Abstract
Heat shock proteins (Hsps) are a set of highly conserved proteins involved in cellular repair and protective mechanisms. They counter protein misfolding and aggregation that are characteristic features of neurodegenerative diseases. Hsps act co-operatively in disaggregation/refolding machines that assemble at sites of protein misfolding and aggregation. Members of the DNAJ (Hsp40) family act as “holdases” that detect and bind misfolded proteins, while members of the HSPA (Hsp70) family act as “foldases” that refold proteins to biologically active states. HSPH1 (Hsp105α) is an important additional member of the mammalian disaggregation/refolding machine that acts as a disaggregase to promote the dissociation of aggregated proteins. Components of a disaggregation/refolding machine were targeted to nuclear speckles after thermal stress in differentiated human neuronal SH-SY5Y cells, namely: HSPA1A (Hsp70-1), DNAJB1 (Hsp40-1), DNAJA1 (Hsp40-4), and HSPH1 (Hsp105α). Nuclear speckles are rich in RNA splicing factors, and heat shock disrupts RNA splicing which recovers after stressful stimuli. Interestingly, constitutively expressed HSPA8 (Hsc70) was also targeted to nuclear speckles after heat shock with elements of a disaggregation/refolding machine. Hence, neurons have the potential to rapidly assemble a disaggregation/refolding machine after cellular stress using constitutively expressed Hsc70 without the time lag needed for synthesis of stress-inducible Hsp70. Constitutive Hsc70 is abundant in neurons in the mammalian brain and has been proposed to play a role in pre-protecting neurons from cellular stress.
Keywords: Heat shock proteins, HSPA1A (Hsp70–1), HSPA8 (Hsc70), DNAJA1 (Hsp40–4), DNAJB1 (Hsp40–1), HSPH1 (Hsp105α), Nuclear speckles, RNA splicing, Human neuronal SH-SY5Y cells
Introduction
Heat shock proteins (Hsps) are a set of highly conserved proteins involved in cellular repair and protective mechanisms that counter protein misfolding and aggregation that are characteristic features of neurodegenerative diseases (Muchowski and Wacker 2005; Asea and Brown 2008; Paul and Mahanta 2014; Duncan et al. 2015; Smith et al. 2015). The classical mammalian protein refolding machine is composed of members of the DNAJ (Hsp40) family that act as “holdases” to detect and bind misfolded proteins, while members of the HSPA (Hsp70) family act as “foldases” that refold proteins to biologically active states (Hageman et al. 2011; Rampelt et al. 2012; Mattoo and Goloubinoff 2014; Clerico et al. 2015; Nillegoda and Bukau 2015; Nillegoda et al. 2015). The Hsp70-40 machine inhibits aggregation of misfolded proteins but cannot dissociate aggregated proteins which accumulate during neurodegenerative diseases and aging (Gao et al. 2015; Nillegoda and Bukau 2015). Bacteria, fungi, and plants express a well-characterized protein disaggregase (Hsp104 in yeast cells and ClpB in Escherichia coli) that can solubilize aggregated proteins, homologs of which are lacking in mammalian cells (Glover and Lindquist 1998; Weibezahn et al. 2005; Bosl et al. 2006; Tyedmers et al. 2010; Nillegoda and Bukau 2015). Recent evidence has revealed that a “disaggregase” function is performed by the mammalian HSPH (Hsp110) family in co-operation with the Hsp70-40 machine (Rampelt et al. 2012; Gao et al. 2015; Nillegoda and Bukau 2015; Nillegoda et al. 2015). This mammalian disaggregation/refolding machine is capable of disassociating amyloid fibrils of α-synuclein in vitro that are associated with Parkinson’s disease (Gao et al. 2015).
Upregulation of Hsps has been suggested as a therapeutic strategy to treat neurodegenerative diseases in which misfolded, aggregation-prone proteins accumulate, disrupt function, and lead to premature death of neuronal cells (Muchowski and Wacker 2005; Asea and Brown 2008; Paul and Mahanta 2014; Smith et al. 2015). Examples include neurofibrillary tangles and amyloid plaques in Alzheimer’s disease (Pei et al. 2008; Seeman and Seeman 2011), α-synuclein aggregates in Parkinson’s disease (Lee 2003), polyglutamine aggregates in Huntington’s disease (Nagai and Popiel 2008), and SOD1 aggregates in amyotrophic lateral sclerosis (ALS) (Blokhuis et al. 2013; Ogawa and Furukawa 2014). Upregulating a set of Hsps by activators of heat shock transcription factor 1 (HSF1), the master regulator of Hsp gene induction, is more effective than manipulation of individual Hsps for the treatment of neurodegenerative diseases which have been characterized as protein misfolding disorders (Asea and Brown 2008).
Recently, we have shown that low-dose co-application of celastrol and arimoclomol to differentiated human neuronal SH-SY5Y cells enhanced the induction of a set of Hsps (Deane and Brown 2016). Celastrol is an HSF1 activator identified in an NIH-sponsored drug screen in search of potential therapeutic compounds that could suppress a hallmark of neurodegenerative diseases, namely protein aggregation (Abbott 2002; Heemskerk et al. 2002). Subsequently, celastrol has been shown to be beneficial in a number of animal models of neurodegenerative diseases, including ALS (Kiaei et al. 2005), Parkinson’s disease (Cleren et al. 2005), polyglutamine disease (Zhang and Sarge 2007), and Alzheimer’s disease (Paris et al. 2010). The mechanism of celastrol involves activation of HSF1 monomers to a trimerized form that binds to heat shock elements (HSEs) in the promoter regions of inducible heat shock genes, resulting in their upregulation (Westerheide et al. 2004; Salminen et al. 2010). Arimoclomol, a co-activator of HSF1 that prolongs the binding of activated HSF1 to the HSE, shows promise in a number of animal models of neurodegenerative diseases (Kalmar et al. 2008; Malik et al. 2013; Parfitt et al. 2014) and is currently in phase IIb/III clinical trials for the treatment of ALS (Genc and Ozdinler 2013).
We have identified stress-sensitive sites in differentiated human neuronal cells by tracking the intracellular localization of HSPA (Hsp70) family members following exposure to heat shock (Khalouei et al. 2014a, b; Shorbagi and Brown 2016). In the present report, we explored the intracellular targeting of members of the mammalian disaggregation/refolding machine following thermal stress in differentiated human neuronal cells. Low-dose co-application of celastrol and arimoclomol was employed to induce a set of Hsps in neuronal cells that were subsequently targeted by heat shock to stress-sensitive intracellular sites (Deane and Brown 2016). Components of a mammalian disaggregation/refolding machine, including HSPA1A (Hsp70-1), DNAJB1 (Hsp40-1), DNAJA1 (Hsp40-4), and HSPH1 (Hsp105α), co-localized following heat shock at nuclear speckles that are rich in RNA splicing factors (Lamond and Spector 2003; Spector and Lamond 2011). The small heat shock protein, HSPB1 (Hsp27), that has been shown to enhance protein disaggregation (Ehrnsperger et al. 1997; Lee et al. 1997; Mogk et al. 2003; Duennwald et al. 2012), was also targeted to nuclear speckles following heat shock. These results indicate that components of a protein disaggregation/refolding machine are targeted to nuclear speckles after thermal stress. Heat shock disrupts RNA splicing which must recover following stressful stimuli (Yost and Lindquist 1986, 1991; Corell and Gross 1992; Marin-Vinader et al. 2006; Biamonti and Caceres 2009).
Interestingly, a constitutively expressed member of the HSPA family, HSPA8 (Hsc70), was also targeted to nuclear speckles with members of the disaggregation/refolding machine following heat shock. HSPA8 is abundant in neuronal cells in vivo relative to non-neuronal tissues and has been proposed to pre-protect neurons from cellular stress (Manzerra et al. 1997; Chen and Brown 2007). This novel observation suggests that differentiated human neurons have the capacity to rapidly form a disaggregation/refolding machine employing constitutive HSPA8 to combat misfolded, aggregation-prone neuronal proteins, without the time lag required to synthesize stress-inducible HSPA1A.
Materials and methods
Cell culture and differentiation
Human neuronal SH-SY5Y cells (American Type Culture Collection, Manassas, VA, USA) were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Wisent, QC, Canada) supplemented with 10 % fetal bovine serum (FBS; Wisent) and cultured at 37 °C in a humidified 5 % CO2 atmosphere. Cells plated at 4.5 × 104 cells per square centimeters on glass coverslips placed inside 6-well plates were allowed to settle onto the growth surface and adhere for 24 h. Neuronal differentiation was induced by treatment with 10 μM all-trans-retinoic acid (R2625; Sigma Aldrich, St. Louis, MO, USA) for 72 h under serum free conditions.
Induction of heat shock proteins
Following 72 h of differentiation, media containing all-trans-retinoic acid was removed and replaced with fresh serum-free DMEM with or without 0.3 μM celastrol plus 50 μM arimoclomol (Deane and Brown 2016). Celastrol (70950; Cayman Chemical, Ann Arbor, MI, USA) dissolved in DMSO was added directly to the media. Arimoclomol (gift from Professor Michael Cheetham, Institute of Ophthalmology, University College London, UK) was prepared fresh for each experiment by dissolving in an appropriate volume of serum-free DMEM and filtering (0.2 μm pore size). DMSO treatment alone was used as a vehicle control for celastrol. After 12 h incubation to allow Hsp induction, the cells were either fixed for immunofluorescence (no HS) or exposed to heat shock (HS). For heat shock, cells were immersed in a water bath equipped with a thermal immersion circulator calibrated at 43 °C (±0.2 °C) for 20 min and subsequently fixed for immunofluorescence.
Immunofluorescence
Cells were fixed with 4 % paraformaldehye (PFA) in phosphate-buffered saline (PBS; pH 7.4) for 30 min. The cells were permeabilized with 0.1 % triton X-100 and 100 mM glycine in PBS for 30 min and blocked with 5 % FBS in PBS for 1 h. Primary antibodies were incubated overnight in 1 % FBS in PBS. HSPA1A (SPA-810), HSPA8 (SPA-815), DNAJB1 (SPA-400), and HSPB1 (SPA-803) antibodies were purchased from Enzo Life Sciences (Farmingdale, NY, USA). DNAJA1 [clone KA2A5.6] (ab3089), HSPH1 (ab109624), BAG-1 (ab7976), SC35 (ab11826), and nucleophosmin (ab37659) primary antibodies were purchased from Abcam (Toronto, ON, CA). Primary antibody for the nuclear speckle marker SON (HPA023535) was purchased from Sigma Aldrich. Cells were then washed and incubated with the appropriate combination of Alexafluor® secondary antibodies (Molecular Probes, Life Technologies, Burlington, ON, CA) and counterstained with 300 nM DAPI (Invitrogen, Life Technologies). Fluorescence images were captured using a Quorum Wave FX-X1 spinning disk confocal microscope (Quorum Technologies, Guelph, ON, CA) equipped with a high resolution Humamatsu Orca R2 camera (Humamatsu Photonics, Japan) using a Plan-APO 63×/1.4 NA oil objective. Excitation lasers = 405, 491, 561, and 644 nm. Emission filters (nm/bandpass): 460/50, 525/50, and 593/40.
Fluorescent image analysis
Image processing and analysis was performed using Volocity 3D image analysis software (PerkinElmer, Waltham, MA, USA). ImageJ software (http://imagej.nih.gov/ij/) was used to perform colocalization analysis using TIFF images exported from Volocity. Background subtracted images were used to generate RGB profile plots representing fluorescence signal intensities in a defined linear region using the RGB Profiler plugin. Images representative of three individual experiments are shown in which 25 cells were analyzed in coverslips harvested from each well of 6-well culture plates.
Results
HSPA and DNAJ components of a protein disaggregation/refolding machine co-localized at nuclear speckles following thermal stress in differentiated human neuronal SH-SY5Y cells
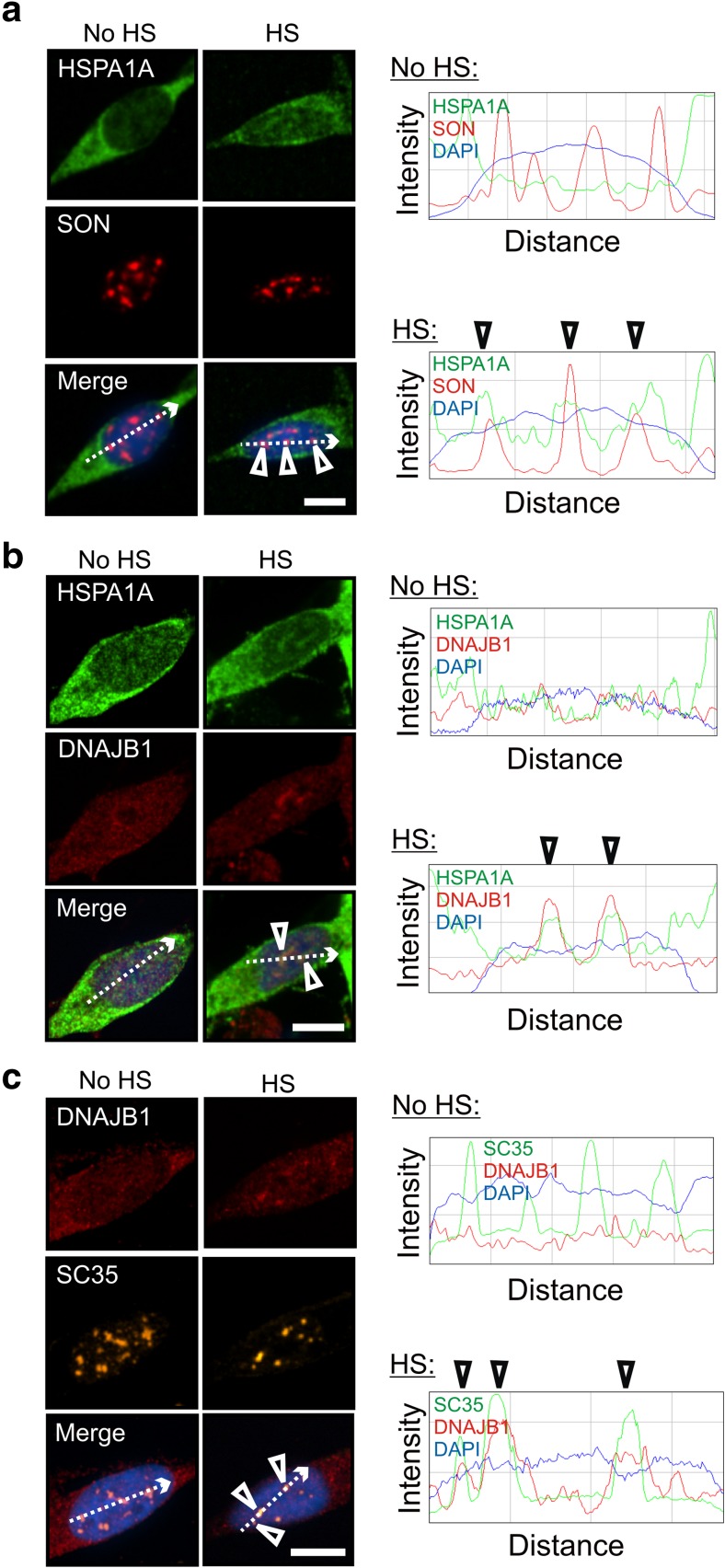
Differentiated human neuronal SH-SY5Y cells were treated with celastrol and arimoclomol to induce Hsps, including HSPA1A (Hsp70-1) and DNAJB1 (Hsp40-1) (Deane and Brown 2016). Prior to heat shock, HSPA1A was distributed diffusely in the neuronal cytoplasm (Fig. 1, no HS panels). Following heat shock (HS), celastrol- and arimoclomol-induced HSPA1A co-localized with nuclear speckles (Fig. 1a, merged panel) identified by the marker protein SON (Sharma et al. 2010; Sytnikova et al. 2011). ImageJ line scans confirmed co-localization of the HSPA1A and SON fluorescent signals (arrowheads shown in panels on the right). After heat shock, DNAJB1 co-localized with HSPA1A (Fig. 1b) and the nuclear speckle marker SC35 (Fig. 1c), confirmed by ImageJ line scans (arrowheads). SC35 and SON have been shown to co-localize with each other in differentiated human neuronal SH-SY5Y cells at nuclear speckles (Khalouei et al. 2014a).
Fig. 1.
HSPA1A (Hsp70-1) and DNAJB1 (Hsp40-1) are targeted to nuclear speckles by heat shock in differentiated human neuronal cells. Differentiated human neuronal SH-SY5Y cells were treated with celastrol and arimoclomol to induce Hsps. a HSPA1A co-localized with the nuclear speckle marker SON after heat shock, confirmed at arrowheads in ImageJ line scan displaying fluorescence intensity across the region indicated by the dotted line. b DNAJB1 co-localized with HSPA1A after heat shock, confirmed at arrowheads in ImageJ line scan. c The nuclear speckle marker SC35 co-localized with DNAJB1 after heat shock (arrowheads in ImageJ line scan). HS heat shock. DAPI staining is used to identify neuronal nuclei (blue). Scale bars represent 5 μm
The Hsp40 holdase, DNAJA1, also targeted nuclear speckles following thermal stress
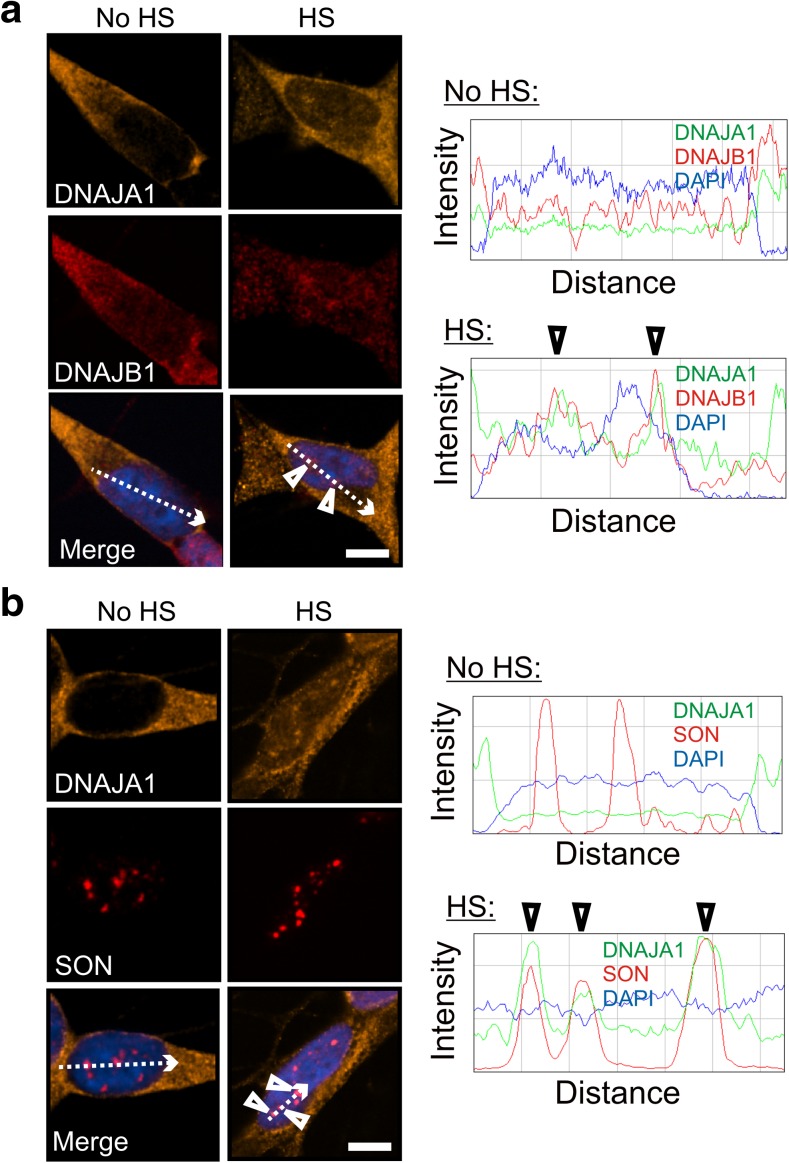
The DNAJ family is a large family of molecular chaperones in mammalian cells and is divided into classes that vary in sequence and structure, but include a characteristic J-domain that mediates interactions with HSPA proteins (Kampinga et al. 2009; Kampinga and Craig 2010). The mammalian disaggregation/refolding machine has been reported to more efficiently promote dissociation of protein aggregates in the joint presence of a class A and B member of the DNAJ family (Nillegoda et al. 2015). As shown in Fig. 2a, a class A member of the DNAJ family, namely DNAJA1 (Hsp40-4), co-localized after heat shock with DNAJB1 at SON-positive nuclear speckles (Fig. 2b), confirmed by ImageJ line scans (arrowheads).
Fig. 2.
The class A protein, DNAJA1 (Hsp40-4), also localized to nuclear speckles after heat shock. a DNAJA1 co-localized with DNAJB1 and b the nuclear speckle marker SON (arrowheads in ImageJ line scans). Scale bars represent 5 μm
The disaggregase HSPH1 (Hsp105α) co-localized with HSPA1A at nuclear speckles after heat shock
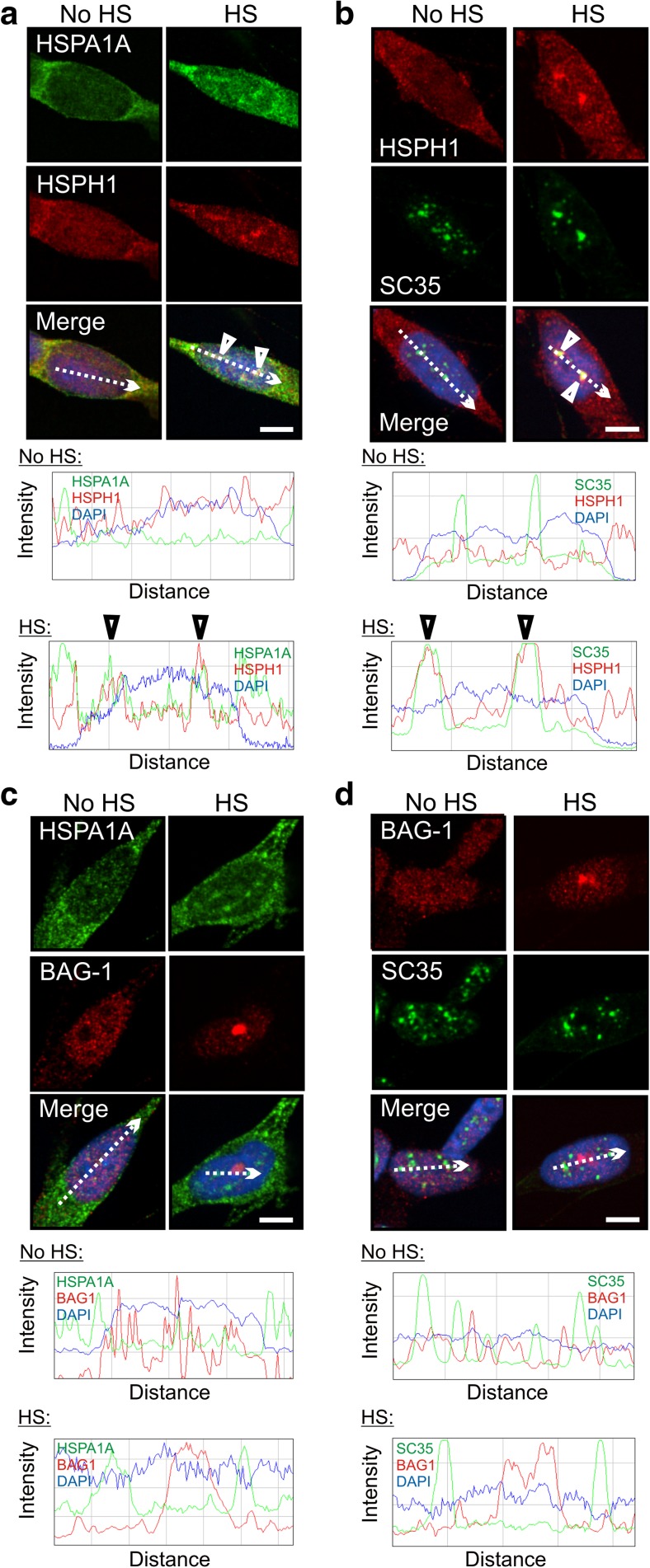
The mammalian disaggregase HSPH1 (Hsp105α) that promotes protein disaggregation in vitro as a member of the disaggregation/refolding machine, acts as a nucleotide exchange factor (NEF) for HSPA proteins (Schuermann et al. 2008; Bracher and Verghese 2015a, b; Nillegoda and Bukau 2015). As shown in the merged panels of Fig. 3, and confirmed by ImageJ line scans, HSPH1 co-localized at nuclear speckles with HSPA1A (Fig. 3a, arrowheads) and SC35 (Fig. 3b, arrowheads) after thermal stress. An alternative Hsp70 NEF that does not participate in the disaggregation/refolding of aggregated proteins, namely BAG-1 (Rampelt et al. 2012; Bracher and Verghese 2015b; Nillegoda and Bukau 2015), did not co-localize with HSPA1A or SC35 (Fig. 3c, d). Targeting of the disaggregase HSPH1 and other components that make up the mammalian disaggregation/refolding machine to nuclear speckles suggests that this machine may contribute to the recovery of RNA splicing following stress-induced inhibition.
Fig. 3.
Targeting of the disaggregase HSPH1 (Hsp105α) to nuclear speckles following thermal stress. a Co-localization of HSPH1 and HSPA1A after heat shock, confirmed by ImageJ line scans (arrowheads). b HSPH1 and SC35 co-localized after heat shock (arrowheads in ImageJ line scan). c BAG-1 did not co-localize with HSPA1A after heat shock, confirmed by ImageJ line scan. d BAG-1 also did not co-localize with SC35 after heat shock. Scale bars represent 5 μm
The small heat shock protein HSPB1 (Hsp27) is targeted to stress-sensitive neuronal sites
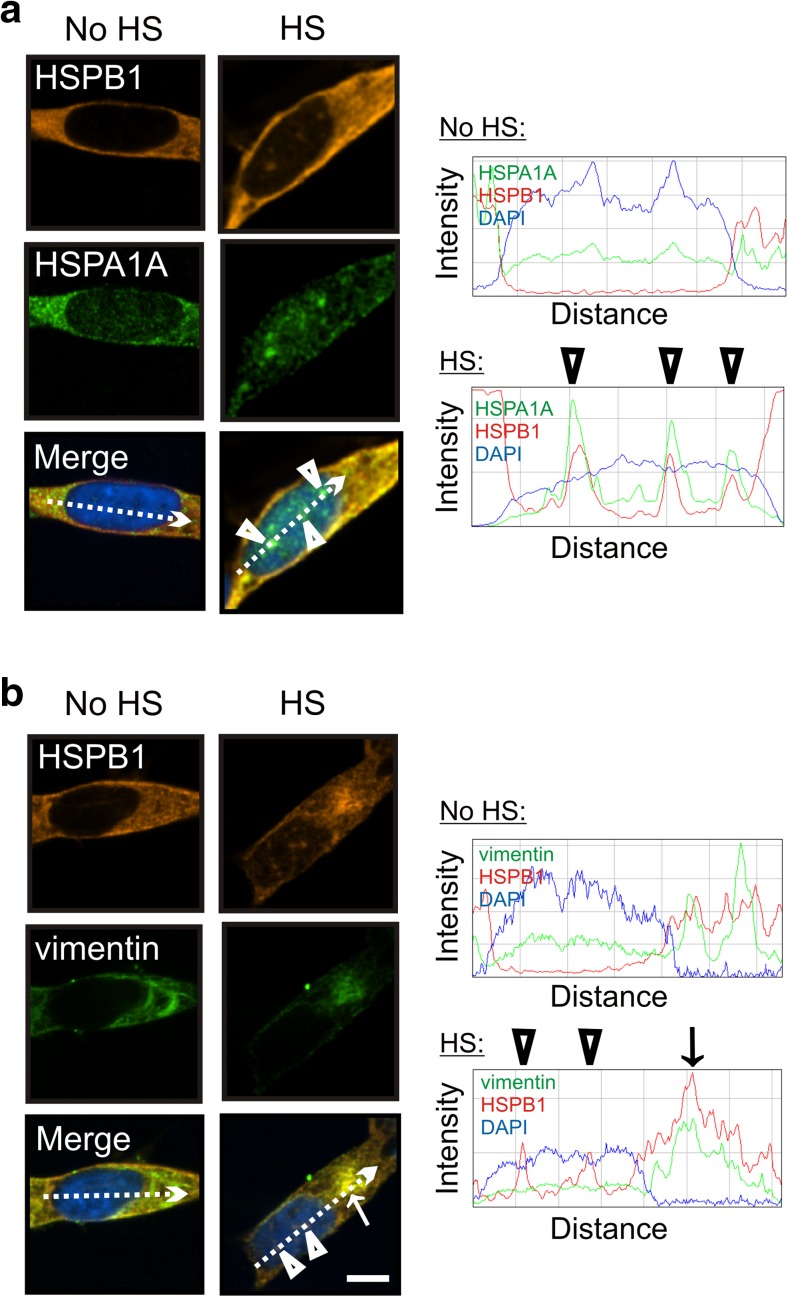
The small heat shock protein HSPB1 (Hsp27) has been shown to facilitate dissociation of protein aggregates (Mogk et al. 2003; Duennwald et al. 2012; Nillegoda and Bukau 2015). As shown in Fig. 4a, HSPB1 (Hsp27) co-localized with HSPA1A (Fig. 4a) which was targeted to nuclear speckles after heat shock (Fig. 1a). HSPB1 may enhance the activity of the disaggregation/refolding machine at nuclear speckles following heat shock. In the cytoplasm, HSPB1 co-localized after heat shock with vimentin, a marker of the cytoskeleton (Fig. 4b). The vimentin cytoskeleton assembles into a cage around aggresomes that form in the cell body during times of stress to sequester misfolded, aggregated proteins and reduce their toxicity (Tyedmers et al. 2010). HSPB1 may be involved in stabilizing the vimentin cage that forms during thermal stress recovery in the cytoplasm (Tyedmers et al. 2010).
Fig. 4.
The small heat shock protein HSPB1 (Hsp27) is targeted by thermal stress to nuclear and cytoplasmic sites in differentiated human neuronal cells. a HSPB1 co-localized with HSPA1A in the nucleus after heat shock (arrowheads). b HSPB1 co-localized with the vimentin cytoskeleton in the cytoplasm after heat shock (arrow). Co-localization was confirmed by ImageJ line scans shown on the right. Scale bars represent 5 μm
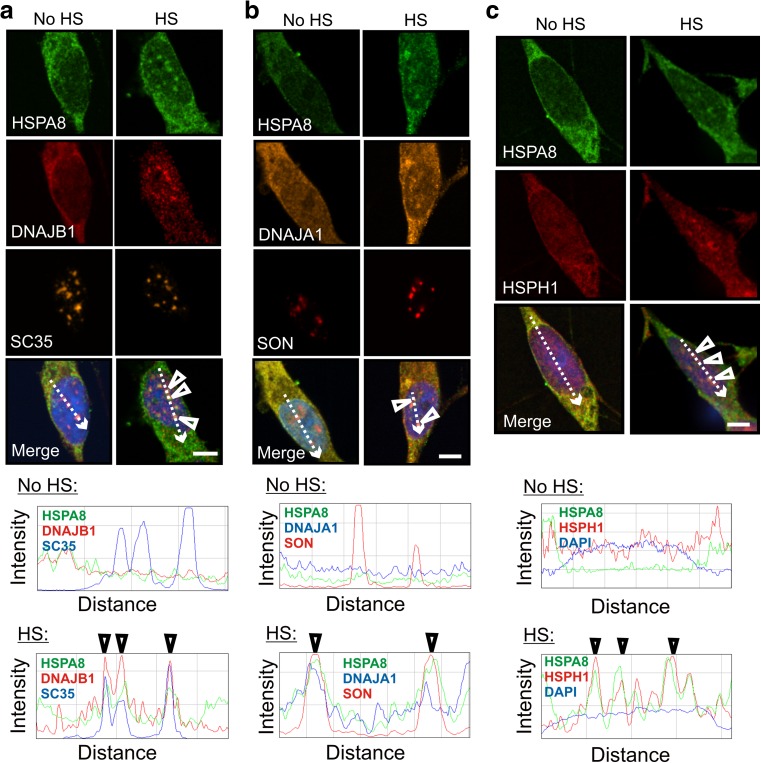
Constitutively expressed HSPA8 (Hsc70) was also targeted to nuclear speckles with components of the disaggregation/refolding machine following heat shock
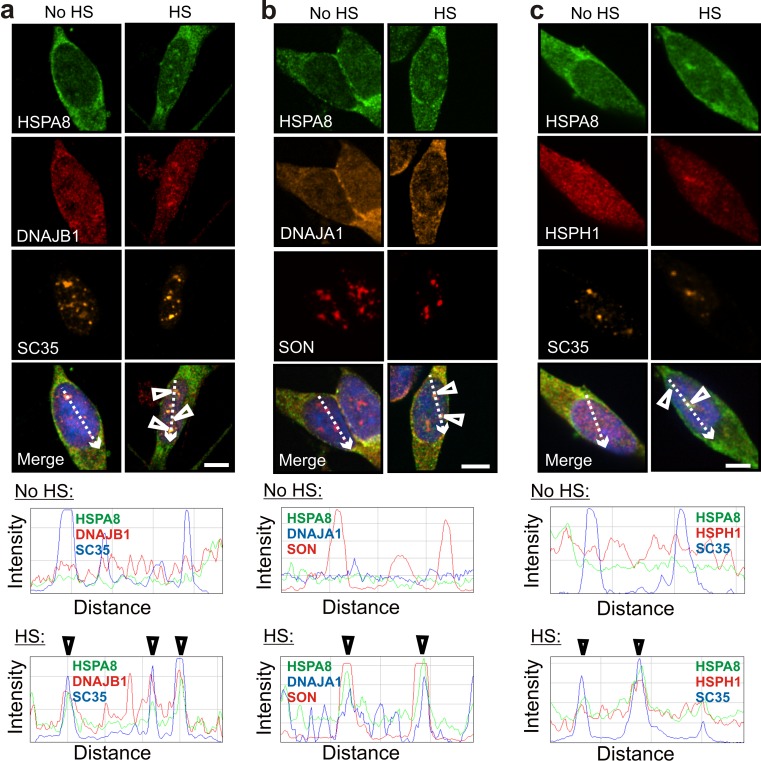
Interestingly, HSPA8 also exhibited stress-induced co-localization at nuclear speckles with DNAJB1, DNAJA1, and HSPH1 (Fig. 5; and confirmed by ImageJ line scans), as was observed for HSPA1A. This suggests that constitutive HSPA8 has the potential to operate in a disaggregation/refolding machine during the recovery of nuclear speckles from thermal stress. Basal levels of DNAJB1, DNAJA1, HSPH1, and HSPA8 are expressed in differentiated human neuronal SH-SY5Y cells (Deane and Brown 2016). Co-localization of these machine components in differentiated human neuronal SH-SY5Y cells at nuclear speckles following heat shock was also observed in the absence of celastrol and arimoclomol co-application (Fig. 6). This observation suggests that neurons have the potential to rapidly assemble a disaggregation/refolding machine following thermal stress utilizing constitutively expressed HSPA8, without the time lag required for synthesis of stress-inducible HSPA1A.
Fig. 5.
Constitutively expressed HSPA8 (Hsc70) targeted nuclear speckles following thermal stress and co-localized with members of the mammalian disaggregation/refolding machine. a HSPA8 co-localized after heat shock with DNAJB1 at SC35-positive nuclear speckles (arrowheads). b HSPA8 also co-localized with DNAJA1 at SON-positive nuclear speckles (arrowheads). c HSPA8 co-localized with HSPH1 (arrowheads). Scale bars represent 5 μm
Fig. 6.
In the absence of prior celastrol and arimoclomol treatment, constitutively expressed HSPA8 co-localized with components of the disaggregation/refolding machine after heat shock. Arrowheads indicate co-localization of HSPA8 after heat shock with a DNAJB1, b DNAJA1, and c HSPH1 at nuclear speckles identified by the marker proteins SC35 (a, c) and SON (b). Scale bars represent 5 μm
Discussion
Recent biochemical studies using purified human Hsps have identified a mammalian disaggregation/refolding machine, composed of members of the HSPA (Hsp70), DNAJ (Hsp40), and HSPH (Hsp110) families, that acts to dissociate and refold aggregated proteins in vitro (Rampelt et al. 2012; Gao et al. 2015; Nillegoda and Bukau 2015; Nillegoda et al. 2015). Novel results are presented in the present report that demonstrate the targeting of members of the mammalian disaggregation/refolding machine to a specific intracellular structure, namely nuclear speckles, following heat shock in differentiated human neuronal SH-SY5Y cells that we have identified as a stress-sensitive neuronal site (Khalouei et al. 2014a; Shorbagi and Brown 2016). Using immunofluorescent confocal imaging, co-localization at nuclear speckles following thermal stress was observed for the stress-inducible foldase HSPA1A, a class A plus a class B member of the DNAJ holdase family of proteins, namely DNAJA1 and DNAJB1, and the mammalian disaggregase HSPH1. Thermal stress targeting and co-localization at nuclear speckles with components of a disaggregation/refolding machine was also observed in the present report for the small heat shock protein HSPB1 that has previously been shown to enhance the dissociation of misfolded protein aggregates in vitro (Mogk et al. 2003; Duennwald et al. 2012; Nillegoda and Bukau 2015).
Nuclear speckles are rich in RNA splicing factors that are sensitive to thermal stress (Lamond and Spector 2003; Spector and Lamond 2011). RNA splicing is disrupted under conditions of thermal stress and Hsps are required for splicing recovery (Yost and Lindquist 1986, 1991; Corell and Gross 1992; Marin-Vinader et al. 2006; Biamonti and Caceres 2009). Co-localization of components of a mammalian disaggregation/refolding machine at nuclear speckles following heat shock suggests that dissociation of misfolded, aggregated proteins may be required for the recovery of RNA splicing following stress-induced inhibition in human neuronal cells. We have previously shown that HSPA-positive nuclear speckles do not co-localize with Sam68, a marker of nuclear stress bodies (Denegri et al. 2001; Biamonti 2004; Khalouei et al. 2014a).
The present results demonstrate that both DNAJB1, a class B member of the DNAJ family, and DNAJA1, a class A member, co-localized at nuclear speckles following heat shock. The DNAJ family of molecular chaperones delivers substrates to HSPA proteins and stimulates their ATPase activity (Kampinga et al. 2009; Kampinga and Craig 2010; Mattoo and Goloubinoff 2014). It is divided into classes that differ structurally but contain a characteristic J-domain that defines this family and mediates interactions with HSPA proteins (Kampinga et al. 2009; Kampinga and Craig 2010). Class A proteins exhibit the greatest degree of similarity with yeast DNAJ proteins while class B proteins lack the zinc finger domain found in class A proteins (Kampinga et al. 2009; Kampinga and Craig 2010). Nillegoda et al. (2015) have recently shown that efficient dissociation of aggregated proteins by the mammalian disaggregation/refolding machine requires the presence of both a class A and a class B member of the DNAJ family and that DNAJA1 and DNAJB1 are effective partners.
HSPA nucleotide exchange factors (NEFs) in humans fall into families that are structurally and functionally distinct, including the HSPH (Hsp110) family, Bcl2-associated athanogene (BAG) family, and Hsp70 binding protein-type (HSPBP-type) NEFs (Schuermann et al. 2008; Kampinga and Craig 2010; Bracher and Verghese 2015a, b). The diversity of HSPA NEFs provides functional specificity by linking HSPA members to distinct cellular pathways (Kampinga and Craig 2010; Hageman et al. 2011; Bracher and Verghese 2015a, b). The present results indicate that HSPH1 co-localized at nuclear speckles with components of the disaggregation/refolding machine following thermal stress, whereas BAG-1 did not. HSPH1 promotes dissociation of both heat and chemically denatured protein aggregates in vitro (Rampelt et al. 2012; Bracher and Verghese 2015b; Nillegoda and Bukau 2015). Furthermore, knockdown of Hsp110 in C. elegans, but not BAG-1, resulted in the persistence of heat shock-induced luciferase aggregates in YFP luciferase-expressing animals (Rampelt et al. 2012). Contrary to the dissociation and refolding activity directed by HSPH1, BAG family NEFs promote the degradation of misfolded protein targets by linking HSPA to protein degradation machinery (Bracher and Verghese 2015a). Co-localization of HSPH1, but not BAG-1, with HSPA1A, DNAJB1, and DNAJA1 at nuclear speckles following thermal stress suggests that disaggregation and refolding of thermally denatured proteins occurs during splicing recovery, rather than protein degradation.
The small heat shock protein HSPB1 is not an essential member of the disaggregation/refolding machine; however, it has been shown to enhance the in vitro activity of the machine (Mogk et al. 2003; Duennwald et al. 2012; Nillegoda and Bukau 2015). The present results demonstrate targeting of HSPB1 to nuclear speckles and to the vimentin cytoskeleton in the cytoplasm following heat shock. HSPB1 may contribute to splicing recovery by enhancing the ability of the machine to dissociate heat-induced aggregates of nuclear speckle proteins that are sensitive to thermal stress (Lamond and Spector 2003; Spector and Lamond 2011). Targeting of HSPB1 to the vimentin cytoskeleton after heat shock suggests that it may be involved in stabilization of the “vimentin cage,” which sequesters aggregated proteins following cellular stress (Tyedmers et al. 2010). Interestingly, a recent literature review aimed at identifying modifiers of proteotoxicity identified small Hsps as modifiers of neuronal proteotoxicity associated with neurodegenerative diseases (Brehme and Voisine 2016).
The current study revealed that constitutively expressed HSPA8 was also targeted to nuclear speckles following heat shock in differentiated human neuronal SH-SY5Y cells and co-localized with DNAJA1, DNAJB1, and HSPH1. This novel observation suggests that neurons have the potential to rapidly deploy HSPA8 in times of stress to assemble a disaggregation/refolding machine at nuclear speckles, without the time lag required for synthesis of stress-inducible HSPA1A. Previous work in our laboratory has shown that HSPA8 is abundant in mammalian neurons in vivo compared to non-neural tissues and has been proposed to pre-protect neurons from cellular stress (Manzerra et al. 1997; Chen and Brown 2007). High levels of HSPA8 (Hsc70) in neurons may explain previous observations that neurons are refractory to HSPA (Hsp70) induction following heat shock (Manzerra et al. 1997; Drujan and De Maio 1999; Chow et al. 2010). Purified HSPA8 act efficiently in vitro as a member of the machine to dissociate α-synuclein amyloid fibrils that form during Parkinson’s disease (Gao et al. 2015). Thus, constitutively expressed HSPA8 could be a key factor in a disaggregation/refolding machine to combat misfolded, aggregation-prone proteins in human neurodegenerative diseases. Our novel results indicate that members of a mammalian disaggregation/refolding machine composed of constitutively expressed HSPA8, DNAJB1, DNAJA1, and HSPH1, rapidly co-localize at nuclear speckles during times of stress in differentiated human neurons and may be involved in recovery of RNA splicing from stress-induced inhibition.
Acknowledgements
Supported by grants from NSERC to I.R.B.
References
- Abbott A. Neurologists strike gold in drug screen effort. Nature. 2002;417:109. doi: 10.1038/417109a. [DOI] [PubMed] [Google Scholar]
- Asea AA, Brown IR (eds) (2008) Heat shock proteins and the brain: implications for neurodegenerative diseases and neuroprotection. Springer Science + Business Media B.V.
- Biamonti G. Nuclear stress bodies: a heterochromatin affair? Nat Rev Mol Cell Biol. 2004;5:493–498. doi: 10.1038/nrm1405. [DOI] [PubMed] [Google Scholar]
- Biamonti G, Caceres JF. Cellular stress and RNA splicing. Trends Biochem Sci. 2009;34:146–153. doi: 10.1016/j.tibs.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Blokhuis AM, Groen EJ, Koppers M, van den Berg LH, Pasterkamp RJ. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125:777–794. doi: 10.1007/s00401-013-1125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosl B, Grimminger V, Walter S. The molecular chaperone Hsp104—a molecular machine for protein disaggregation. J Struct Biol. 2006;156:139–148. doi: 10.1016/j.jsb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Bracher A, Verghese J. GrpE, Hsp110/Grp170, HspBP1/Sil1 and BAG domain proteins: nucleotide exchange factors for Hsp70 molecular chaperones. Subcell Biochem. 2015;78:1–33. doi: 10.1007/978-3-319-11731-7_1. [DOI] [PubMed] [Google Scholar]
- Bracher A, Verghese J. The nucleotide exchange factors of Hsp70 molecular chaperones. Front Mol Biosci. 2015;2:10. doi: 10.3389/fmolb.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehme M, Voisine C. Model systems of protein-misfolding diseases reveal chaperone modifiers of proteotoxicity. Dis Model Mech. 2016;9:823–838. doi: 10.1242/dmm.024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Brown IR. Translocation of constitutively expressed heat shock protein Hsc70 to synapse-enriched areas of the cerebral cortex after hyperthermic stress. J Neurosci Res. 2007;85:402–409. doi: 10.1002/jnr.21124. [DOI] [PubMed] [Google Scholar]
- Chow AM, Mok P, Xiao D, Khalouei S, Brown IR. Heteromeric complexes of heat shock protein 70 (HSP70) family members, including Hsp70B, in differentiated human neuronal cells. Cell Stress Chaperones. 2010;15:545–553. doi: 10.1007/s12192-009-0167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleren C, Calingasan NY, Chen J, Beal MF. Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. J Neurochem. 2005;94:995–1004. doi: 10.1111/j.1471-4159.2005.03253.x. [DOI] [PubMed] [Google Scholar]
- Clerico EM, Tilitsky JM, Meng W, Gierasch LM. How Hsp70 molecular machines interact with their substrates to mediate diverse physiological functions. J Mol Biol. 2015;427:1575–1588. doi: 10.1016/j.jmb.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corell RA, Gross RH. Splicing thermotolerance maintains pre-mRNA transcripts in the splicing pathway during severe heat shock. Exp Cell Res. 1992;202:233–242. doi: 10.1016/0014-4827(92)90070-O. [DOI] [PubMed] [Google Scholar]
- Deane CA, Brown IR. Induction of heat shock proteins in differentiated human neuronal cells following co-application of celastrol and arimoclomol. Cell Stress Chaperones. 2016 doi: 10.1007/s12192-016-0708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denegri M, Chiodi I, Corioni M, Cobianchi F, Riva S, Biamonti G. Stress-induced nuclear bodies are sites of accumulation of pre-mRNA processing factors. Mol Biol Cell. 2001;12:3502–3514. doi: 10.1091/mbc.12.11.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drujan D, De Maio A. Expression of HSP70 is impaired at the transcriptional level in stressed murine neuroblastoma cells. Shock. 1999;12:443–448. doi: 10.1097/00024382-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Duennwald ML, Echeverria A, Shorter J. Small heat shock proteins potentiate amyloid dissolution by protein disaggregases from yeast and humans. PLoS Biol. 2012;10:e1001346. doi: 10.1371/journal.pbio.1001346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan EJ, Cheetham ME, Chapple JP, van der Spuy J. The role of HSP70 and its co-chaperones in protein misfolding, aggregation and disease. Subcell Biochem. 2015;78:243–273. doi: 10.1007/978-3-319-11731-7_12. [DOI] [PubMed] [Google Scholar]
- Ehrnsperger M, Graber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, et al. Human Hsp70 disaggregase reverses Parkinson's-linked alpha-synuclein amyloid fibrils. Mol Cell. 2015;59:781–793. doi: 10.1016/j.molcel.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc B, Ozdinler PH (2013) Moving forward in clinical trials for ALS: motor neurons lead the way please Drug. Discov Today [DOI] [PMC free article] [PubMed]
- Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998;94:73–82. doi: 10.1016/S0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- Hageman J, van Waarde MA, Zylicz A, Walerych D, Kampinga HH. The diverse members of the mammalian HSP70 machine show distinct chaperone-like activities. Biochem J. 2011;435:127–142. doi: 10.1042/BJ20101247. [DOI] [PubMed] [Google Scholar]
- Heemskerk J, Tobin AJ, Bain LJ (2002) Teaching old drugs new tricks. Meeting of the neurodegeneration drug screening consortium, 7–8 April 2002, Washington, DC, USA Trends Neurosci 25:494–496 [DOI] [PubMed]
- Kalmar B, Novoselov S, Gray A, Cheetham ME, Margulis B, Greensmith L. Late stage treatment with arimoclomol delays disease progression and prevents protein aggregation in the SOD1 mouse model of ALS. J Neurochem. 2008;107:339–350. doi: 10.1111/j.1471-4159.2008.05595.x. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalouei S, Chow AM, Brown IR. Localization of heat shock protein HSPA6 (HSP70B') to sites of transcription in cultured differentiated human neuronal cells following thermal stress. J Neurochem. 2014;131:743–754. doi: 10.1111/jnc.12970. [DOI] [PubMed] [Google Scholar]
- Khalouei S, Chow AM, Brown IR. Stress-induced localization of HSPA6 (HSP70B') and HSPA1A (HSP70-1) proteins to centrioles in human neuronal cells. Cell Stress Chaperones. 2014;19:321–327. doi: 10.1007/s12192-013-0459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiaei M, Kipiani K, Petri S, Chen J, Calingasan NY, Beal MF. Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:246–254. doi: 10.1159/000090364. [DOI] [PubMed] [Google Scholar]
- Lamond AI, Spector DL. Nuclear speckles: a model for nuclear organelles. Nat Rev Mol Cell Biol. 2003;4:605–612. doi: 10.1038/nrm1172. [DOI] [PubMed] [Google Scholar]
- Lee GJ, Roseman AM, Saibil HR, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ. Alpha-synuclein aggregation: a link between mitochondrial defects and Parkinson's disease? Antioxid Redox Signal. 2003;5:337–348. doi: 10.1089/152308603322110904. [DOI] [PubMed] [Google Scholar]
- Malik B, Nirmalananthan N, Gray AL, La Spada AR, Hanna MG, Greensmith L. Co-induction of the heat shock response ameliorates disease progression in a mouse model of human spinal and bulbar muscular atrophy: implications for therapy. Brain. 2013;136:926–943. doi: 10.1093/brain/aws343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzerra P, Rush SJ, Brown IR. Tissue-specific differences in heat shock protein hsc70 and hsp70 in the control and hyperthermic rabbit. J Cell Physiol. 1997;170:130–137. doi: 10.1002/(SICI)1097-4652(199702)170:2<130::AID-JCP4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Marin-Vinader L, Shin C, Onnekink C, Manley JL, Lubsen NH. Hsp27 enhances recovery of splicing as well as rephosphorylation of SRp38 after heat shock. Mol Biol Cell. 2006;17:886–894. doi: 10.1091/mbc.E05-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo RU, Goloubinoff P. Molecular chaperones are nanomachines that catalytically unfold misfolded and alternatively folded proteins. Cell Mol Life Sci. 2014;71:3311–3325. doi: 10.1007/s00018-014-1627-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogk A, Deuerling E, Vorderwulbecke S, Vierling E, Bukau B. Small heat shock proteins, ClpB and the DnaK system form a functional triade in reversing protein aggregation. Mol Microbiol. 2003;50:585–595. doi: 10.1046/j.1365-2958.2003.03710.x. [DOI] [PubMed] [Google Scholar]
- Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Popiel HA. Conformational changes and aggregation of expanded polyglutamine proteins as therapeutic targets of the polyglutamine diseases: exposed beta-sheet hypothesis. Curr Pharm Des. 2008;14:3267–3279. doi: 10.2174/138161208786404164. [DOI] [PubMed] [Google Scholar]
- Nillegoda NB, Bukau B. Metazoan Hsp70-based protein disaggregases: emergence and mechanisms. Front Mol Biosci. 2015;2:57. doi: 10.3389/fmolb.2015.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nillegoda NB, et al. Crucial HSP70 co-chaperone complex unlocks metazoan protein disaggregation. Nature. 2015;524:247–251. doi: 10.1038/nature14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Furukawa Y. A seeded propagation of Cu, Zn-superoxide dismutase aggregates in amyotrophic lateral sclerosis. Front Cell Neurosci. 2014;8:83. doi: 10.3389/fncel.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt DA, et al. The heat-shock response co-inducer arimoclomol protects against retinal degeneration in rhodopsin retinitis pigmentosa. Cell Death Dis. 2014;5:e1236. doi: 10.1038/cddis.2014.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D, et al. Reduction of beta-amyloid pathology by celastrol in a transgenic mouse model of Alzheimer's disease. J Neuroinflammation. 2010;7:17. doi: 10.1186/1742-2094-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Mahanta S. Association of heat-shock proteins in various neurodegenerative disorders: is it a master key to open the therapeutic door? Mol Cell Biochem. 2014;386:45–61. doi: 10.1007/s11010-013-1844-y. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Sjogren M, Winblad B. Neurofibrillary degeneration in Alzheimer’s disease: from molecular mechanisms to identification of drug targets. Curr Opin Psychiatry. 2008;21:555–561. doi: 10.1097/YCO.0b013e328314b78b. [DOI] [PubMed] [Google Scholar]
- Rampelt H, Kirstein-Miles J, Nillegoda NB, Chi K, Scholz SR, Morimoto RI, Bukau B. Metazoan Hsp70 machines use Hsp110 to power protein disaggregation. EMBO J. 2012;31:4221–4235. doi: 10.1038/emboj.2012.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Lehtonen M, Paimela T, Kaarniranta K. Celastrol: molecular targets of thunder god vine. Biochem Biophys Res Commun. 2010;394:439–442. doi: 10.1016/j.bbrc.2010.03.050. [DOI] [PubMed] [Google Scholar]
- Schuermann JP, et al. Structure of the Hsp110:Hsc70 nucleotide exchange machine. Mol Cell. 2008;31:232–243. doi: 10.1016/j.molcel.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Seeman N. Alzheimer’s disease: beta-amyloid plaque formation in human brain. Synapse. 2011;65:1289–1297. doi: 10.1002/syn.20957. [DOI] [PubMed] [Google Scholar]
- Sharma A, Takata H, Shibahara K, Bubulya A, Bubulya PA. Son is essential for nuclear speckle organization and cell cycle progression. Mol Biol Cell. 2010;21:650–663. doi: 10.1091/mbc.E09-02-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorbagi S, Brown IR. Dynamics of the association of heat shock protein HSPA6 (Hsp70B') and HSPA1A (Hsp70-1) with stress-sensitive cytoplasmic and nuclear structures in differentiated human neuronal cells. Cell Stress Chaperones. 2016 doi: 10.1007/s12192-016-0724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HL, Li W, Cheetham ME. Molecular chaperones and neuronal proteostasis Semin. Cell Dev Biol. 2015;40:142–152. doi: 10.1016/j.semcdb.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector DL, Lamond AI (2011) Nuclear speckles Cold Spring. Harb Perspect Biol 3 doi:10.1101/cshperspect.a000646 [DOI] [PMC free article] [PubMed]
- Sytnikova YA, Kubarenko AV, Schafer A, Weber AN, Niehrs C. Gadd45a is an RNA binding protein and is localized in nuclear speckles. PLoS One. 2011;6:e14500. doi: 10.1371/journal.pone.0014500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyedmers J, Mogk A, Bukau B. Cellular strategies for controlling protein aggregation. Nat Rev Mol Cell Biol. 2010;11:777–788. doi: 10.1038/nrm2993. [DOI] [PubMed] [Google Scholar]
- Weibezahn J, Schlieker C, Tessarz P, Mogk A, Bukau B. Novel insights into the mechanism of chaperone-assisted protein disaggregation. Biol Chem. 2005;386:739–744. doi: 10.1515/BC.2005.086. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, et al. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–56060. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- Yost HJ, Lindquist S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell. 1986;45:185–193. doi: 10.1016/0092-8674(86)90382-X. [DOI] [PubMed] [Google Scholar]
- Yost HJ, Lindquist S. Heat shock proteins affect RNA processing during the heat shock response of Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:1062–1068. doi: 10.1128/MCB.11.2.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Sarge KD. Celastrol inhibits polyglutamine aggregation and toxicity though induction of the heat shock response. J Mol Med (Berl) 2007;85:1421–1428. doi: 10.1007/s00109-007-0251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]