Abstract
Membrane lipid composition is altered in the brain during the pathogenesis of several age-related neurodegenerative diseases, including Alzheimer’s disease. The entorhinal cortex is one of the first regions of the brain to display the neuropathology typical of Alzheimer’s disease, yet little is known about the changes that occur in membrane lipids within this brain region during normal aging (i.e., in the absence of dementia). In the present study, the phospholipid composition of mitochondrial and microsomal membranes from human entorhinal cortex was examined for any changes over the adult lifespan (18–98 years). Overall, changes in several molecular phospholipids were seen with age in the entorhinal cortex across both membranes. The proportion of total phosphatidylcholine within the mitochondrial fraction increased within the entorhinal cortex with age, while total mitochondrial phosphatidylethanolamine decreased. Many mitochondrial phosphatidylethanolamines containing docosahexaenoic acid increased with age; however, this did not translate into an overall age-related increase in total mitochondrial docosahexaenoic acid. The most abundant phospholipid present within the human brain, PC 16:0_18:1, also increased with age within the mitochondrial membranes of the entorhinal cortex. When compared to other regions of the brain, the phospholipid composition of the entorhinal cortex remains relatively stable in adults over the lifespan in the absence of dementia.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-017-9961-2) contains supplementary material, which is available to authorized users.
Keywords: Lipids, DHA, Docosahexaenoic acid, Phosphatidylcholine, Phosphatidylethanolamine; mass spectrometry, Lipidomics
Introduction
Phospholipids are essential components of the human brain, comprising a quarter of its dry mass (O’Brien and Sampson 1965). Phospholipids have a high capacity for structural variety within membranes, with different permutations of head group and fatty acids generating up to 10,000 theoretical molecular species (Yetukuri et al. 2008). Phospholipid composition is highly altered within many regions of the human brain in Alzheimer’s disease (AD) (Kosicek and Hecimovic 2013), often within the earliest stages (Han 2005). The incidence of AD is highly correlated with advanced age, but despite this relationship, little is known about what changes occur to the phospholipid composition of the human brain over the adult lifespan.
The entorhinal cortex (EC) is located within the medial temporal lobe in the anterior portion of the parahippocampal gyrus and forms the interface between the hippocampal formation and associated neocortical regions. Superficial layers of the EC project to the dentate gyrus and hippocampus, forming the perforant pathway involved in encoding episodic memory (Gallagher and Koh 2011). The neurofibrillary tangles typical of AD are deposited within the EC in the earliest stages of the disease (Braak and Braak 1991). Alongside this neuropathology, the EC experiences significant atrophy that is detectable even in the preclinical stages of AD (Duara et al. 2015; Schröder and Pantel 2016), as well as a substantial loss in neuron number and density (Gómez-Isla et al. 1996; Price et al. 2001; von Gunten et al. 2005; von Gunten et al. 2006). In respect to phospholipids, Chan et al. (2012) observed numerous changes to phospholipid species in the EC with AD. Changes to the phospholipid composition of the entire parahippocampal gyrus with AD have also been reported, with alterations in several polyunsaturated fatty acids being observed (Skinner et al. 1993; Prasad et al. 1998).
Considerably less is known about what occurs to the EC during normal aging (i.e., in the absence of dementia). Several studies have reported substantial declines in cortical volume and/or thickness with age (Jiang et al. 2014; Fjell et al. 2014), but the rate of atrophy within this region is thought to be lower than other related regions such as the hippocampus (Raz et al. 2004; Raz et al. 2005). Recent studies have observed age-related alterations in the composition of phospholipids within the mitochondrial and microsomal membranes of the human prefrontal cortex (Norris et al. 2015) and hippocampus (Hancock et al. 2015), but to date, no studies have examined the changes occurring in the phospholipid composition of the EC during normal aging. Given the important role that the EC plays in the pathogenesis of AD, understanding any age-related changes occurring within this brain region could potentially lead to a better understanding of the mechanisms underlying the development of AD.
Therefore, the aim of this study was to establish a baseline on the age-related changes occurring to phospholipids of the EC in neurologically normal humans aged 18–98. The lipidomic workflow used allowed us to characterize any age-related changes to EC phospholipids at the fatty acyl/alkyl positional level. Additionally, the tissue was fractionated into mitochondria-enriched and microsomal membranes (plasma membrane and endomembranous system consisting of the endoplasmic reticulum, Golgi, etc.) to isolate any age-related changes to a particular subcellular compartment.
Methods
Brain tissue
Neurologically normal frozen postmortem brain tissue from the EC was obtained from the New South Wales Brain Tissue Resource Centre at the University of Sydney, Australia, with detailed demographics of the cohort being previously published (Norris et al. 2015). The status of donor brains was assessed by the brain tissue resource centre as outlined in Sutherland et al. (2016). All donors over the age of 60 underwent a modified Braak staging screen, with either stage I or II being observed in most of the elderly donors (Supplementary table S1). Due to the loss of one sample in this region, there were 35 samples in the EC cohort (11 female, 24 male) with an average age of 57.6 years (±3.6, range 18–98). No differences were observed between brain pH or postmortem interval between the two sexes, but females were significantly older than males (68.5 ± 6.9 years versus 52.7 ± 3.9 respectively, p < 0.05). All experiments were conducted with the approval of the Human Research Ethics Committee of the University of Wollongong (HE11/267).
Subcellular fractionation and lipids extraction
Subcellular fractionation followed that previously reported (Norris et al. 2015; Hancock et al. 2015). Briefly, 100 mg of frozen, pulverized tissue was homogenized by a bead homogenizer (FastPrep®-24, MP Biomedicals, NSW, Australia) at 6.0 m/s for 40 s using 1.4-mm zirconium oxide beads in 2 mL of an ice-cold 20 mM Tris buffer (pH 7.4) containing 250 mM sucrose, 2 mM EDTA, 2 mM DTT, and complete protease inhibitor. Large cellular debris and nuclei were removed from each sample by a short centrifuge step (10 min, 1000×g). The supernatant was then sequentially centrifuged to obtain a mitochondria-enriched (35 min, 10,000×g, 4 °C) and microsomal (mixed membrane) pellet (40 min, 100,000×g, 4 °C). Total protein was determined for both the mitochondrial and microsomal fractions (BCA Protein Assay Kit, Thermo Fisher Scientific, VIC, Australia). Lipids were extracted from 75 μg of protein as described previously (Deeley et al. 2008) in the presence of internal standards (20 μM of PC 19:0/19:0, PE 17:0/17:0 and PS 17:0/17:0; 10 μM of lyso-PC 17:0 and lyso-PE 14:0). Extracted lipids were stored in chloroform: methanol (1 mL, 1:2 v/v with 0.01% butylated hydroxytoluene) at −20 °C until analysis.
Mass spectrometry
Nano-electrospray ionization mass spectrometry of lipid extracts was performed using a hybrid triple quadrupole linear ion trap mass spectrometer (QTRAP® 5500 SCIEX, MA, USA) equipped with an automated chip-based nanoelectrospray source (TriVersa Nanomate™, Advion Biosciences, NY, USA) using parameters and targeted ion scans described previously (Norris et al. 2015). Phospholipids were quantified from internal standards by Lipidview™ software (version 1.2, SCIEX, MA, USA). Briefly, phospholipids at the sum composition level (i.e., head group and sum fatty acid composition) were quantified from positive precursor ion (PC, m/z 184) and neutral loss (PE, 141 Da; PS, 185 Da) scans, and molecular phospholipids (i.e., head group and fatty acids) were determined from complementary negative precursor ion scans for fatty acids (Norris et al. 2015). This method cannot determine sn position of fatty acids (i.e., their position on the glycerol backbone), and so phospholipid structure was reported with an underscore separating the two fatty acids as recommended by Liebisch et al. (2013).
The mass spectrometry method used in this study was also unable to distinguish alkyl and plasmenyl ether isobars, and so these are reported as being monounsaturated alkyl ether phospholipids. Due to this, no correction factor for PE-plasmenyl ethers was applied (Berry and Murphy 2004), and any PE-plasmenyl ethers will be underestimated by approximately 29% (Mitchell et al. 2007). Small amounts of saturated odd-chain acyl isobars were detected alongside all reported alkyl ether phospholipids. The exact proportion of each isobar could not be determined quantitatively, and so these isobars were reported as being potentially either molecular phospholipid.
Statistical analysis
Statistical analysis was performed using SPSS Statistics (version 19, IBM Corp., NY, USA) and R (v. 3.1.1). The Wilcoxon signed-rank test was used to compare the amount within phospholipid classes between the two membrane fractions. Linear regression was performed between phospholipids and age with sex as a second independent variable, with a significance level, was set at p < 0.05. Influential data points were identified by a Cook’s distance of greater than one and were removed from the analysis. Normality of the dependent variable was assessed by examining histograms of the residuals. Outliers were identified as being more than three standard deviations from the mean by examining histograms of the residuals and were first treated by transformation of the dependent variable or by removal from analysis if transformation was unsuccessful or violated the assumptions of normality. Values reported for ages 20 and 100 are derived from the slope of the regression line. Parameters for all statistically significant linear regression results are included in the supplementary material (Table S2–4).
Results
Phospholipid composition of mitochondrial and microsomal membranes
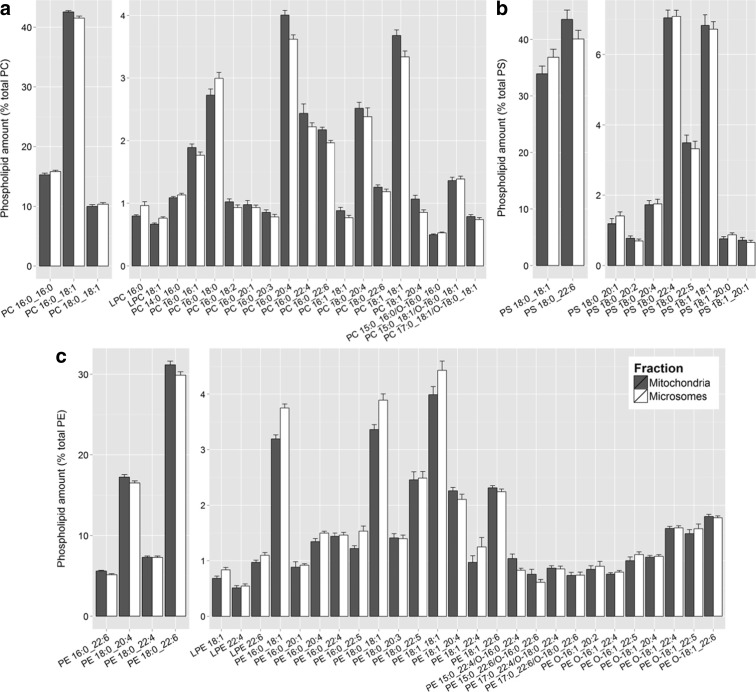
Examination of the phospholipid composition of the mitochondrial and microsomal membranes revealed a similar profile of molecular phospholipids between the two fractions (Fig. 1). Over half of the phospholipid present within each class was made up of only two to four molecular phospholipids. These highly abundant molecular phospholipids did not differ significantly in amount between the two membrane fractions. Indeed, most of the differences present between the mitochondrial and microsomal membranes were small and occurred in molecular phospholipids comprising less than 5% of each phospholipid class.
Fig. 1.
Phospholipids detected within PC (a), PS (b), and PE (c) in the mitochondrial (dark gray) and microsomal (white) membranes of the human entorhinal cortex (as a percent of total phospholipid within each class). Phospholipids were quantified as described in materials and methods. Values are mean across the cohort ± SEM
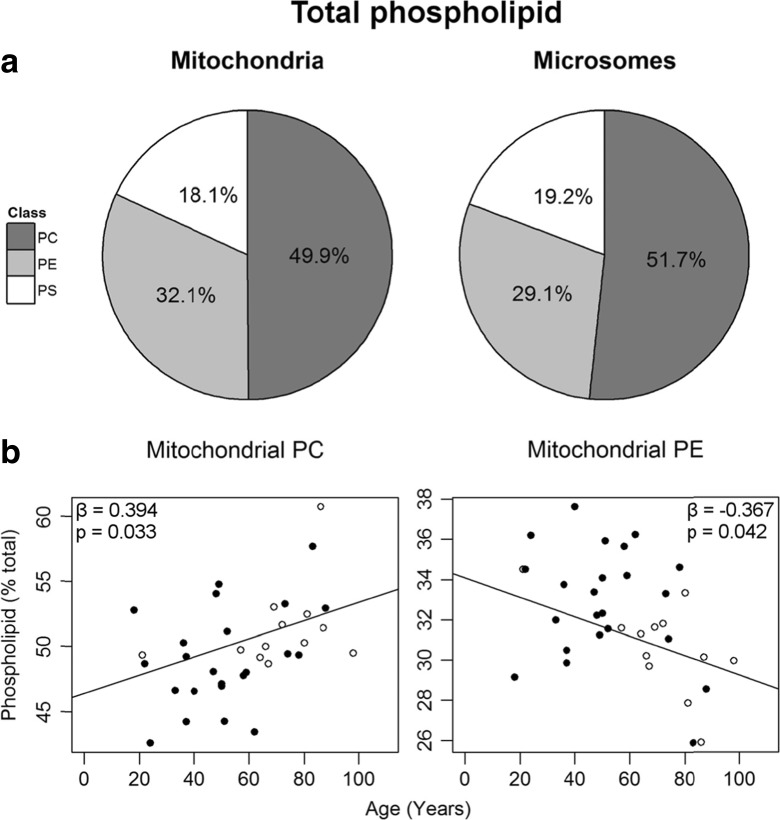
Small, statistically significant differences were seen between the two membranes in the proportion of total phospholipid within each class (Fig. 2a, Table S2). Mitochondrial membranes contained more PE than microsomal (29.1 ± 0.4% vs. 32.1 ± 0.5%; p < 0.001), while the microsomes had more PC (49.9 ± 0.6% vs. 51.7 ± 0.6%; p < 0.005). No differences were observed between the two membrane fractions for total PS (18.1 ± 0.5% vs. 19.2 ± 0.4%).
Fig. 2.
Phospholipid classes within the mitochondrial and microsomal membranes of the entorhinal cortex. a The percent composition of each class within the mitochondrial (left) and microsomal (right) fractions. Values shown are the mean for the entire cohort. b Two phospholipid classes changed significantly with age within the mitochondrial membranes (n = 35), mitochondrial PC (left) and mitochondrial PE (right). Regression model was adjusted for sex: males (black circle), females (white circle), and parameters are available in Table S2
Changes in mitochondrial phospholipid with age
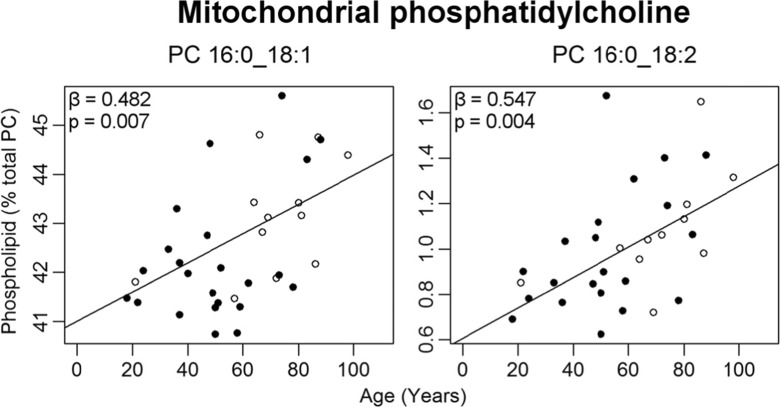
Two phospholipid classes changed in abundance with age within the mitochondrial membranes (Fig. 2b). PC increased by 12% over the 80-year period, while PE declined by 11%. Several mitochondrial molecular phospholipids also changed in abundance with age within PC and PE only (Table S3). An age-related increase was observed in the most abundant phospholipid of the mitochondrial fraction, PC 16:0_18:1, which increased from 42% of total mitochondrial PC at age 20 to 44% at age 100 (Fig. 3). Due to the high abundance of this phospholipid, this represents one of the largest age-related changes for any single mitochondrial phospholipid in the human EC. PC 16:0_18:2 was the only other PC to change with age in this fraction, increasing from 0.7 to 1.3% from ages 20 to 100.
Fig. 3.
Mitochondrial PCs changing significantly with age (as a percent of total PC) in normal human entorhinal cortex (n = 30–35). Regression model was adjusted for sex: males (black circle), females (white circle). Regression parameters are available in the Table S3
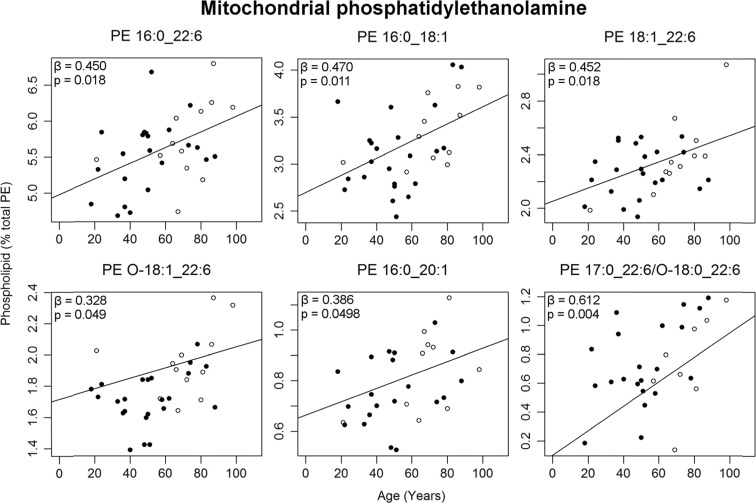
Six mitochondrial PEs increased in abundance over the 80-year period, with four of these containing a 22:6 fatty acid: PE 16:0_22:6, PE 18:1_22:6, PE O-18:1_22:6, and PE 17:0_22:6/O-18:0_22:6 (Fig. 4). The most abundant of this type, PE 16:0_22:6, increased by 17% from ages 20 to 100. The remaining two PEs to increase with age in this fraction were PE 16:0_18:1 by 25% and PE 16:0_20:1 by 30%.
Fig. 4.
Mitochondrial PEs changing significantly with age (as a percent of total PE) in normal human entorhinal cortex (n = 30–35). Regression model was adjusted for sex: males (black circle), females (white circle). Regression parameters are available in Table S3
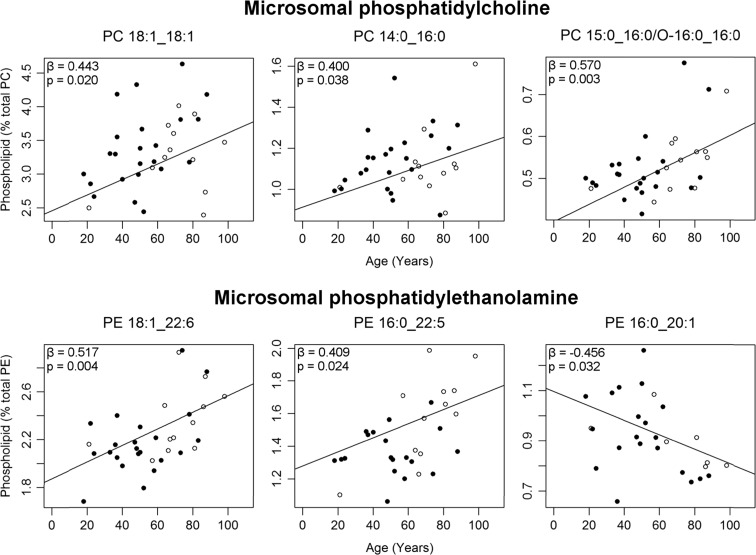
Changes in microsomal phospholipid with age
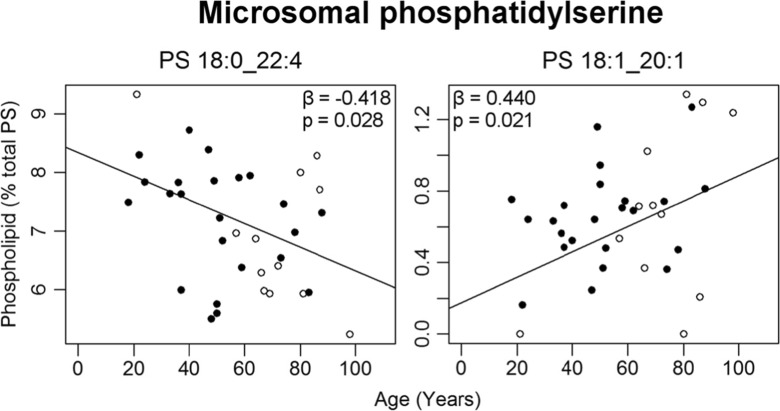
There were no age-related changes seen in the total amount of any phospholipid class within the microsomal membranes. A total of eight molecular phospholipids changed with age within the EC microsomes (Table S4). Three PCs increased with age: PC 18:1_18:1 by 4%, PC 14:0_16:0 by 23%, and PC 15:0_16:0/O-16:0_16:0 by 38% from ages 20 to 100 (Fig. 5). Within PE, both PE 18:1_22:6 and PE 16:0_22:5 increased with age while PE 16:0_20:1 decreased over the 80-year period. In contrast to the mitochondrial membranes, two microsomal PS phospholipids changed with age: PS 18:0_22:4 decreased by 20% from ages 20 to 100, whereas PS 18:1_20:1 increased by 180% (Fig. 6). While a seemingly large age-related increase was reported PS 18:1_20:1, this phospholipid is of low abundance in microsomal membranes making up less than 0.7% of total PS (Fig. 1).
Fig. 5.
Microsomal PCs (top) and PEs (bottom) changing significantly with age (as a percent of total PC and PE respectively) in normal human entorhinal cortex (n = 30–35). Regression model was adjusted for sex: males (black circle), females (white circle). Regression parameters are available in Table S4
Fig. 6.
Microsomal PSs changing significantly with age (as a percent of total PS) in normal human entorhinal cortex (n = 30–35). Regression model was adjusted for sex: males (black circle), females (white circle). Regression parameters are available in Table S4
Discussion
Overall, several changes occurred to molecular phospholipids within the mitochondrial and microsomal membrane of the EC with age; however, these were fewer in number compared to that recently reported for the dorsolateral prefrontal cortex (Norris et al. 2015) and hippocampus (Hancock et al. 2015). At present, no other studies of age-related changes in phospholipid composition of the EC exist, preventing any comparisons from being made.
The phospholipid that underwent the largest age-related increase within the EC was mitochondrial PC 16:0_18:1, the most abundant phospholipid present within this membrane fraction (Fig. 1). This phospholipid was also previously found to increase with age within the mitochondria of the prefrontal cortex (Norris et al. 2015) and hippocampus (Hancock et al. 2015). Increased amounts of this phospholipid can attenuate the oxidation of polyunsaturated fatty acids within model membranes (Cortie and Else 2015), a mechanism that may support longevity (Dei et al. 2002; Montine et al. 2011; Guest et al. 2014). Additionally, PC 18:1/16:0 is enriched in the protrusion tips of PC12 and neuro-2A cultures as well as in the neuronal synapses of mouse brain (Kuge et al. 2014), indicating that this phospholipid could be important for establishing and maintaining neural connections. However, the lipidomics method used in the present study was unable to separate these two isomers (PC 16:0/18:1 versus PC 18:1/16:0), and therefore understanding the role that PC 18:1/16:0 may play in the aging brain will require further investigation.
Within the present work, a number of phospholipids containing a 22:6 fatty acid increased with age within the EC, including mitochondrial PE 16:0_22:6, PE O-18:1_22:6, and PE 17:0_22:6/O-18:0_22:6, as well as both mitochondrial and microsomal PE 18:1_22:6 (Tables S2–3). Despite these changes to individual phospholipids with age, no significant changes were seen with age in total 22:6 (data not shown). The 22:6 fatty acid within these phospholipids can be putatively identified as docosahexaenoic acid (DHA), the principal omega-3 fatty acid present in the brain. DHA is important in the developing brain, accumulating in the earliest months of life (Martínez and Mougan 1998). DHA has a number of pro-cell survival roles within cell membranes, including the production of anti-inflammatory D-series resolvins and neuroprotectins (Weylandt et al. 2012). DHA is known to be severely reduced in many regions of the brain affected by AD pathology (Söderberg et al. 1991; Martín et al. 2010; Igarashi et al. 2011; Cunnane et al. 2012; Fabelo et al. 2014); however, there are very few studies of this type looking specifically at the changes to the EC. Only a single study has examined the EC for changes in its phospholipids in AD, finding no changes in either total PC, PE or PS (Chan et al. 2012). Two further studies examined the parahippocampal gyrus (Skinner et al. 1993; Prasad et al. 1998), but only Prasad et al. (1998) observed a loss of DHA with AD within the PE class of phospholipids. Although the current evidence is relatively sparse, if the loss of PE-DHA in the EC and greater parahippocampal gyrus is indeed involved in the progression of AD, then the increase in PE-DHA during normal aging could be protective against further development of AD neuropathology; however, much work remains to be done to explore this theory.
Considering that decreases in DHA with AD have been documented in many affected regions of the brain, the question remains as to how to maintain general levels of DHA within the brain over the lifespan. The inefficient conversion of the long-chain omega-3 fatty acids DHA and eicosapentaenoic acid (EPA, 20:5 n-3) from shorter chain precursors in humans suggests that both should be considered to essential dietary fatty acids (Parletta et al. 2013). But supplementation with DHA and/or EPA in the healthy elderly, elderly with mild cognitive impairment, and elderly with neurodegenerative disease have shown mixed results regarding improvement or retention of cognitive status (Sydenham et al. 2012; Janssen and Kiliaan 2014; Burckhardt et al. 2016). More recently it has been suggested that dietary consumption of seafood as a source of DHA may be more important for prevention of AD than supplementation, with APOEε4 carriers who consumed seafood at least once per week showing a lower burden of neuritic plaques and neurofibrillary tangles at death (Morris et al. 2016). However, the discrepancies between intervention studies could also be the product of an alteration to DHA metabolism even in normal, healthy adults with advanced age (Astarita et al. 2010; Plourde et al. 2011). Needless to say, much work remains to be done in this area to determine the best way of maintaining DHA levels within the brain over the lifespan.
In summary, although many molecular phospholipids changed with age within the mitochondrial and microsomal membranes of the EC, the number of molecular phospholipids affected was far fewer than those reported for other brain regions. PC 16:0_18:1, a phospholipid which may be involved in the growth and maintenance of neuronal synapses as well as the attenuation of peroxidation within the membrane, showed substantial age-related increases within the mitochondrial membranes. We also observed an increase in several DHA-containing PE phospholipids within the mitochondrial membranes of the EC, but no age-related change in total DHA levels was observed. Despite this, the maintenance of PE-DHA levels within the entorhinal cortex over the adult lifespan could be neuroprotective. Overall, these findings suggest that the phospholipid composition of the EC remains relatively stable in adults over the course of normal aging without dementia.
Electronic supplementary material
(DOCX 31 kb)
Abbreviations
- AD
Alzheimer’s disease
- DHA
Docosahexaenoic acid
- EC
Entorhinal cortex
- PC
Phosphatidylcholine
- PE
Phosphatidylethanolamine
- PS
Phosphatidylserine
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
All experiments were conducted with the approval of the Human Research Ethics Committee of the University of Wollongong (HE11/267).
Funding
This work was supported by a grant from the National Health and Medical Research Council of Australia (1008667). TWM is supported by an Australia Research Council Future Fellowship (FT110100249). Human brain tissue was received from the New South Wales Tissue Resource Centre at the University of Sydney, which is supported by the National Health and Medical Research Council of Australia, Schizophrenia Research Institute, and the National Institute of Alcohol Abuse and Alcoholism (NIH (NIAAA) R24AA012725).
References
- Astarita G, Jung K-M, Berchtold NC, Nguyen VQ, Gillen DL, Head E, Cotman CW, Piomelli D. Deficient liver biosynthesis of docosahexaenoic acid correlates with cognitive impairment in Alzheimer’s disease. PLoS One. 2010;5:e12538. doi: 10.1371/journal.pone.0012538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry KAZ, Murphy RC. Electrospray ionization tandem mass spectrometry of glycerophosphoethanolamine plasmalogen phospholipids. J Am Soc Mass Spectrom. 2004;15:1499–1508. doi: 10.1016/j.jasms.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Burckhardt M, Herke M, Wustmann T, Watzke S, Langer G, Fink A (2016) Omega-3 fatty acids for the treatment of dementia. In: Cochrane database of systematic reviews. Wiley [DOI] [PMC free article] [PubMed]
- Chan RB, Oliveira TG, Cortes EP, Honig LS, Duff KE, Small SA, Wenk MR, Shui G, Di Paolo G. Comparative lipidomic analysis of mouse and human brain with Alzheimer disease. J Biol Chem. 2012;287:2678–2688. doi: 10.1074/jbc.M111.274142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortie CH, Else PL. An antioxidant-like action for non-peroxidisable phospholipids using ferrous iron as a peroxidation initiator. Biochim Biophys Acta BBA - Biomembr. 2015;1848:1303–1307. doi: 10.1016/j.bbamem.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Cunnane SC, Schneider JA, Tangney C, Tremblay-Mercier J, Fortier M, Bennett DA, Morris MC. Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis JAD. 2012;29:691–697. doi: 10.3233/JAD-2012-110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeley JM, Mitchell TW, Wei X, Korth J, Nealon JR, Blanksby SJ, Truscott RJW. Human lens lipids differ markedly from those of commonly used experimental animals. Biochim Biophys Acta. 2008;1781:288–298. doi: 10.1016/j.bbalip.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Dei R, Takeda A, Niwa H, Li M, Nakagomi Y, Watanabe M, Inagaki T, Washimi Y, Yasuda Y, Horie K, Miyata T, Sobue G. Lipid peroxidation and advanced glycation end products in the brain in normal aging and in Alzheimer’s disease. Acta Neuropathol (Berl) 2002;104:113–122. doi: 10.1007/s00401-002-0523-y. [DOI] [PubMed] [Google Scholar]
- Duara R, Barker W, Loewenstein D, Greig MT, Rodriguez R, Goryawala M, Zhou Q, Adjouadi M. Insights into cognitive aging and Alzheimer’s disease using amyloid PET and structural MRI scans. Clin Transl Imaging. 2015;3:65–74. doi: 10.1007/s40336-015-0110-6. [DOI] [Google Scholar]
- Fabelo N, Martín V, Marín R, Moreno D, Ferrer I, Díaz M. Altered lipid composition in cortical lipid rafts occurs at early stages of sporadic Alzheimer’s disease and facilitates APP/BACE1 interactions. Neurobiol Aging. 2014;35:1801–1812. doi: 10.1016/j.neurobiolaging.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Dale AM, Walhovd KB. Accelerating cortical thinning: unique to dementia or universal in aging? Cereb Cortex. 2014;24:919–934. doi: 10.1093/cercor/bhs379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Koh MT. Episodic memory on the path to Alzheimer’s disease. Curr Opin Neurobiol. 2011;21:929–934. doi: 10.1016/j.conb.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Isla T, Price JL, Mckeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J, Grant R, Mori TA, Croft KD. Changes in oxidative damage, inflammation and [NAD(H)] with age in cerebrospinal fluid. PLoS One. 2014;9:e85335. doi: 10.1371/journal.pone.0085335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X. Lipid alterations in the earliest clinically recognizable stage of Alzheimer’s disease: implication of the role of lipids in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2005;2:65–77. doi: 10.2174/1567205052772786. [DOI] [PubMed] [Google Scholar]
- Hancock SE, Friedrich MG, Mitchell TW, Truscott RJW, Else PL. Decreases in phospholipids containing Adrenic and arachidonic acids occur in the human hippocampus over the adult lifespan. Lipids. 2015;50:861–872. doi: 10.1007/s11745-015-4030-z. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Ma K, Gao F, Kim H-W, Rapoport SI, Rao JS. Disturbed choline plasmalogen and phospholipid fatty acid concentrations in Alzheimer’s disease prefrontal cortex. J Alzheimers Dis JAD. 2011;24:507–517. doi: 10.3233/JAD-2011-101608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen CIF, Kiliaan AJ. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog Lipid Res. 2014;53:1–17. doi: 10.1016/j.plipres.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Jiang J, Sachdev P, Lipnicki DM, Zhang H, Liu T, Zhu W, Suo C, Zhuang L, Crawford J, Reppermund S, Trollor J, Brodaty H, Wen W. A longitudinal study of brain atrophy over two years in community-dwelling older individuals. NeuroImage. 2014;86:203–211. doi: 10.1016/j.neuroimage.2013.08.022. [DOI] [PubMed] [Google Scholar]
- Kosicek M, Hecimovic S. Phospholipids and Alzheimer’s disease: alterations, mechanisms and potential biomarkers. Int J Mol Sci. 2013;14:1310–1322. doi: 10.3390/ijms14011310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge H, Akahori K, Yagyu K, Honke K. Functional compartmentalization of the plasma membrane of neurons by a unique acyl chain composition of phospholipids. J Biol Chem. 2014;289:26783–26793. doi: 10.1074/jbc.M114.571075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebisch G, Vizcaíno JA, Köfeler H, Trötzmüller M, Griffiths WJ, Schmitz G, Spener F, Wakelam MJO. Shorthand notation for lipid structures derived from mass spectrometry. J Lipid Res. 2013;54:1523–1530. doi: 10.1194/jlr.M033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín V, Fabelo N, Santpere G, Puig B, Marín R, Ferrer I, Díaz M. Lipid alterations in lipid rafts from Alzheimer’s disease human brain cortex. J Alzheimers Dis JAD. 2010;19:489–502. doi: 10.3233/JAD-2010-1242. [DOI] [PubMed] [Google Scholar]
- Martínez M, Mougan I. Fatty acid composition of human brain phospholipids during normal development. J Neurochem. 1998;71:2528–2533. doi: 10.1046/j.1471-4159.1998.71062528.x. [DOI] [PubMed] [Google Scholar]
- Mitchell TW, Buffenstein R, Hulbert AJ. Membrane phospholipid composition may contribute to exceptional longevity of the naked mole-rat (Heterocephalus glaber): a comparative study using shotgun lipidomics. Exp Gerontol. 2007;42:1053–1062. doi: 10.1016/j.exger.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Peskind ER, Quinn JF, Wilson AM, Montine KS, Galasko D. Increased cerebrospinal fluid F2-isoprostanes are associated with aging and latent Alzheimer’s disease as identified by biomarkers. Neruomol Med. 2011;13:37–43. doi: 10.1007/s12017-010-8126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Brockman J, Schneider JA, Wang Y, Bennett DA, Tangney CC, van de Rest O. Association of seafood consumption, brain mercury level, and APOE ε4 status with brain neuropathology in older adults. JAMA. 2016 doi: 10.1001/jama.2015.19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris SE, Friedrich MG, Mitchell TW, Truscott RJW, Else PL. Human prefrontal cortex phospholipids containing docosahexaenoic acid increase during normal adult aging, whereas those containing arachidonic acid decrease. Neurobiol Aging. 2015;36:1659–1669. doi: 10.1016/j.neurobiolaging.2015.01.002. [DOI] [PubMed] [Google Scholar]
- O’Brien JS, Sampson EL. Lipid composition of the normal human brain: gray matter, white matter, and myelin. J Lipid Res. 1965;6:537–544. [PubMed] [Google Scholar]
- Parletta N, Milte CM, Meyer BJ. Nutritional modulation of cognitive function and mental health. J Nutr Biochem. 2013;24:725–743. doi: 10.1016/j.jnutbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Plourde M, Chouinard-Watkins R, Vandal M, Zhang Y, Lawrence P, Brenna JT, Cunnane SC. Plasma incorporation, apparent retroconversion and β-oxidation of 13C-docosahexaenoic acid in the elderly. Nutr Metab. 2011;8:5. doi: 10.1186/1743-7075-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad MR, Lovell MA, Yatin M, Dhillon H, Markesbery WR. Regional membrane phospholipid alterations in Alzheimer’s disease. Neurochem Res. 1998;23:81–88. doi: 10.1023/A:1022457605436. [DOI] [PubMed] [Google Scholar]
- Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and ca1 in preclinical alzheimer disease. Arch Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62:433–438. doi: 10.1212/01.WNL.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Schröder J, Pantel J. Neuroimaging of hippocampal atrophy in early recognition of Alzheimer’s disease—a critical appraisal after two decades of research. Psychiatry Res Neuroimaging. 2016;247:71–78. doi: 10.1016/j.pscychresns.2015.08.014. [DOI] [PubMed] [Google Scholar]
- Skinner ER, Watt C, Besson JAO, Best PV. Differences in the fatty acid composition of the grey and white matter of different regions of the brains of patients with Alzheimer’s disease and control subjects. Brain. 1993;116:717–725. doi: 10.1093/brain/116.3.717. [DOI] [PubMed] [Google Scholar]
- Söderberg M, Edlund C, Kristensson K, Dallner G. Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids. 1991;26:421–425. doi: 10.1007/BF02536067. [DOI] [PubMed] [Google Scholar]
- Sutherland GT, Sheedy D, Stevens J, McCrossin T, Smith CC, van Roijen M, Kril JJ. The NSW brain tissue resource centre: banking for alcohol and major neuropsychiatric disorders research. Alcohol. 2016;52:33–39. doi: 10.1016/j.alcohol.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydenham E, Dangour AD, Lim W-S (2012) Omega 3 fatty acid for the prevention of cognitive decline and dementia. In: Cochrane database of systematic reviews. Wiley [DOI] [PMC free article] [PubMed]
- von Gunten A, Kövari E, Rivara C-B, Bouras C, Hof PR, Giannakopoulos P. Stereologic analysis of hippocampal Alzheimer’s disease pathology in the oldest-old: evidence for sparing of the entorhinal cortex and CA1 field. Exp Neurol. 2005;193:198–206. doi: 10.1016/j.expneurol.2004.12.005. [DOI] [PubMed] [Google Scholar]
- von Gunten A, Kövari E, Bussière T, Rivara C-B, Gold G, Bouras C, Hof PR, Giannakopoulos P. Cognitive impact of neuronal pathology in the entorhinal cortex and CA1 field in Alzheimer’s disease. Neurobiol Aging. 2006;27:270–277. doi: 10.1016/j.neurobiolaging.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Weylandt KH, Chiu C-Y, Gomolka B, Waechter SF, Wiedenmann B. Omega-3 fatty acids and their lipid mediators: towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012;97:73–82. doi: 10.1016/j.prostaglandins.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Yetukuri L, Ekroos K, Vidal-Puig A, Orešič M. Informatics and computational strategies for the study of lipids. Mol BioSyst. 2008;4:121–127. doi: 10.1039/B715468B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 31 kb)