac aging process is characterized by a loss of 38 million cardiomyocyte nuclei per year in human LVs (Olivetti et al. 1991).
The endocrine system has an impact on cardiomyocytes during aging, especially the renin angiotensin system (RAS). Angiotensin II (Ang II) and angiotensin converting enzyme (ACE) are increased in cardiac tissue with age (Dai et al. 2009; Lakatta and Levy 2003). Ang II can directly induce cardiomyocyte hypertrophy, fibrosis, apoptosis, LV stiffness, and diastolic dysfunction (Domenighetti et al. 2005). Furthermore, treatment with the ACE inhibitor enalapril and the angiotensin II type 1 receptor antagonist losartan ameliorates age-related cardiac changes (Basso et al. 2007). ACE inhibitors have been shown to inhibit MMP-9 activity by interacting with the proteolytic site (Yamamoto et al. 2007a; Yamamoto et al. 2007b), which can partially explain the beneficial effects of ACE inhibitors on cardiac aging.
Aging effects on cardiac macrophage physiology
An enhanced chronic inflammatory status is a hallmark of cardiac aging (Franceschi 2007). Mitochondrial DNA (mtDNA) from dead cells may play a role in cardiac inflammation by stimulating endogenous cardiac cell inflammatory gene expression and by serving as a direct or indirect chemoattractant for inflammatory cells. Unlike nuclear DNA, mtDNA is not methylated and triggers macrophage activation by engaging with toll-like receptors (Sun et al. 2016). Monocyte chemoattractant protein-1 (MCP-1 or CCL2) is a chemokine with an age-dependent increase in circulating levels and LV expression (Chiao et al. 2011; Deshmane et al. 2009). Macrophage content in the LV and MMP-9 expression in the LV and plasma are also elevated in aging mice (Chiao et al. 2011). Plasma MCP-1 and MMP-9 positively correlate with end-diastolic dimension, indicating MCP-1 and MMP-9 are circulating biomarkers of cardiac aging (Chiao et al. 2011).
Beyond macrophage quantity, LV macrophage polarization (M1 vs. M2 phenotype) is also altered by aging (Ma et al. 2015). MMP-9 deletion suppresses these phenotypic changes by increasing macrophage mRNA levels of CD206 and Fizz1 and by preventing the age-related shift from F4/80+CD206+ M2 cells to F4/80+CD206− M1 cells (Ma et al. 2015). Macrophage accumulation is also decreased in the LVs of old SPARC-null mice (Toba et al. 2015). Isolated peritoneal macrophage stimulated with SPARC recombinant protein showed increased expression of M1 pro-inflammatory polarization markers (Ccl3, Ccl5, TNF-α, and IL-12) and decreased expression of anti-inflammatory M2 polarization markers (Arg1 and Mrc1) (Toba et al. 2015). Therefore, in addition to mediating ECM events in cardiac aging, MMP-9 and SPARC regulate cell physiology and signaling.
Aging effects on myocardial endothelial cell physiology
Endothelial dysfunction is highly associated with age. Impaired nitric oxide (NO) bioavailability is a major cause of diminished vasodilation, and endothelial NO synthase (eNOS) expression is decreased with age (Brandes et al. 2005). Moreover, reactive oxygen species (ROS), including superoxide radical (O2 −), are increased with age (Herrera et al. 2010). ROS can rapidly scavenge NO, decreasing its bioavailability and producing other free radicals such as reactive nitrogen species (RNS). In addition, ROS can uncouple eNOS by depleting tetrahydrobiopterin (BH4) stores. This uncoupled eNOS converts L-arginine to O2 − instead of NO, creating a positive feedback loop to free-radical production (Zweier et al. 2011). ROS and RNS can promote protein structural modifications by reacting with amino acid residues. Protein oxidation or nitrosylations can enhance or diminish activity. For example, free radicals can disrupt the cysteine switch between the inhibitory and catalytic domains of MMPs, resulting in an active full-length enzyme (Chow et al. 2007). Unbalanced ROS production can activate nuclear factor-kappa B (NFκB), leading to a pro-inflammatory shift in the endothelial gene expression profile (Donato et al. 2007). Increased ROS contributes to activation of the TNF-α signaling pathway and impaired mitochondrial activity (Herrera et al. 2010). Thus, oxidative stress can accelerate endothelial cell senescence by decreasing proliferative responses to mitotic stimuli (Toussaint et al. 2002).
One index of endothelial dysfunction is vascular permeability. Increased vascular permeability and cadherin-1 expression were observed in the LVs of 15–18-month-old mice compared to 6–8-month-old mice (Yabluchanskiy et al. 2014). Cadherin-1 is a transmembrane protein that forms adherens junctions between endothelial cells, and an increase in cadherin-1 may indicate an attempt to preserve vascular permeability in the aged LV. Moreover, integrin αV decreases with age and in line with the observed vessel rarefaction. MMP-9 gene deletion blunts vascular permeability in 15–18-month-old mice, indicating a role for MMP-9 in maintaining vessel integrity (Yabluchanskiy et al. 2014).
Vascular endothelial growth factor (VEGF) is an essential factor that regulates angiogenesis by inducing endothelial cell growth, migration, and tube formation (Yabluchanskiy et al. 2014). While VEGF mRNA increases in the LVs of both 15–18-month-old wild-type and MMP-9-null mice, vessel numbers assessed by griffonia simplicifolia lectin I staining only increase in the null group (Yabluchanskiy et al. 2014). These results indicate an age-related disconnect between angiogenic mediators released by myocardial cells and their effectiveness (Yabluchanskiy et al. 2014). Human dermal microvascular endothelial cells derived from elderly and neonatal individuals showed decreased VEGF mRNA and protein in the elderly group, indicating likely organ specific differences (Ahluwalia et al. 2014).
Coronary blood flow is decreased in the hearts of 30-month-old rats compared to 6-month-old young rats, and capillary density was decreased in the mid and apex regions (Khan et al. 2001). Decreased blood perfusion, decreased new vessel formation, and impaired vasodilation are associated with age to generate an environment that can maintain basal function but has reduced reserve potential.
Aging effects on associated cardiovascular pathologies
Aging and hypertension
Cardiovascular aging affects the walls of large arteries in humans, particularly the aorta. Over time, the aorta becomes thicker and loses its elastic nature. This process results in arterial stiffness and increases in pulse wave velocity. Of note, the age-related increase in blood pressure does not occur in mice (Lin et al. 2008). Thus, the mouse is a particularly interesting species to study aging effects on the heart without confounding age-related blood pressure effects. Intrinsic cardiac aging in the murine model closely recapitulates age-related changes in humans who do not have accompanying hypertension, including LV hypertrophy, fibrosis, and diastolic dysfunction (Dai and Rabinovitch 2009).
Aging-induced hypertension has been related to adverse remodeling with accompanying endothelial dysfunction, LV hypertrophy, and diastolic dysfunction (Wang and Shah 2015). In aging Wistar rats, mRNA transcription is decreased for type III collagen, fibronectin, and β1 integrin in comparison to young Wistar rats. In contrast, increased transcription and expression of type III collagen, fibronectin, and β1 integrin are observed in aging spontaneously hypertensive rats (SHR) compared to young SHR (Mamuya et al. 1992). These data suggest that in the rat, aging may not increase transcription of ECM components when blood pressure is normal. In this case, post-transcriptional modifications may be contributing to the increased ECM deposition in aging hearts.
Aging and MI
Aging worsens post-MI LV remodeling outcomes. There is reduced collagen scar deposition in healing infarcts of old mice (>24 month old) compared to young mice (2–3 month old); collagen deposition in resolving infarctions is similar, indicating delayed kinetics (Yang et al. 2008). Mice >2 years old have decreased expression of osteopontin mRNA compared to young mice 2–3 months old. TGF-β1, TGF-β2, and TGF-β3 levels are not significantly different in the infarcted/reperfused regions of both old and young mice (Bujak et al. 2008). The upregulation of TGF-β stimulates the synthesis of fibrous connective tissue, which reduces the flexibility of the myocardium and increases myocardial stiffness. In addition, an imbalance between MMPs and TIMPs leads to ECM changes that stimulate cardiac dilatation due to excessive ECM degradation. These changes can lead to diastolic dysfunction and eventually heart failure. Aging, therefore, changes the ECM environment, such that the response to MI injury is less effective (Nguyen et al. 2014). The positive correlation between age and LV dilation in post-MI wild-type mice is not observed with MMP-9 deletion (Yabluchanskiy et al. 2015). In addition, MMP-9 deletion improves post-MI survival rates. Moreover, MMP-9 deletion does not change the number of macrophages in the LV, but does increase the expression of M2 polarization markers, suggesting an improved post-MI repair profile.
Future directions and conclusion
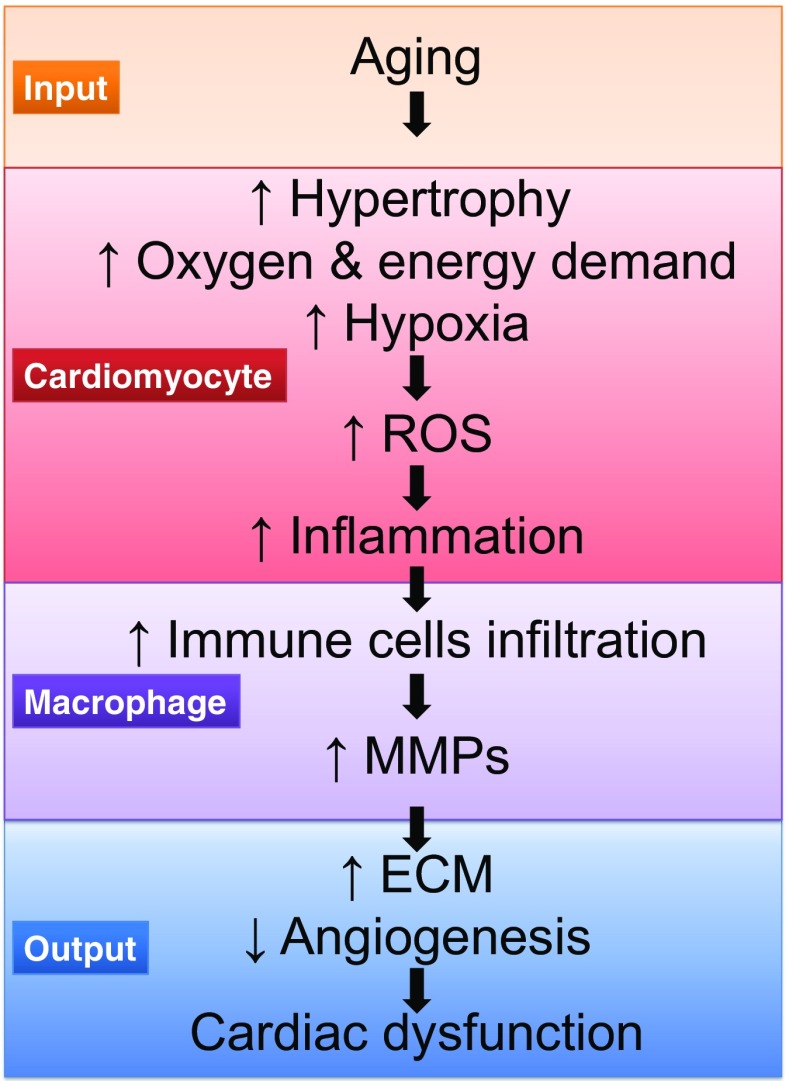
The myocardium undergoes a number of cellular and extracellular responses during aging, leading to increased LV stress and diastolic dysfunction (Fig. 2). While much knowledge has been obtained, there are several avenues fruitful for future examinations. One direction is to better understand how MMP activities could be modified to prevent or slow the development of excessive cardiomyocyte hypertrophy and ECM deposition. Aging is a resetting of baseline values to set a new homeostasis, and attempts to delay or prevent this shift may improve the cardiac aging phenotype.
Fig. 2.
Aging-related events involved in the development of cardiac dysfunction. Aging leads to increased afterload and decreased vasodilation, and these are the inputs for cardiomyocyte hypertrophy. Increased oxygen and energy demand results in hypoxia, which favors an increase of reactive oxygen species (ROS) levels and release of pro-inflammatory factors. As a result, there is an increase in immune cell infiltration, such as macrophages. Macrophages secrete matrix metalloproteinases (MMPs), which leads to increased extracellular matrix (ECM) deposition, decreased angiogenesis, and cardiac dysfunction
Proteomics is a powerful tool with multiple applications for cardiac aging studies. Proteomics is used to catalogue MMP substrates to further elucidate cell signaling pathways modified by MMPs (Cauwe et al. 2009; Eckhard et al. 2016; Ma et al. 2012; Iyer et al. 2016). Unbiased proteomics explorations will continue to elucidate molecular pathways involved in cardiac aging and identify useful biomarkers of cardiac aging. Genomics screens also provide a method of identifying gene pathways important for aging (Johnson et al. 2011; Yamamoto and Takai 2009). As we amass big data on cardiac aging, computational models at the molecular, cellular, and organ levels will be useful. For example, a computational model of fibroblast changes during the time course of aging will be useful for understanding intracellular fibroblast communication, as well as intercellular communication that includes fibroblast connections to macrophages, cardiomyocyte, and endothelial cells (Saucerman 2016). Understanding the relationship between myocyte hypertrophy, inflammation, and fibrosis will also be an important future avenue of research (Nahrendorf 2016; Turner 2016).
While this review focused on aging-related changes in ECM and MMPs and their relationship with hypertension and MI, a similar template can be applied for other relevant molecular components and as a broad application to other cardiovascular diseases that involve MMPs and have aging as a risk factor, such as diabetes, hyperlipoproteinemia, renal failure, and also cardiovascular events as stroke and aneurysms. In conclusion, understanding the dynamic ECM changes that occur over the time continuum of cardiac aging will provide us novel insight into the aging process that has implications for both physiology and pathophysiology.
Acknowledgements
This work was supported by the National Institute of Health HL075360, HL129823, HL051971, T32HL105324, GM114833, and GM104357 and by the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Award 5I01BX000505.
References
- Ahluwalia A, Jones MK, Szabo S, Tarnawski AS. Aging impairs transcriptional regulation of vascular endothelial growth factor in human microvascular endothelial cells: implications for angiogenesis and cell survival. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2014;65:209–215. [PubMed] [Google Scholar]
- Annoni G, et al. Age-dependent expression of fibrosis-related genes and collagen deposition in the rat myocardium. Mech Ageing Dev. 1998;101:57–72. doi: 10.1016/S0047-6374(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Antia M, Baneyx G, Kubow KE, Vogel V. Fibronectin in aging extracellular matrix fibrils is progressively unfolded by cells and elicits an enhanced rigidity response. Faraday Discuss. 2008;139:229–249. doi: 10.1039/b718714a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anversa P, Hiler B, Ricci R, Guideri G, Olivetti G. Myocyte cell loss and myocyte hypertrophy in the aging rat heart. J Am Coll Cardiol. 1986;8:1441–1448. doi: 10.1016/S0735-1097(86)80321-7. [DOI] [PubMed] [Google Scholar]
- Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, Capasso JM. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res. 1990;67:871–885. doi: 10.1161/01.RES.67.4.871. [DOI] [PubMed] [Google Scholar]
- Anversa P, et al. Myocardial aging. Basic Res Cardiol. 2005;100:482–493. doi: 10.1007/s00395-005-0554-3. [DOI] [PubMed] [Google Scholar]
- Asif M, et al. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc Natl Acad Sci U S A. 2000;97:2809–2813. doi: 10.1073/pnas.040558497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. Protective effect of long-term angiotensin II inhibition. Am J Phys Heart Circ Phys. 2007;293:H1351–H1358. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- Biernacka A, Frangogiannis NG. Aging and cardiac fibrosis. Aging and disease. 2011;2:158–173. [PMC free article] [PubMed] [Google Scholar]
- Bildyug NB, Voronkina IV, Smagina LV, Yudintseva NM, Pinaev GP. Matrix metalloproteinases in primary culture of cardiomyocytes. Biochemistry Biokhimiya. 2015;80:1318–1326. doi: 10.1134/S0006297915100132. [DOI] [PubMed] [Google Scholar]
- Bokov AF, Lindsey ML, Khodr C, Sabia MR, Richardson A. Long-lived Ames dwarf mice are resistant to chemical stressors the journals of gerontology series A. Biological sciences and medical sciences. 2009;64:819–827. doi: 10.1093/gerona/glp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnema DD, et al. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) J Card Fail. 2007;13:530–540. doi: 10.1016/j.cardfail.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD. The role of SPARC in extracellular matrix assembly. Journal of cell communication and signaling. 2009;3:239–246. doi: 10.1007/s12079-009-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Bonnema DD, Zile MR. Age-dependent alterations in fibrillar collagen content and myocardial diastolic function: role of SPARC in post-synthetic procollagen processing. Am J Phys Heart Circ Phys. 2010;298:H614–H622. doi: 10.1152/ajpheart.00474.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol. 2008;51:1384–1392. doi: 10.1016/j.jacc.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess ML, McCrea JC, Hedrick HL. Age-associated changes in cardiac matrix and integrins. Mech Ageing Dev. 2001;122:1739–1756. doi: 10.1016/S0047-6374(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Burkauskiene A. Age-related changes in the structure of myocardial collagen network of auricle of the right atrium in healthy persons and ischemic heart disease patients. Medicina. 2005;41:145–154. [PubMed] [Google Scholar]
- Campbell DJ, et al. Diastolic dysfunction of aging is independent of myocardial structure but associated with plasma advanced glycation end-product levels. PLoS One. 2012;7:e49813. doi: 10.1371/journal.pone.0049813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauwe B, Martens E, Proost P, Opdenakker G. Multidimensional degradomics identifies systemic autoantigens and intracellular matrix proteins as novel gelatinase B/MMP-9 substrates. Integrative biology : quantitative biosciences from nano to macro. 2009;1:404–426. doi: 10.1039/b904701h. [DOI] [PubMed] [Google Scholar]
- Cauwe B, Opdenakker G. Intracellular substrate cleavage: a novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2010;45:351–423. doi: 10.3109/10409238.2010.501783. [DOI] [PubMed] [Google Scholar]
- Cauwe B, Van den Steen PE, Opdenakker G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2007;42:113–185. doi: 10.1080/10409230701340019. [DOI] [PubMed] [Google Scholar]
- Chiao YA, et al. Multi-analyte profiling reveals matrix metalloproteinase-9 and monocyte chemotactic protein-1 as plasma biomarkers of cardiac aging. Circ Cardiovasc Genet. 2011;4:455–462. doi: 10.1161/CIRCGENETICS.111.959981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao YA, et al. Matrix metalloproteinase-9 deletion attenuates myocardial fibrosis and diastolic dysfunction in ageing mice. Cardiovasc Res. 2012;96:444–455. doi: 10.1093/cvr/cvs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow AK, Cena J, Schulz R. Acute actions and novel targets of matrix metalloproteinases in the heart and vasculature. Br J Pharmacol. 2007;152:189–205. doi: 10.1038/sj.bjp.0707344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Rabinovitch PS. Cardiac aging in mice and humans: the role of mitochondrial oxidative stress. Trends in cardiovascular medicine. 2009;19:213–220. doi: 10.1016/j.tcm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro Bras LE, Toba H, Baicu CF, Zile MR, Weintraub ST, Lindsey ML, Bradshaw AD. Age and SPARC change the extracellular matrix composition of the left ventricle. Biomed Res Int. 2014;2014:810562. doi: 10.1155/2014/810562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenighetti AA, Wang Q, Egger M, Richards SM, Pedrazzini T, Delbridge LM. Angiotensin II-mediated phenotypic cardiomyocyte remodeling leads to age-dependent cardiac dysfunction and failure. Hypertension. 2005;46:426–432. doi: 10.1161/01.HYP.0000173069.53699.d9. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Eckhard U, et al. Active site specificity profiling of the matrix metalloproteinase family: proteomic identification of 4300 cleavage sites by nine MMPs explored with structural and synthetic peptide cleavage analyses matrix biology. Journal of the International Society for Matrix Biology. 2016;49:37–60. doi: 10.1016/j.matbio.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Eghbali M, et al. Localization of types I, III and IV collagen mRNAs in rat heart cells by in situ hybridization. J Mol Cell Cardiol. 1989;21:103–113. doi: 10.1016/0022-2828(89)91498-3. [DOI] [PubMed] [Google Scholar]
- Eghbali M, Eghbali M, Robinson TF, Seifter S, Blumenfeld OO. Collagen accumulation in heart ventricles as a function of growth and aging. Cardiovasc Res. 1989;23:723–729. doi: 10.1093/cvr/23.8.723. [DOI] [PubMed] [Google Scholar]
- Finkel T. The metabolic regulation of aging. Nat Med. 2015;21:1416–1423. doi: 10.1038/nm.3998. [DOI] [PubMed] [Google Scholar]
- Flack EC, et al. Alterations in cultured myocardial fibroblast function following the development of left ventricular failure. J Mol Cell Cardiol. 2006;40:474–483. doi: 10.1016/j.yjmcc.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev. 2007;65:S173–S176. doi: 10.1301/nr.2007.dec.S173-S176. [DOI] [PubMed] [Google Scholar]
- Gazoti Debessa CR, Mesiano Maifrino LB, Rodrigues de Souza R. Age related changes of the collagen network of the human heart. Mech Ageing Dev. 2001;122:1049–1058. doi: 10.1016/S0047-6374(01)00238-X. [DOI] [PubMed] [Google Scholar]
- Graf K, et al. Myocardial osteopontin expression is associated with left ventricular hypertrophy. Circulation. 1997;96:3063–3071. doi: 10.1161/01.CIR.96.9.3063. [DOI] [PubMed] [Google Scholar]
- Gunning JF, Coleman HN., 3rd Myocardial oxygen consumption during experimental hypertrophy and congestive heart failure. J Mol Cell Cardiol. 1973;5:25–38. doi: 10.1016/0022-2828(73)90033-3. [DOI] [PubMed] [Google Scholar]
- Hartog JW, Voors AA, Bakker SJ, Smit AJ, van Veldhuisen DJ. Advanced glycation end-products (AGEs) and heart failure: pathophysiology and clinical implications. Eur J Heart Fail. 2007;9:1146–1155. doi: 10.1016/j.ejheart.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Johansson B, Engstrom-Laurent A. Hyaluronan and its receptor CD44 in the heart of newborn and adult rats. The anatomical record part A. Discoveries in molecular, cellular, and evolutionary biology. 2006;288:587–592. doi: 10.1002/ar.a.20332. [DOI] [PubMed] [Google Scholar]
- Herrera MD, Mingorance C, Rodriguez-Rodriguez R, Alvarez de Sotomayor M. Endothelial dysfunction and aging: an update. Ageing Res Rev. 2010;9:142–152. doi: 10.1016/j.arr.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Horn MA, et al. Age-related divergent remodeling of the cardiac extracellular matrix in heart failure: collagen accumulation in the young and loss in the aged. J Mol Cell Cardiol. 2012;53:82–90. doi: 10.1016/j.yjmcc.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Horn MA, Trafford AW. Aging and the cardiac collagen matrix: novel mediators of fibrotic remodelling. J Mol Cell Cardiol. 2016;93:175–185. doi: 10.1016/j.yjmcc.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet E, et al. Deletion of extracellular matrix metalloproteinase inducer/CD147 induces altered cardiac extracellular matrix remodeling in aging mice. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2015;66:355–366. [PubMed] [Google Scholar]
- Iyer RP, Chiao YA, Flynn ER, Hakala K, Cates CA, Weintraub ST, de Castro Bras LE (2016) Matrix metalloproteinase-9-dependent mechanisms of reduced contractility and increased stiffness in the aging heart. Proteomics Clin Appl 10:92–107. doi:10.1002/prca.201500038 [DOI] [PMC free article] [PubMed]
- Johnson JL, et al. A selective matrix metalloproteinase-12 inhibitor retards atherosclerotic plaque development in apolipoprotein E-knockout mice. Arterioscler Thromb Vasc Biol. 2011;31:528–535. doi: 10.1161/ATVBAHA.110.219147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugdutt BI, Jelani A, Palaniyappan A, Idikio H, Uweira RE, Menon V, Jugdutt CE. Aging-related early changes in markers of ventricular and matrix remodeling after reperfused ST-segment elevation myocardial infarction in the canine model: effect of early therapy with an angiotensin II type 1 receptor blocker. Circulation. 2010;122:341–351. doi: 10.1161/CIRCULATIONAHA.110.948190. [DOI] [PubMed] [Google Scholar]
- Kajstura J, et al. Necrotic and apoptotic myocyte cell death in the aging heart of Fischer 344 rats. Am J Phys. 1996;271:H1215–H1228. doi: 10.1152/ajpheart.1996.271.3.H1215. [DOI] [PubMed] [Google Scholar]
- Kandasamy AD, Chow AK, Ali MAM, Schulz R. Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res. 2010;85:413–423. doi: 10.1093/cvr/cvp268. [DOI] [PubMed] [Google Scholar]
- Kaplan P, et al. Effect of aging on the expression of intracellular Ca(2+) transport proteins in a rat heart. Mol Cell Biochem. 2007;301:219–226. doi: 10.1007/s11010-007-9414-9. [DOI] [PubMed] [Google Scholar]
- Khan AS, Lynch CD, Sane DC, Willingham MC, Sonntag WE. Growth hormone increases regional coronary blood flow and capillary density in aged rats. The journals of gerontology series A. Biological sciences and medical sciences. 2001;56:B364–B371. doi: 10.1093/gerona/56.8.B364. [DOI] [PubMed] [Google Scholar]
- Khan AS, Sane DC, Wannenburg T, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res. 2002;54:25–35. doi: 10.1016/S0008-6363(01)00533-8. [DOI] [PubMed] [Google Scholar]
- Kwak HB, Kim JH, Joshi K, Yeh A, Martinez DA, Lawler JM. Exercise training reduces fibrosis and matrix metalloproteinase dysregulation in the aging rat heart. FASEB J. 2011;25:1106–1117. doi: 10.1096/fj.10-172924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG. Cardiovascular reserve capacity in healthy older humans. Aging (Milano) 1994;6:213–223. doi: 10.1007/BF03324244. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health. Links to Heart Disease Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- Lin J, Lopez E, Jin Y, Van Remmen H, Bauch T, Han H, Lindsey M. Age-related cardiac muscle sarcopenia: combining experimental and mathematical modeling to identify mechanisms. Exp Gerontol. 2008;43:296–306. doi: 10.1016/j.exger.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey ML, et al. Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc Res. 2005;66:410–419. doi: 10.1016/j.cardiores.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Lindsey ML, Iyer RP, Jung M, De Leon-Pennell KY, Ma Y. Matrix metalloproteinases as input and output signals for post-myocardial infarction remodeling. J Mol Cell Cardiol. 2016;91:134–140. doi: 10.1016/j.yjmcc.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Finkel T. Aging: the blurry line between life and death. Curr Biol. 2014;24:R610–R613. doi: 10.1016/j.cub.2014.05.057. [DOI] [PubMed] [Google Scholar]
- Liu J, et al. Glycation end-product cross-link breaker reduces collagen and improves cardiac function in aging diabetic heart. Am J Phys Heart Circ Phys. 2003;285:H2587–H2591. doi: 10.1152/ajpheart.00516.2003. [DOI] [PubMed] [Google Scholar]
- Luther DJ, et al. Absence of type VI collagen paradoxically improves cardiac function, structure, and remodeling after myocardial infarction. Circ Res. 2012 doi: 10.1161/CIRCRESAHA.111.252734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, et al. Deriving a cardiac ageing signature to reveal MMP-9-dependent inflammatory signalling in senescence. Cardiovasc Res. 2015;106:421–431. doi: 10.1093/cvr/cvv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Chiao YA, Zhang J, Manicone AM, Jin YF, Lindsey ML. Matrix metalloproteinase-28 deletion amplifies inflammatory and extracellular matrix responses to cardiac aging. Microsc Microanal. 2012;18:81–90. doi: 10.1017/S1431927611012220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamuya W, Chobanian A, Brecher P. Age-related changes in fibronectin expression in spontaneously hypertensive. Wistar-Kyoto, and Wistar rat hearts Circulation research. 1992;71:1341–1350. doi: 10.1161/01.res.71.6.1341. [DOI] [PubMed] [Google Scholar]
- Martin-Fernandez B, Gredilla R (2016) Mitochondria and oxidative stress in heart aging Age doi:10.1007/s11357-016-9933-y [DOI] [PMC free article] [PubMed]
- Mendes AB, Ferro M, Rodrigues B, Souza MR, Araujo RC, Souza RR. Quantification of left ventricular myocardial collagen system in children, young adults, and the elderly. Medicina (B Aires) 2012;72:216–220. [PubMed] [Google Scholar]
- Nahrendorf M (2016) Monocyte and macrophage contributions to cardiac remodeling Journal of molecular and cellular cardiology in press (JMCC9602) [DOI] [PMC free article] [PubMed]
- Nguyen NT, Yabluchanskiy A, de Castro Brás LE, Jin Y-F, Lindsey ML (2014) Aging-related changes in extracellular matrix: implications for ventricular remodeling following myocardial infarction
- Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circulation research. 1991;68:1560–1568. doi: 10.1161/01.RES.68.6.1560. [DOI] [PubMed] [Google Scholar]
- Olivetti G, Ricci R, Anversa P. Hyperplasia of myocyte nuclei in long-term cardiac hypertrophy in rats. J Clin Invest. 1987;80:1818–1821. doi: 10.1172/JCI113278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parati G, Frattola A, Di Rienzo M, Castiglioni P, Mancia G. Broadband spectral analysis of blood pressure and heart rate variability in very elderly subjects. Hypertension. 1997;30:803–808. doi: 10.1161/01.HYP.30.4.803. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- Riches K, Morley ME, Turner NA, O'Regan DJ, Ball SG, Peers C, Porter KE. Chronic hypoxia inhibits MMP-2 activation and cellular invasion in human cardiac myofibroblasts. J Mol Cell Cardiol. 2009;47:391–399. doi: 10.1016/j.yjmcc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, et al. The effect of aging on the specialized conducting system: a telemetry ECG study in rats over a 6 month period. PLoS One. 2014;9:e112697. doi: 10.1371/journal.pone.0112697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack MN, Finkel T (2012) Mitochondrial metabolism, sirtuins, and aging. Cold Spring Harb Perspect Biol:4. doi:10.1101/cshperspect.a013102 [DOI] [PMC free article] [PubMed]
- Saucerman J (2016) Computational modeling of cardiac fibroblasts and fibrosis. Journal of molecular and cellular cardiology in press (JMCC9598) [DOI] [PMC free article] [PubMed]
- Sternlicht M, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait JB, Lakatta EG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart failure clinics. 2012;8:143–164. doi: 10.1016/j.hfc.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Youle RJ, Finkel T. The mitochondrial basis of aging. Mol Cell. 2016;61:654–666. doi: 10.1016/j.molcel.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen M, et al. Absence of thrombospondin-2 causes age-related dilated cardiomyopathy. Circulation. 2009;120:1585–1597. doi: 10.1161/CIRCULATIONAHA.109.863266. [DOI] [PubMed] [Google Scholar]
- Thomas DP, Cotter TA, Li X, McCormick RJ, Gosselin LE. Exercise training attenuates aging-associated increases in collagen and collagen crosslinking of the left but not the right ventricle in the rat. Eur J Appl Physiol. 2001;85:164–169. doi: 10.1007/s004210100447. [DOI] [PubMed] [Google Scholar]
- Thomas DP, McCormick RJ, Zimmerman SD, Vadlamudi RK, Gosselin LE. Aging- and training-induced alterations in collagen characteristics of rat left ventricle and papillary muscle. Am J Phys. 1992;263:H778–H783. doi: 10.1152/ajpheart.1992.263.3.H778. [DOI] [PubMed] [Google Scholar]
- Thomas DP, Zimmerman SD, Hansen TR, Martin DT, McCormick RJ. Collagen gene expression in rat left ventricle: interactive effect of age and exercise training. J Appl Physiol. 2000;89:1462–1468. doi: 10.1152/jappl.2000.89.4.1462. [DOI] [PubMed] [Google Scholar]
- Toba H, de Castro Bras LE, Baicu CF, Zile MR, Lindsey ML, Bradshaw AD (2015) Secreted protein acidic and rich in cysteine facilitates age-related cardiac inflammation and macrophage M1 polarization. American Journal of Physiology Cell Physiology 308:C972–C982 doi:10.1152/ajpcell.00402.2014 [DOI] [PMC free article] [PubMed]
- Toba H, de Castro Bras LE, Baicu CF, Zile MR, Lindsey ML, Bradshaw AD. Increased ADAMTS1 mediates SPARC-dependent collagen deposition in the aging myocardium. Am J Phys Endocrinol Metab. 2016;310:E1027–E1035. doi: 10.1152/ajpendo.00040.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toprak A, Reddy J, Chen W, Srinivasan S, Berenson G. Relation of pulse pressure and arterial stiffness to concentric left ventricular hypertrophy in young men (from the Bogalusa Heart Study) Am J Cardiol. 2009;103:978–984. doi: 10.1016/j.amjcard.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Toussaint O, Royer V, Salmon M, Remacle J. Stress-induced premature senescence and tissue ageing. Biochem Pharmacol. 2002;64:1007–1009. doi: 10.1016/S0006-2952(02)01170-X. [DOI] [PubMed] [Google Scholar]
- Turner N (2016) Inflammatory and fibrotic responses of cardiac fibroblasts to myocardial damage associated molecular patterns (DAMPs). Journal of molecular and cellular cardiology in press (JMCC9542) [DOI] [PubMed]
- Vanhoutte D, Heymans S. TIMPs and cardiac remodeling: ‘embracing the MMP-independent-side of the family’. J Mol Cell Cardiol. 2010;48:445–453. doi: 10.1016/j.yjmcc.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Voorhees AP, et al. Building a better infarct: modulation of collagen cross-linking to increase infarct stiffness and reduce left ventricular dilation post-myocardial infarction. J Mol Cell Cardiol. 2015;85:229–239. doi: 10.1016/j.yjmcc.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Shah AM. Age-associated pro-inflammatory remodeling and functional phenotype in the heart and large arteries. J Mol Cell Cardiol. 2015;83:101–111. doi: 10.1016/j.yjmcc.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KT, Janicki JS, Shroff SG, Pick R, Chen RM, Bashey RI (1988) Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium, Circulation research 62:757–765 [DOI] [PubMed]
- Wohlgemuth SE, Calvani R, Marzetti E. The interplay between autophagy and mitochondrial dysfunction in oxidative stress-induced cardiac aging and pathology. J Mol Cell Cardiol. 2014;71:62–70. doi: 10.1016/j.yjmcc.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Wu JJ, et al. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep. 2013;4:913–920. doi: 10.1016/j.celrep.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Finkel T. A role for mitochondria as potential regulators of cellular life span. Biochem Biophys Res Commun. 2002;294:245–248. doi: 10.1016/S0006-291X(02)00464-3. [DOI] [PubMed] [Google Scholar]
- Yabluchanskiy A, et al. Cardiac aging is initiated by matrix metalloproteinase-9-mediated endothelial dysfunction American journal of physiology. Heart and circulatory physiology. 2014;306:H1398–H1407. doi: 10.1152/ajpheart.00090.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabluchanskiy A, et al. Myocardial infarction superimposed on aging: MMP-9 deletion promotes M2 macrophage polarization. The journals of gerontology series A. Biological sciences and medical sciences. 2015 doi: 10.1093/gerona/glv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D, Takai S. Pharmacological implications of MMP-9 inhibition by ACE inhibitors. Curr Med Chem. 2009;16:1349–1354. doi: 10.2174/092986709787846514. [DOI] [PubMed] [Google Scholar]
- Yamamoto D, Takai S, Jin D, Inagaki S, Tanaka K, Miyazaki M. Molecular mechanism of imidapril for cardiovascular protection via inhibition of MMP-9. J Mol Cell Cardiol. 2007;43:670–676. doi: 10.1016/j.yjmcc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Yamamoto D, Takai S, Miyazaki M. Prediction of interaction mode between a typical ACE inhibitor and MMP-9 active site. Biochem Biophys Res Commun. 2007;354:981–984. doi: 10.1016/j.bbrc.2007.01.088. [DOI] [PubMed] [Google Scholar]
- Yang Y, et al. Age-related differences in postinfarct left ventricular rupture and remodeling American journal of physiology. Heart and circulatory physiology. 2008;294:H1815–H1822. doi: 10.1152/ajpheart.00831.2007. [DOI] [PubMed] [Google Scholar]
- Zweier JL, Chen CA, Druhan LJ. S-glutathionylation reshapes our understanding of endothelial nitric oxide synthase uncoupling and nitric oxide/reactive oxygen species-mediated signaling. Antioxid Redox Signal. 2011;14:1769–1775. doi: 10.1089/ars.2011.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]