Abstract
Enterotoxigenic Escherichia coli (ETEC) causes substantial diarrheal morbidity and mortality in young children in countries with limited resources. We determined the phenotypic profiles of 915 ETEC diarrheal isolates derived from Egyptian children under 3 years of age who participated in a 3-year population-based study. For each strain, we ascertained enterotoxin and colonization factor (CF) expression, the O:H serotype, and antimicrobial susceptibility. Sixty-one percent of the strains expressed heat-stable enterotoxin (ST) only, 26% expressed heat-labile enterotoxin (LT) alone, and 12% expressed both toxins. The most common CF phenotypes were colonization factor antigen I (CFA/I) (10%), coli surface antigen 6 (CS6) (9%), CS14 (6%), and CS1 plus CS3 (4%). Fifty-nine percent of the strains did not express any of the 12 CFs included in our test panel. Resistance of ETEC strains to ampicillin (63%), trimethoprim-sulfamethoxazole (52%), and tetracycline (43%) was common, while resistance to quinolone antibiotics was rarely detected. As for the distribution of observed serotypes, there was an unusually wide diversity of O antigens and H types represented among the 915 ETEC strains. The most commonly recognized composite ETEC phenotypes were ST CS14 O78:H18 (4%), ST (or LTST) CFA/I O128:H12 (3%), ST CS1+CS3 O6:H16 (2%), and ST CFA/I O153:H45 (1.5%). Temporal plots of diarrheal episodes associated with ETEC strains bearing common composite phenotypes were consistent with discrete community outbreaks either within a single or over successive warm seasons. These data suggest that a proportion of the disease that is endemic to young children in rural Egypt represents the confluence of small epidemics by clonally related ETEC strains that are transiently introduced or that persist in a community reservoir.
Enterotoxigenic Escherichia coli (ETEC) is an important human and animal pathogen (13, 20). Young children in areas of the world with limited resources and travelers to these regions are the most affected human populations. This pathotype of diarrheagenic E. coli characteristically causes secretory diarrhea and dehydration. In the very young, disease can lead to death if patients are not properly treated with rehydration therapy, while in older children and adults it generally results in temporary incapacitation.
Several ETEC attributes confer virulence or otherwise serve as useful clinical or epidemiologic markers. The expression of intestinal adhesins and enterotoxins is central to ETEC disease pathogenesis. The former exhibit host-restricted receptor-binding specificities, while the latter are biologically active in a range of mammalian hosts. Both of these virulence traits are typically plasmid encoded. Most of the 22 known colonization factors (CFs) associated with human disease comprise filamentous surface structures (11). The two enterotoxin types that are the defining features of ETEC are the heat-labile enterotoxin (LT), an oligomeric protein that is structurally and functionally related to cholera toxin, and the heat-stable enterotoxin (STa), a peptide toxin that has two closely related variants, STp and STh (21). ETEC strains, like other pathogenic and nonpathogenic E. coli strains, express somatic (lipopolysaccharide) and flagellar antigens on the bacterial surface, which together constitute the basis for the standard E. coli O:H serotype system (16). While these chromosomally encoded antigens have not been clearly shown to contribute to virulence, they serve as useful genetic markers in that ETEC strains often display distinctive serotypes. From a clinical standpoint, the carriage rates of plasmid-encoded antimicrobial resistance genes among ETEC have implications for treatment recommendations, particularly as they relate to travelers' diarrhea.
Colonization factor antigen I (CFA/I), CFA/II, and CFA/IV, the first anthrotropic CFs to be described, are common worldwide. CFA/II consists of subtype antigens, coli surface antigen 1 (CS1), CS2, and CS3, of which CS3 is expressed either alone or concomitantly with CS1 or CS2. Similarly, CFA/IV is composed of CS4, CS5, and CS6, with CS6 being expressed alone or in conjunction with either CS4 or CS5. Putative colonization factor antigens that are found with varying frequencies include CS7, CS8 (CFA/III), CS12 (PCFO159), CS14 (PCFO166), and CS17. Other more recently recognized CFs have not been routinely evaluated due to the lack of standardized test reagents.
Our knowledge base on ETEC's phenotypic features and their relationships is derived from numerous studies that have been reported over the past 30 years. In general, these reports have been weighted towards the characterization of ETEC strains that have been isolated during the course of clinic- and hospital-based studies over limited time periods. While considerable variation in the distribution of CF types and serotypes has been noted, some common associations between toxins, CFs, and serotypes have been recognized across populations and regions. There have been modifications to testing methods, particularly those for CF detection, with a shift towards a reliance on monoclonal rather than polyclonal antibody-based immunodetection and the incorporation of an expanding battery of CF-specific reagents. Until very recently (32), standardized assays for genotypic detection of the majority of the known CFs have been lacking. In contrast, both genotypic and phenotypic assays for ETEC enterotoxin detection have been widely used for many years. Studies to comprehensively characterize ETEC strains that have been systematically derived from pediatric population-based surveillance have been underrepresented, particularly for the region of North Africa.
The present study was undertaken to determine the phenotypic profiles of an extensive collection of diarrhea-associated ETEC isolates obtained from infants and young children participating in a longitudinal, community-based study (29). Specifically, we examined the distribution of recognized ETEC virulence factors, O:H serotypes, and susceptibilities to common antibiotics, as well as the relationships between each of these phenotypic traits. We also examined temporal-spatial trends of diarrheal episodes associated with these features over the 3-year duration of the study.
MATERIALS AND METHODS
Population and study features.
We conducted a community-based study of diarrhea in children from early life until 3 years of age in two rural villages of the Abu Homos District, Beheira Governorate, Egypt, situated in the Nile Delta. A full description of the study design and definitions, population makeup, and surveillance procedures has been published elsewhere (29). Upon initiation of the study in February 1995, all eligible children under 2 years of age were enrolled, and thereafter all newborn infants were enrolled until 6 months before study closure in February 1998. Active disease surveillance was performed by semiweekly home visits, at which time data were collected on gastrointestinal symptoms and fecal specimens were obtained from all children with loose stools. The 397 subjects were monitored for a total of 670 person-years, over which time 3,477 episodes of diarrhea were detected. Nine hundred thirty-three (27%) of these diarrheal episodes were associated with the excretion of ETEC, either alone (n = 729 [78%]) or with one or more copathogens (n = 204 [22%]), including Shigella, Salmonella, Campylobacter, rotavirus, and astrovirus.
ETEC virulence factor assessment.
As part of the prospective study, five E. coli colonies from each rectal swab specimen were picked from primary isolation plates (MacConkey medium), cultured overnight in brain heart infusion broth, and frozen in 15% glycerol at −70°C until use. Generally within 1 month of initial isolation, strains were subcultured from frozen stocks onto MacConkey medium and batch tested for enterotoxin expression by GM1 enzyme-linked immunosorbent assays (34, 36). All enterotoxin-positive isolates were subcultured onto CFA agar with and without bile salts (Difco, Detroit, Mich.) (9, 19) and were tested for CF expression by a colony dot blot assay that employed monoclonal antibodies against CFA/I, CS1 to -7, CS8, CS12, CS14, and CS17 (5, 17, 37).
Strain selection.
For the purposes of this study, we systematically selected a single representative ETEC isolate or, in a small number of cases, two isolates from each episode of diarrhea associated with the excretion of ETEC. When individual ETEC isolates displaying different toxin and CF phenotypes were obtained from one or more fecal samples that were collected during a single diarrheal episode, an isolate corresponding to the most numerous composite phenotype was selected. For mixed ETEC episodes for which isolates of the same toxin phenotype differed by the presence or absence of CF expression, a CF-positive isolate was selected for inclusion. For a small number of episodes (n = 12) for which there were equal numbers of colonies detected that displayed either of two distinct ETEC toxin and CF phenotypes, one representative isolate of each phenotype was selected. Nine hundred twenty-one representative isolates, each from a single episode, and paired representative isolates from the other 12 episodes (n = 24) were subcultured from their original frozen stocks onto MacConkey agar. Nutrient agar slants were inoculated with single isolated colonies, grown overnight at 37°C, and sent for serotype analysis. Upon subculture from nutrient agar at the serotype reference laboratory at the National Autonomous University of Mexico, Mexico City, 30 of the unique representative isolates were either nonviable or contaminated and hence were not serotyped.
Serotype methods.
E. coli serotypes were determined for the 915 ETEC isolates (from 891 episodes) in 96-well round-bottomed plates by a microagglutination assay using specific rabbit antisera against 175 somatic (O) and 56 flagellar (H) antigens (24). For the latter, strains were grown repeatedly in Craigie tubes containing semisolid medium to enhance the development of flagella (2).
Antimicrobial susceptibility testing.
Of the 915 ETEC disease isolates that were characterized by a virulence phenotype and serotype, 859 (94%) were accessible for a determination of antimicrobial susceptibility. The remainder were either nonviable or contaminated upon subculture of their frozen stocks. Antimicrobial susceptibility testing was conducted by the disk diffusion method (3). E. coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as quality control strains. The antibiotic test panel included ampicillin, aztreonam, ceftriaxone, ciprofloxacin, trimethoprim-sulfamethoxazole (SXT), naladixic acid, and tetracycline (Becton Dickinson Microbiology Systems, Sparks, Md.). Isolates were defined as susceptible, intermediately susceptible, or resistant according to standardized guidelines (22). For data analyses, isolates that showed either intermediate susceptibility or resistance were classified as nonsusceptible.
RESULTS
For a community-based study of diarrhea in Egyptian children under 3 years of age, a total of 933 episodes of diarrhea associated with the excretion of ETEC were observed during a 3-year follow-up period of a dynamic cohort of 397 subjects. We determined the serotypes of single representative isolates from 891 of these episodes and of two isolates each from 12 episodes, adding to the toxin and CF expression data collected during the course of the study. Antimicrobial susceptibility testing was performed with 859 of these isolates.
The distribution of toxin and associated CF expression for the 915 ETEC isolates is shown in Table 1. The most common toxin type was ST-only (61.3%), followed by LT-only (26.4%) and LTST (12.2%). The most commonly expressed ETEC CFs were CFA/IV (12.6%), CFA/I (9.7%), CFA/II (7.3%), and CS14 (5.8%). Fewer than 2% of the isolates expressed the other CFs included in our immunodetection test panel, namely, CS7, CS8, CS12, and CS17. A large proportion (59%) of the ETEC isolates failed to express any of the 12 CFs tested. The proportion of strains that expressed a CF was largest for LTST (72 of 112 [64%]), intermediate for ST-only (269 of 561 [48%]), and smallest for LT-only (35 of 242 [14%]) ETEC strains (P < 0.0001 by a test for trends).
TABLE 1.
Distribution of toxin and CF phenotypes for representative ETEC strains isolated from Egyptian children with ETEC-associated diarrhea from February 1995 to February 1998
| CF type | No. (%) of isolates with toxin type
|
No. (%) of all ETEC isolates with CF type (n = 915) | ||
|---|---|---|---|---|
| LT-only (n = 242) | ST-only (n = 561) | LTST (n = 112) | ||
| CFA/I | 4 (1.6) | 73 (13.0) | 12 (10.7) | 89 (9.7) |
| CFA/II | 4 (1.6) | 47 (8.4) | 16 (14.3) | 67 (7.3) |
| CS1 | 2 | 2 | ||
| CS1 + CS3 | 1 | 32 | 4 | 37 |
| CS2 | 1 | 1 | 2 | |
| CS2 + CS3 | 2 | 7 | 4 | 13 |
| CS3 | 6 | 7 | 13 | |
| CFA/IV | 6 (2.5) | 86 (15.3) | 23 (20.5) | 115 (12.6) |
| CS4 | 2 | 2 | ||
| CS4 + CS6 | 3 | 12 | 15 | |
| CS5 | 2 | 3 | 5 | |
| CS5 + CS6 | 6 | 8 | 14 | |
| CS6 | 6 | 73 | 79 | |
| CS7 | 8 (3.3) | 3 (0.5) | 11 (1.2) | |
| CS8 | 5 (2.1) | 2 (0.4) | 1 (0.9) | 8 (0.9) |
| CS12 | 2 (0.8) | 5 (0.9) | 4 (3.6) | 11 (1.2) |
| CS14 | 0 | 39 (7.0) | 14 (12.5) | 53 (5.8) |
| CS17 | 5 (2.1) | 4 (0.7) | 1 (0.9) | 10 (1.1) |
| Mixed CFa | 1 (0.4) | 10 (1.8) | 1 (0.9) | 12 (1.3) |
| No detectable CF | 207 (85.5) | 292 (52.0) | 40 (35.7) | 539 (58.9) |
The mixed CF combinations observed were as follows: ST CS2+CS6 (n = 3), ST CS12+CS14 (n = 2), LTST CS1+CS2+CS17 (n = 1), ST CS1+CS6 (n = 1), ST CS1+CS14 (n = 1), LT CS6+CS8 (n = 1), ST CS6+CS14 (n = 2), and ST CS5+CS6+CS7 (n = 1).
We observed a remarkably wide array of O serogroups and H types among the ETEC disease isolates tested. One hundred twenty-one O antigens were represented, although a large majority of these (n = 99) were detected in fewer than 10 isolates each. Table 2 presents the 22 O serogroups that were identified in 10 or more isolates, altogether comprising 56% of the total, or nearly two-thirds of the O-typeable strains. Serogroups O78, O6, O8, O128, O27, and O114 were the most prevalent, with each identified in >3% of the isolates. A minority of the ETEC isolates were either non-O-typeable (n = 84 [9%]) or rough (n = 23 [2%]). A total of 37 H types were detected among the ETEC isolates. Collectively, the 21 most prevalent H types (Table 3) were detected in 63% of the 915 ETEC isolates, while the other 19 H types accounted for 8% of the isolates. No H type was detected (H negative) in 261 (28%) of the isolates.
TABLE 2.
Most common O serogroups expressed among 915 ETEC isolates from community-acquired diarrheal episodes
| O group | % of strains (total no. of strains) |
|---|---|
| 78 | 5.7 (52) |
| 6 | 5.5 (50) |
| 8 | 4.8 (44) |
| 128 | 4.7 (43) |
| 27 | 3.5 (32) |
| 114 | 3.5 (32) |
| 153 | 3.0 (27) |
| 159 | 2.6 (24) |
| 15 | 2.5 (23) |
| 71 | 2.4 (22) |
| 20 | 2.4 (22) |
| 25 | 2.0 (18) |
| 39 | 1.8 (17) |
| 86 | 1.5 (14) |
| 132 | 1.5 (14) |
| 44 | 1.4 (13) |
| 21 | 1.4 (13) |
| 169 | 1.4 (13) |
| 88 | 1.3 (12) |
| 168 | 1.2 (11) |
| 7 | 1.1 (10) |
| 80 | 1.1 (10) |
TABLE 3.
Most common H flagellar antigen types expressed among 915 ETEC isolates from community-acquired diarrheal episodes
| H type | % of strains (total no. of strains) |
|---|---|
| 18 | 8.7 (80) |
| 12 | 7.4 (68) |
| 45 | 5.6 (51) |
| 4 | 5.5 (50) |
| 10 | 5.1 (47) |
| 16 | 4.7 (43) |
| 9 | 4.4 (40) |
| 5 | 2.4 (22) |
| 7 | 2.2 (20) |
| 20 | 1.9 (17) |
| 25 | 1.9 (17) |
| 19 | 1.8 (16) |
| 30 | 1.6 (15) |
| 11 | 1.4 (13) |
| 1 | 1.3 (12) |
| 41 | 1.3 (12) |
| 40 | 1.2 (11) |
| 49 | 1.2 (11) |
| 2 | 1.1 (10) |
| 28 | 1.1 (10) |
| 6 | 1.1 (10) |
ETEC strains that expressed a commonly identified O serogroup were significantly more likely to also express one of the 12 CFs in our diagnostic test panel. Only 5% (2 of 40) of the strains that expressed a unique O serogroup were CF positive, compared to 24% (61 of 252) of strains that expressed an O serogroup that was detected in 2 to 9 isolates and 55% (283 of 516) of strains characterized by an O group that was detected in 10 or more isolates (P < 0.0001 by a test for trends). Thus, those ETEC strains that displayed a common O group were more likely to have the full genetic package (i.e., both enterotoxin and CF genes) that is required to achieve a pathogenic lifestyle.
The serotypes that were most commonly found in conjunction with each CF type (Table 4) were primarily involved in associations that have been observed for other geographic regions (41), with a few notable exceptions. The most prevalent composite phenotypes included ST CS14 O78:H18, ST CFA/I O128:H12, ST CS1+CS3 O6:H16, ST CFA/I O153:H45, and ST CS6 O169:H41. While O71 has rarely been reported in association with ETEC (4), we identified several ST CFA/I ETEC isolates that displayed serotypes O71:H− and O71:H45. O39:H12, the most common serotype associated with the expression of CS5+CS6, is another phenotype that has heretofore rarely, if ever, been reported from ETEC surveys. Several serotypes that were detected in a number of ETEC isolates did not express any of the CFs in our diagnostic panel, which may be indicative of a lack of in vitro expression or, in some cases, the presence of other known CFs that were not detected by our panel or of heretofore unrecognized CFs.
TABLE 4.
Serotypes and phenotypes identified for multiple diarrhea-associated ETEC isolates
| CF phenotype | Serotype | n | Toxin phenotype (no. of isolates with phenotype) |
|---|---|---|---|
| CFA/I | O128:H12 | 26 | ST (22), LTST (4) |
| O153:H45 | 14 | All ST | |
| O71:H− | 8 | All ST | |
| O71:H45 | 7 | ST (6), LT (1) | |
| CS1+CS3 | O6:H16 | 18 | All ST |
| CS2+CS3 | O6:H− | 6 | ST (5), LTST (1) |
| CS3 | O8:H9 | 8 | LTST (6), ST (2) |
| CS4+CS6 | O25:H− | 6 | All LTST |
| O78:H18 | 3 | All LTST | |
| CS5+CS6 | O39:H12 | 4 | All LTST |
| CS6 | O169:H41 | 10 | All ST |
| O27:H7 | 9 | All ST | |
| O27:H20 | 7 | All ST | |
| O27:H− | 7 | All ST | |
| O159:H− | 7 | All ST | |
| O159:H20 | 3 | All ST | |
| O78:H18 | 3 | All ST | |
| CS7 | O114:H49 | 9 | LT (8), ST (1) |
| CS8 | O25:H− | 4 | All LT |
| CS12 | O68:H12 | 3 | ST (2), LTST (1) |
| O80:H26 | 3 | ST (2), LTST (1) | |
| CS14 | O78:H18 | 34 | ST (33), LTST (1) |
| O78:H− | 8 | All LTST | |
| CS17 | O8:H9 | 3 | All LT |
| No CF detected | O6:H1 | 10 | ST (6), LT (4) |
| O159:H4 | 10 | LT (9), ST (1) | |
| O44:H18 | 9 | ST (5), LT (4) | |
| O114:H− | 9 | ST (5), LTST (4) | |
| O153:H45 | 8 | ST (6), LT (2) | |
| O168:H− | 8 | ST (7), LT (1) | |
| O8:H− | 7 | LT (3), ST (3), LTST (1) | |
| O20:H34 | 7 | All ST | |
| O128:H12 | 7 | All ST | |
| O88:H25 | 6 | LT (5), ST (1) | |
| O2:H4 | 5 | LT (2), ST (2), LTST (1) | |
| O8:H9 | 5 | ST (2), LTST (2), LT (1) | |
| O20:H5 | 5 | ST (4), LT (1) | |
| O77:H18 | 5 | ST (3), LT (2) | |
| O157:H− | 5 | ST (4), LTST (1) |
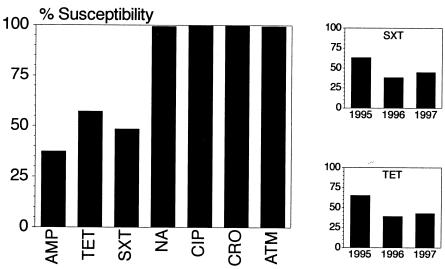
Antimicrobial susceptibility testing of 859 ETEC isolates revealed high levels of resistance to ampicillin (63%), SXT (51%), and tetracycline (43%) (Fig. 1). Few of the isolates displayed resistance to naladixic acid (n = 6), ciprofloxacin (n = 1), and aztreonam (n = 5), while universal susceptibility to ceftriaxone was demonstrated. Resistance rates to ampicillin, tetracycline, or SXT did not vary significantly between ETEC isolates expressing different toxin types or between CF-positive and CF-negative isolates (data not shown). There were no changes in the level of ETEC resistance to ampicillin over time. On the other hand, there was a statistically significant trend towards increasing resistance to SXT and tetracycline observed over the 3-year period, as shown in Fig. 1 (P < 0.001 for a linear trend, comparing SXT and ampicillin proportional susceptibilities over time).
FIG. 1.
Antimicrobial susceptibility of 859 representative ETEC diarrhea-associated strains from Egyptian children under 3 years of age. The graph on the left indicates the susceptibilities of all isolates to different antibiotics, including ampicillin (AMP), tetracycline (TET), trimethoprim-sulfamethoxazole (SXT), naladixic acid (NA), ciprofloxacin (CIP), ceftriaxone (CRO), and aztreonam (ATM). The smaller graphs to the right show the changes in susceptibility to SXT and tetracycline by year (for 1995, n = 271; for 1996, n = 264; for 1997, n = 324) over the 3-year course of the study.
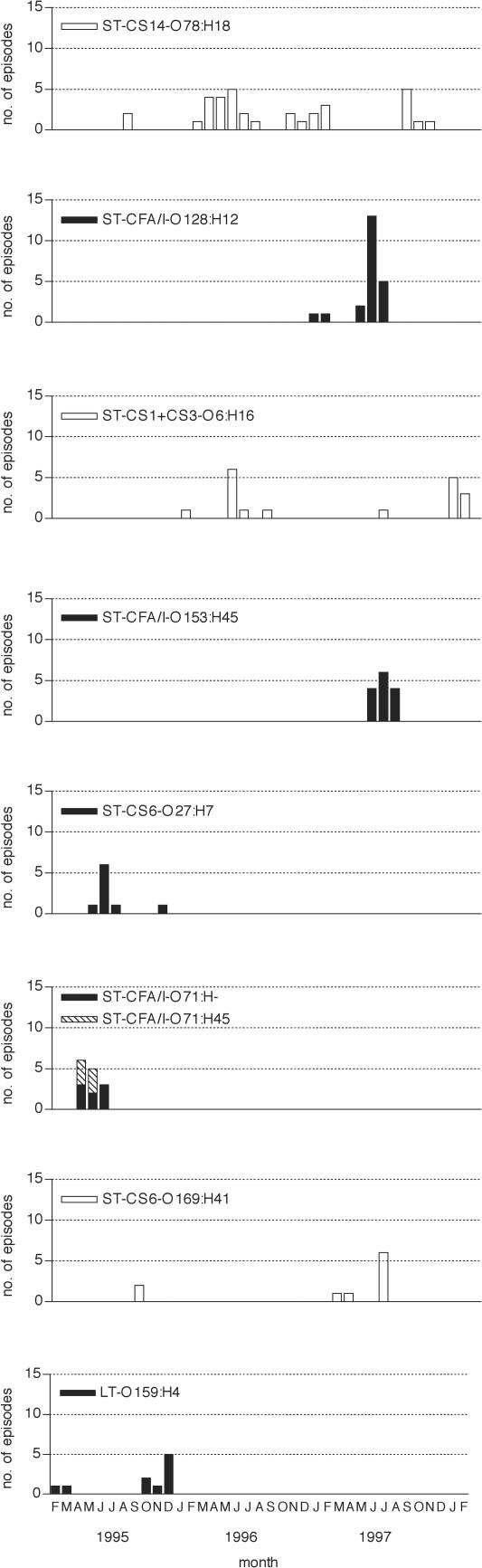
As a surrogate for clonality, we assembled ETEC diarrheal isolates that shared the same composite phenotype (a “superphenotype” comprising the same toxin, CF, and O:H serotype) and plotted the episodes associated with each of the most common superphenotypes. Time plots showed that diarrheal episodes caused by clonally related ETEC strains emerged in temporal clusters mostly during the warm season (Fig. 2). Some appeared transiently in a single season and others reoccurred in successive years. Reflecting the high overall rate of antimicrobial resistance, the majority of ETEC isolates representing all but one clonal group manifested resistance to one or more antibiotics. More than two-thirds of the disease-related isolates displayed triple resistance to ampicillin, SXT, and tetracycline for two of the superphenotypes, namely, ST CS14 O78:H18 ETEC (21 of 31 [68%]) and ST CFA/I O153:H45 ETEC (10 of 14 [71%]).
FIG. 2.
Temporal clustering of childhood diarrheal episodes characterized by the excretion of ETEC with common serotype, toxin, and colonization factor antigen profiles in two rural Egyptian villages. Open bars are shown for phenotypes that appeared in more than 1 year, and filled bars are shown for those that appeared during 1 year only.
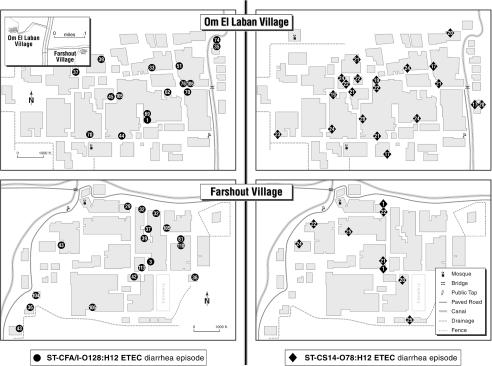
While there were too few episodes associated with any one ETEC superphenotype to perform statistically meaningful cluster analyses, we charted diarrhea episodes associated with the two most common composite phenotypes for descriptive purposes (Fig. 3). For ST CS14 O78:H18 ETEC, the sentinel diarrheal episodes emerged within a few weeks of one another in both villages late in the first warm season of surveillance. In each of the subsequent two warm seasons, a cluster of cases appeared concurrently over several months in both villages. The vast majority of cases appeared in the second year. Diarrheal episodes due to ST CFA/I O128:H12 ETEC were confined to a single season. The initial cases occurred in one village and were followed by a large cluster of cases several weeks later in both villages. The temporal appearance of associated cases did not show any remarkable spatial pattern for either superphenotype. The common usage of interconnected local canals for both social (e.g., laundry and swimming) and sanitation (e.g., bathing) purposes raises the possibility that outbreaks were associated with general contamination of such waterways, although no microbiological investigation was performed to support this prospect.
FIG. 3.
Temporospatial patterns of diarrhea episode appearance for disease associated with the two most common ETEC superphenotypes. Household locations and the weeks of appearances of cases associated with ST CS14 O78:H18 ETEC (denoted by filled circles) are shown in the two village maps on the left, and those associated with ST CFA/I O128:H12 ETEC (denoted by filled diamonds) are shown on the right. The inset (upper left) shows the relative location and distance between the two catchment villages.
DISCUSSION
In Egypt and many other areas of the developing world, ETEC is the most common etiologic agent associated with childhood diarrhea. In the longitudinal study from which the strains described herein were obtained, the overall incidence of ETEC-associated diarrhea in children from birth to 3 years of age was 1.5 episodes per child-year (29). In fact, more than one-quarter of the 3,477 episodes of diarrhea were associated with excretion of this E. coli pathotype. A comprehensive phenotypic characterization of such a large collection of ETEC diarrheal isolates from a population-based study has not previously been performed. This undertaking has provided an informative assessment of the pool of ETEC strains circulating in a geographically restricted population over a 3-year period. Indeed, our findings on toxin and CF distribution as well as antimicrobial resistance validate much of what has been found in smaller studies from this region. One finding of particular interest was that 59% of the ETEC strains did not express any of the 12 CFs represented in our diagnostic test panel, a proportion towards the upper bounds of those observed in other studies in which the same test methods were used (27, 28, 31, 38). As for the distribution of observed serotypes, there was an unusually wide diversity of O antigens and H types represented among the 915 ETEC strains. Finally, an evaluation of diarrheal episodes associated with the most commonly detected composite phenotypes revealed temporal patterns of pathogen persistence that were indicative of a confluence of localized miniepidemics that would not otherwise have been appreciated had it not been for our use of a combination of plasmid and chromosomal markers to identify these apparently clonal pathogenic strains.
The distribution of enterotoxin phenotypes among ETEC diarrheal isolates was similar to those in most previously reported studies of populations in areas where the disease is endemic and of travelers in Egypt and other regional countries in that ST-only ETEC predominated (12, 15, 26, 27, 40, 43). Findings among U.S. military personnel deployed to Egypt and Saudi Arabia represented an exception in that LTST was detected in half of all ETEC diarrheal isolates (42). Our finding that LTST (followed by ST-only) ETEC isolates were most likely to express a CF was consistent with reports from various locations around the world (23, 28, 41). These data, in conjunction with the well-documented association between ST-positive ETEC and symptomatic disease (7, 8, 28, 38), must be taken into account for the development of ETEC vaccine strategies, in which a primary reliance on LT-based antitoxic immunity is disadvantaged wherever there is a preponderance of ST-only ETEC.
CFA/I, CFA/II, and CFA/IV were among the most prevalent CF types detected in this community-based setting, reflecting their historical predominance (11, 41). Regarding discrete antigenic subtypes, CFA/I was the most prevalent CF type, accounting for 10% of all ETEC isolates, followed closely by CS6-only, a phenotype that is now recognized as highly prevalent in many settings (14, 33, 40). This underscores a need to fill in existing gaps in our knowledge of the pathogenic role of CS6, for which nominal evidence exists to substantiate its function as a colonization factor (35) and a clear epidemiologic association with disease has yet to be established. CS14 was the one other CF that was detected in a relatively large proportion of ETEC isolates. Since the introduction of a CS14-specific monoclonal antibody into diagnostic batteries, a high prevalence of this antigen has been documented for Latin America (25) and South Asia (28, 31). In this study, the expression of the ST CS14 O78:H18 phenotype defined the most prevalent composite ETEC phenotype detected in diarrheal isolates, which was associated with a few cases during the first summer season and reappeared with a higher prevalence during the second and third years of the study. Noting that the same composite phenotype has been recognized in Argentina, this phenotype represents either a newly recognized or emergent pathogenic clone of global significance (38).
The conspicuously large proportion of ETEC diarrheal isolates that did not express any of the CFs in our diagnostic panel falls at the upper end of the range of findings from recent studies that employed the same CF test methods, in which 24 to 73% of ETEC isolates from symptomatic children were negative for CF (27, 28, 31, 38). Several explanations may each account, in part, for these findings. Some of the ETEC isolates may have in fact lacked the ability to express CFs in vitro on an artifactual (e.g., loss of positive regulatory or structural genetic elements upon passage) or natural (i.e., a true lack of a CF making for a less fit pathogenic lifestyle) basis. Other known CFs for which routine diagnostic reagents are not yet widely available may represent some proportion of the apparent CF-negative ETEC isolates. Lastly, as yet unidentified CFs may account for some proportion of the CF-negative ETEC isolates. To explain this finding, we have undertaken more detailed analyses of the CF-negative ETEC strains archived from this study to determine the actual reasons for the large proportion of apparently CF-negative isolates.
There is a relative dearth of systematically collected data on ETEC antimicrobial resistance from Egypt and the surrounding region, and the findings herein help to fill this information gap. While their relevance to the treatment of pediatric diarrhea is limited due to recommendations against routine antibiotics for uncomplicated secretory diarrhea (1, 44), the data presented here bear some relevance for regional treatment advice for travelers' diarrhea. The high prevalence of antimicrobial resistance to SXT, ampicillin, and tetracycline reflects well-documented global trends. The low prevalence of quinolone resistance among ETEC diarrheal isolates suggests the validity of using fluoroquinolones as the treatment of choice for diarrhea in travelers to the region. Vigilance in the ongoing collection of antimicrobial resistance data should be maintained, however, in light of recent reports documenting the emergence of quinolone-resistant ETEC (18, 39). The universal sensitivity of ETEC isolates to ceftriaxone in this study suggests that this antibiotic is a rational choice for the treatment of complicated or prolonged childhood diarrhea in which ETEC is a primary etiologic consideration.
A wide variety of serotypes were represented among the ETEC diarrheal isolates obtained from children for this study. Among the most commonly detected serotypes were ones that have been shown to be prevalent elsewhere. These included O128:H12 and O153:H45, which are typically associated with ST or LTST and CFA/I expression, and O6:H16, which is expressed in association with CS1+CS3 (6, 30, 41). All of the CS1+CS3 O6:H16 ETEC strains in this study were found to express ST only, contrasting with the typical expression of both LT and ST (41). Certain other relatively common serotypes in this study have rarely, if ever, been reported in association with ETEC, among which are O71:H− and O71:H45 isolates, most of which expressed CFA/I and ST; and O39, which was associated with different H types, including H12 (the most common serotype associated with the expression of CS5+CS6) (4). A few of the more commonly identified serotypes have been linked with other E. coli pathotypes, such as O44:H18, a typical serotype of enteroaggregative E. coli (21). Interestingly, among the 12 O serogroups that were most commonly detected in this study were 8 (O6, O8, O128, O27, O25, O153, O159, and O20) that were previously shown to be among the 10 most prevalent ETEC serogroups isolated from cases of diarrhea among military personnel deployed to Egypt or Saudi Arabia from 1989 to 1991 (42). This correlation clearly implies that within Egypt and the Middle East, certain pathogenic clones do in fact cocirculate among children and travelers, two distinct populations at high risk for ETEC diarrhea.
We found that ETEC diarrheal strains characterized by the expression of O serogroups that were commonly found in this collection were also more likely to express a CF antigen. This finding indirectly offers some insight about the phenotypic profiles that correlate with pathogen fitness and epidemic disease potential. Given that many of the common O serogroups detected here were those that have been associated with ETEC elsewhere and that these isolates expressed both a toxin and CF more often, these strains appear to be most fit for a pathogenic lifestyle. On the other hand, strains that expressed less common or unique serogroups tended to lack the full package of ETEC virulence determinants and perhaps transiently acquired an enterotoxin plasmid by conjugal transfer in vivo (10). This finding is consistent with published data showing that certain serotypes maintain ETEC virulence plasmids more stably (10). In any case, their lack of association with more than a few cases of childhood diarrhea in this community-based setting may be viewed as an indicator of their lack of robustness as pathogens.
Based on phenotypic analyses of ETEC strains inclusive of determinants that are generally encoded by plasmids (toxin and CF) and the chromosome (O:H), we defined composite phenotypes as a reasonable proxy for clonal relatedness. Using this marker, we tracked the temporal and spatial trends in the appearance of associated diarrheal episodes. Episodes associated with the most prevalent composite phenotypes typically manifested small outbreaks of illness in a single warm season or in successive seasons, almost always involving contemporaneous cases in both study villages (data not shown). These findings suggest that the most prevalent ETEC clones circulate in a village in what appear to be small epidemics. The spatial patterns of occurrence are not highly specific for a given transmission pattern, although they appear to be most consistent with contamination and spread from a common source, such as the local canal system, to which most villagers lives are intimately connected.
In summary, this study represents by far the largest comprehensive phenotypic evaluation of ETEC isolates ever undertaken in the context of a population-based study performed in a circumscribed geographic location over several years. The findings presented here represent the results of an initial survey of strains and their superphenotypes. Further analyses of this valuable collection of strains have been initiated to explore specific hypotheses raised by our findings, the most productive of which to date has been the search for putative new ETEC CFs that may be important in this and other regions of the world (S. B. Khalil, F. J. Cassels, H. I. Shaheen, L. K. Pannel, N. M. El-Ghorab, K. A. Kamal, M. M. Mansour, S. J. Savarino, and L. F. Peruski, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. B/D-58, 1999; S. B. Khalil, F. J. Cassels, H. I. Shaheen, L. K. Pannell, K. A. Kamal, B. T. Pittner, M. Mansour, R. Frenck, S. J. Savarino, and L. F. Peruski, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. B-79, 2000).
>
Acknowledgments
This research received financial support from the former Naval Medical Research and Development Command under Navy Research Work Unit no. 3270, the National Institute of Child Health and Human Development under interagency agreement Y1-HD-0026-01, and the WHO Global Programme for Vaccines and Immunization/Vaccine Research and Development.
The study from which the bacterial strains characterized herein were derived was performed in strict conformity with all ethical guidelines of the U.S. government. Informed consent was obtained from a parent of each subject before screening.
We thank Laila Salah and Iman Touni for their excellent technical assistance and David Deis for preparation of the cartographic illustration.
The opinions and assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the views of the Navy Department, Department of Defense, the U.S. Government, or the Egyptian Ministry of Health and Population.
REFERENCES
- 1.American Academy of Pediatrics. 1996. Practice parameter: the management of acute gastroenteritis in young children. American Academy of Pediatrics, Provisional Committee on Quality Improvement, Subcommittee on Acute Gastroenteritis. Pediatrics 97:424-435. [PubMed] [Google Scholar]
- 2.Barrow, G. I., and R. K. A. Feltham (ed.). 1993. Cowan and Steel's manual for the identification of medical bacteria, 3rd ed., p. 26. Cambridge University Press, Cambridge, United Kingdom.
- 3.Bauer, A. W., M. M. W. Kirby, J. C. Sherris, and M. Turck. 1966. Antibiotic susceptibility testing by a standard single disk method. Am. J. Clin. Pathol. 45:493-496. [PubMed] [Google Scholar]
- 4.Berry, R. J., K. A. Bettelheim, and M. Gracey. 1983. Studies on enterotoxigenic Escherichia coli isolated from persons without diarrhoea in Western Australia. J. Hyg. (London) 90:99-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binsztein, N., M. J. Jouve, G. I. Viboud, L. Lopez-Maral, M. Rivas, I. Orskov, C. Ahren, and A. M. Svennerholm. 1991. Colonization factors of enterotoxigenic Escherichia coli in children with diarrhea in Argentina. J. Clin. Microbiol. 29:1893-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco, J., M. Blanco, E. A. Gonzalez, J. E. Blanco, M. P. Alonso, J. I. Garabal, and W. H. Jansen. 1993. Serotypes and colonization factors of enterotoxigenic Escherichia coli isolated in various countries. Eur. J. Epidemiol. 9:489-496. [DOI] [PubMed] [Google Scholar]
- 7.Clemens, J., S. Savarino, R. Abu-Elyazeed, M. Safwat, M. Rao, T. Wierzba, A. M. Svennerholm, J. Holmgren, R. Frenck, E. Park, and A. Naficy. 2004. Development of pathogenicity-driven definitions of outcomes for a field trial of a killed oral vaccine against enterotoxigenic Escherichia coli in Egypt: application of an evidence-based method. J. Infect. Dis. 189:2299-2307. [DOI] [PubMed] [Google Scholar]
- 8.Echeverria, P., D. N. Taylor, U. Lexsomboon, M. Bhaibulaya, N. R. Blacklow, K. Tamura, and R. Sakazaki. 1989. Case-control study of endemic diarrheal disease in Thai children. J. Infect. Dis. 159:543-548. [DOI] [PubMed] [Google Scholar]
- 9.Evans, D. G., D. J. Evans, Jr., and W. Tjoa. 1977. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect. Immun. 18:330-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans, D. J., Jr., D. G. Evans, H. L. DuPont, F. Orskov, and I. Orskov. 1977. Patterns of loss of enterotoxigenicity by Escherichia coli isolated from adults with diarrhea: suggestive evidence for an interrelationship with serotype. Infect. Immun. 17:105-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaastra, W., and A. M. Svennerholm. 1996. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 4:444-452. [DOI] [PubMed] [Google Scholar]
- 12.Goldhar, J., R. Peri, R. Zilberberg, and M. Lahav. 1980. Enterotoxigenic Escherichia coli (ETEC) isolated in the Tel-Aviv (Israel) area. Med. Microbiol. Immunol. 169:53-61. [DOI] [PubMed] [Google Scholar]
- 13.Huilan, S., L. G. Zhen, M. M. Mathan, M. M. Mathew, J. Olarte, R. Espejo, U. Khin Maung, M. A. Ghafoor, M. A. Khan, Z. Sami, et al. 1991. Etiology of acute diarrhoea among children in developing countries: a multicentre study in five countries. Bull. W. H. O. 69:549-555. [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang, Z. D., B. Lowe, M. P. Verenkar, D. Ashley, R. Steffen, N. Tornieporth, F. von Sonnenburg, P. Waiyaki, and H. L. DuPont. 2002. Prevalence of enteric pathogens among international travelers with diarrhea acquired in Kenya (Mombasa), India (Goa), or Jamaica (Montego Bay). J. Infect. Dis. 185:497-502. [DOI] [PubMed] [Google Scholar]
- 15.Katouli, M., A. Jaafari, A. A. Farhoudi-Moghaddam, and G. R. Ketabi. 1990. Aetiological studies of diarrhoeal diseases in infants and young children in Iran. J. Trop. Med. Hyg. 93:22-27. [PubMed] [Google Scholar]
- 16.Lior, H. 1996. Classification of Escherichia coli, p. 31-72. In C. L. Gyles (ed.), Escherichia coli in domestic animals and humans. CAB International, Wallingford, United Kingdom.
- 17.Lopez-Vidal, Y., and A. M. Svennerholm. 1990. Monoclonal antibodies against the different subcomponents of colonization factor antigen II of enterotoxigenic Escherichia coli. J. Clin. Microbiol. 28:1906-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushita, S., M. Kawamura, M. Takahashi, K. Yokoyama, N. Konishi, K. Hatakeyama, A. Kai, S. Morozumi, K. Morita, N. Watanabe, M. Kanamori, and Y. Kudoh. 2001. Increasing fluoroquinolone low-sensitivity in enterotoxigenic Escherichia coli isolated from diarrhea of overseas travelers in Tokyo. Kansenshogaku Zasshi 75:785-791. [DOI] [PubMed] [Google Scholar]
- 19.McConnell, M. M., H. Chart, A. M. Field, M. Hibberd, and B. Rowe. 1989. Characterization of a putative colonization factor (PCFO166) of enterotoxigenic Escherichia coli of serogroup O166. J. Gen. Microbiol. 135:1135-1144. [DOI] [PubMed] [Google Scholar]
- 20.Nagy, B., and P. Z. Fekete. 1999. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet. Res. 30:259-284. [PubMed] [Google Scholar]
- 21.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NCCLS. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A2, 3rd ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 23.Nirdnoy, W., O. Serichatalergs, A. Cravioto, C. LeBron, M. Wolf, C. W. Hoge, A. M. Svennerholm, D. N. Taylor, and P. Echeverria. 1997. Distribution of colonization factor antigens among enterotoxigenic Escherichia coli strains isolated from patients with diarrhea in Nepal, Indonesia, Peru, and Thailand. J. Clin. Microbiol. 35:527-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orskov, F., and I. Orskov. 1975. Escherichia coli O:H serotypes isolated from human blood. Prevalence of the K1 antigen with technical details of O and H antigenic determination. Acta Pathol. Microbiol. Scand. 83(Suppl.):595-600. [PubMed] [Google Scholar]
- 25.Paniagua, M., F. Espinoza, M. Ringman, E. Reizenstein, A. M. Svennerholm, and H. Hallander. 1997. Analysis of incidence of infection with enterotoxigenic Escherichia coli in a prospective cohort study of infant diarrhea in Nicaragua. J. Clin. Microbiol. 35:1404-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peltola, H., A. Siitonen, H. Kyronseppa, I. Simula, L. Mattila, P. Oksanen, M. J. Kataja, and M. Cadoz. 1991. Prevention of travellers' diarrhoea by oral B-subunit/whole-cell cholera vaccine. Lancet 338:1285-1289. [DOI] [PubMed] [Google Scholar]
- 27.Peruski, L. F., Jr., B. A. Kay, R. A. El-Yazeed, S. H. El-Etr, A. Cravioto, T. F. Wierzba, M. Rao, N. El-Ghorab, H. Shaheen, S. B. Khalil, K. Kamal, M. O. Wasfy, A. M. Svennerholm, J. D. Clemens, and S. J. Savarino. 1999. Phenotypic diversity of enterotoxigenic Escherichia coli strains from a community-based study of pediatric diarrhea in periurban Egypt. J. Clin. Microbiol. 37:2974-2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qadri, F., S. K. Das, A. S. Faruque, G. J. Fuchs, M. J. Albert, R. B. Sack, and A. M. Svennerholm. 2000. Prevalence of toxin types and colonization factors in enterotoxigenic Escherichia coli isolated during a 2-year period from diarrheal patients in Bangladesh. J. Clin. Microbiol. 38:27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao, M. R., R. Abu-Elyazeed, S. J. Savarino, A. B. Naficy, T. F. Wierzba, I. Abdel-Messih, H. Shaheen, R. W. Frenck, A. M. Svennerholm, and J. D. Clemens. 2003. High disease burden due to enterotoxigenic Escherichia coli diarrhea in early life among rural Egyptian children. J. Clin. Microbiol. 41:4862-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reis, M. H., A. F. Castro, M. R. Toledo, and L. R. Trabulsi. 1979. Production of heat-stable enterotoxin by the O128 serogroup of Escherichia coli. Infect. Immun. 24:289-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sommerfelt, H., H. Steinsland, H. M. S. Grewal, G. I. Viboud, N. Bhandari, W. Gaastra, A. M. Svennerholm, and M. K. Bhan. 1996. Colonization factors of enterotoxigenic Escherichia coli isolated from children in north India. J. Infect. Dis. 174:1142. [DOI] [PubMed] [Google Scholar]
- 32.Steinsland, H., P. Valentiner-Branth, H. M. Grewal, W. Gaastra, K. K. Molbak, and H. Sommerfelt. 2003. Development and evaluation of genotypic assays for the detection and characterization of enterotoxigenic Escherichia coli. Diagn. Microbiol. Infect. Dis. 45:97-105. [DOI] [PubMed] [Google Scholar]
- 33.Steinsland, H., P. Valentiner-Branth, M. Perch, F. Dias, T. K. Fischer, P. Asby, K. Molbak, and H. Sommerfelt. 2002. Enterotoxigenic Escherichia coli infections and diarrhea in a cohort of young children in Guinea-Bissau. J. Infect. Dis. 186:1740-1747. [DOI] [PubMed] [Google Scholar]
- 34.Svennerholm, A. M., and J. Holmgren. 1978. Identification of Escherichia coli heat-labile enterotoxin by means of a ganglioside immunosorbent assay (GM1-ELISA) procedure. Curr. Microbiol. 1:19-23. [Google Scholar]
- 35.Svennerholm, A. M., Y. L. Vidal, J. Holmgren, M. M. McConnell, and B. Rowe. 1988. Role of PCF8775 antigen and its coli surface subcomponents for colonization, disease, and protective immunogenicity of enterotoxigenic Escherichia coli in rabbits. Infect. Immun. 56:523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svennerholm, A. M., M. Wikstrom, M. Lindblad, and J. Holmgren. 1986. Monoclonal antibodies against Escherichia coli heat-stable toxin (STa) and their use in a diagnostic ST ganglioside GM1-enzyme-linked immunosorbent assay. J. Clin. Microbiol. 24:585-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viboud, G. I., N. Binsztein, and A. M. Svennerholm. 1993. Characterization of monoclonal antibodies against putative colonization antigens of enterotoxigenic Escherichia coli and their use in an epidemiological study. J. Clin. Microbiol. 31:558-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viboud, G. I., M. J. Jouve, N. Binsztein, M. Vergara, M. Rivas, M. Quiroga, and A. M. Svennerholm. 1999. Prospective cohort study of enterotoxigenic Escherichia coli infections in Argentinean children. J. Clin. Microbiol. 37:2829-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vila, J., M. Vargas, J. Ruiz, M. Corachan, M. T. Jimenez De Anta, and J. Gascon. 2000. Quinolone resistance in enterotoxigenic Escherichia coli causing diarrhea in travelers to India in comparison with other geographical areas. Antimicrob. Agents Chemother. 44:1731-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willshaw, G. A., T. Cheasty, B. Rowe, H. R. Smith, D. N. Faithfull-Davies, and T. G. Brooks. 1995. Isolation of enterotoxigenic Escherichia coli from British troops in Saudi Arabia. Epidemiol. Infect. 115:455-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf, M. K. 1997. Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin. Microbiol. Rev. 10:569-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolf, M. K., D. N. Taylor, E. C. Boedeker, K. C. Hyams, D. R. Maneval, M. M. Levine, K. Tamura, R. A. Wilson, and P. Echeverria. 1993. Characterization of enterotoxigenic Escherichia coli isolated from U.S. troops deployed to the Middle East. J. Clin. Microbiol. 31:851-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolk, M., E. Ohad, R. Shafran, I. Schmid, and E. Jarjoui. 1997. Enterotoxigenic Escherichia coli (ETEC) in hospitalised Arab infants from Judea area—west bank, Israel. Public Health 111:11-17. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. 1995. The treatment of diarrhoea: a manual for physicians and other senior health workers, W.H.O./CDR/95.3 ed. World Health Organization, Geneva, Switzerland.