Abstract
There are different views of how the immune system participates in the reaction to cancer. Here, we evaluated expression of DAMP proteins HSP70 and cancer-testis antigen SPAG9 in patients with hepatocellular carcinoma (HCC) and lung cancer to explore tumor immunity. Our analysis showed that levels of HSP70 and SPAG9 antibody were significantly higher in the serum of lung cancer and HCC patients than in the serum of healthy subjects (P < 0.001), but there were no differences in levels of HSP70 antibody in patients and controls. Levels of serum SPAG9 antibody in newly diagnosed lung cancer patients were significantly higher than in treated lung cancer patients (P < 0.05), but there were no differences in levels of HSP70 or HSP70 antibody. Levels of serum HSP70 and SPAG9 antibody, but not HSP70 antibody, were also higher in hepatitis/cirrhosis patients than in healthy subjects (P = 0.005, P < 0.001). Levels of serum SPAG9 antibody were significantly higher in HCC patients than in hepatitis/cirrhosis patients, but there were no differences in HSP70 or HSP70 antibody levels. Finally, levels of serum HSP70 and SPAG9 antibody were significantly higher in HCC patients than in lung cancer patients (P < 0.05, P < 0.001). These results indicate that cancer-testis antigen SPAG9 induces a strong humoral immune response in cancer patients but HSP70 does not. These results show that SPAG9 has potential as a tumor-specific biomarker.
Keywords: Heat shock protein 70, Sperm-associated antigen 9, Auto-antibody, Hepatocellular carcinoma, Lung cancer
Introduction
According to the “danger” theory of immunology, damage-associated molecular patterns (DAMPs) are released by the body’s cells as a signal that there is endogenous danger (Srikrishna and Freeze 2009). DAMPs, such as high mobility group protein B1 and heat shock proteins (HSPs), are released from damaged or necrotic tissue and by some activated immune cells (Pradeu and Cooper 2012). DAMPs and pathogen-associated molecular patterns (PAMPs) signal through toll-like receptors (TLRs) activate antigen presenting cells (APCs) and initiate the adaptive immune response (Miyaji et al. 2011). This theory is a challenge to the traditional self/non-self discrimination (SNSD) theory, which holds that activation of the immune system occurs strictly based on whether or not an entity is foreign. The danger theory, in contrast, posits that the immune system is only triggered by “dangerous” entities.
Extracellular HSP70 and SPAG9 are proteins released by tumor cells (Li et al. 2013; Sinha et al. 2013a), but their characteristics and the roles played in the development of cancer are different. HSP70 is produced in response to internal and external stresses such as trauma, infection, and cancer (Radons 2016; Ren et al. 2016a). HSP70 has a dual role: When present inside cells, it can induce resistance to apoptosis; outside the cell, it can promote apoptosis (Lanneau et al. 2007). Extracellular HSP70 as an endogenous DAMP protein participates in the innate immune response and in the adaptive immune response (Chen and Cao 2010). The ability of HSP70 to accelerate the elimination of tumor cells has been the focus of considerable research (Kumar et al. 2016; Murphy 2013; Meng et al. 2011).
SPAG9 is a member of the cancer-testis antigen (CTA) family, which, as a scaffold protein, plays an important role in the process of sperm-egg fusion (Jagadish et al. 2005a; Jagadish et al. 2005b). In addition to expression in germ cells, it is also expressed in a variety of tumor tissues and can induce a strong humoral immune response indicated by production of auto-antibodies (Garg et al. 2007; Agarwal et al. 2014a; Kanojia et al. 2013; Xie et al. 2014; Ren et al. 2016b). That SPAG9 can induce an immune response in the context of danger (that is, cancer) supports the SNSD theory.
Like SPAG9, HSP70 levels are upregulated in certain cancer patients (Luk et al. 2006; Alaiya et al. 2001). In this study, levels of HSP70, HSP70 antibodies, and SPAG9 antibodies were compared in peripheral blood of patients with lung cancer and hepatocellular carcinoma (HCC) and healthy subjects. We compared the similarities and differences between expression profiles of antigens and auto-antibodies and evaluate the value of these markers in the diagnosis of lung cancer and HCC. We also speculate on how immune responses to these two proteins support the SNSD and danger theories of the immune response to cancer.
Material and methods
Patients and sample collection
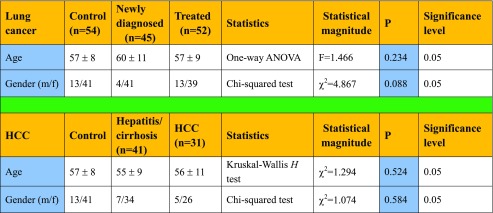
Patients were treated in the Tumor Hospital and Second People’s Hospital of Hunan Province. Diagnoses were confirmed by a pathologist. Patients consented to specimen collection, and the study was approved by the ethics committee of Second People’s Hospital of Hunan Province. Serum samples were obtained from 97 lung cancer patients; the 45 patients who had not yet received treatment were classified as the newly diagnosed group. The 52 lung cancer patients who had received radiotherapy and chemotherapy were classified as the treated group. Lung cancer cases were diagnosed by CT, MRI, fiber-optic bronchoscopy, and percutaneous lung biopsy.
Samples from HCC patients with cancers caused by HBV or by HCV who had not received any treatment and 41 patients with hepatitis/cirrhosis caused by HBV or HCV without HCC were analyzed. HCC was diagnosed as previously reported (Song et al. 2013). HCC inclusion criteria were no prior anti-cancer treatment (drug, radiotherapy, or chemotherapy) and HBV or HCV positive or both positive. Exclusion criteria were pregnancy, reproductive system embryonic tumors, or metastatic liver cancer.
Fifty-four healthy subjects were recruited from the Health Screening Center of Hunan Second People’s Hospital. There were no significant differences in age or gender among groups. Characteristics of the patients are shown in Table 1. Peripheral blood was collected, and the serum was separated by centrifuging blood at 1000×g for 10 min. The serum was stored at −80 °C until analysis.
Table 1.
Comparison of gender and age between groups
ELISA
Recombinant human SPAG9 protein (r-hSPAG9, Abnova) was used as antigen in an ELISA to detect serum anti-SPAG9 IgG antibody levels (Kanojia et al. 2009). Basal levels in the ELISA were established using the serum from 35 healthy donors, and the cutoff signal intensity (mean ± 1.96 SD) was an OD of 0.416 (0.187 ± 0.229). The absorbance was read at 450 nm with 630 nm as reference filter, and the intra-assay and inter-assay coefficients of variation were 2.3 and 8.6%, respectively. HSP70 quantitative analysis was performed on the Multiskan MK3 automatic enzyme immunoassay instrument from Labsystems with reagents from Adlitteram Diagnostic Laboratories. HSP70 quantitative analysis was performed on the Labsystems Multiskan MK3 with reagents from Adlitteram Diagnostic Laboratories.
Statistical analyses
The Pearson chi-squared test, Fisher’s exact test, Student’s t test for unpaired data, Wilcoxon signed-rank test, Mann-Whitney U test, and Kruskal-Wallis test were performed using the SPSS 16.0 statistical software. Results are expressed as means ± standard deviations (SD). All P values were two-sided, and a P value <0.05 was considered statistically significant.
Results
Levels of serum SPAG9 antibody, HSP70, and HSP70 antibody in lung cancer and HCC patients
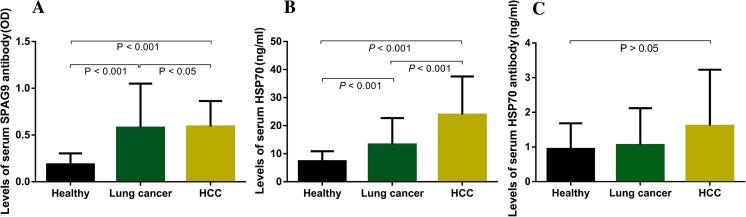
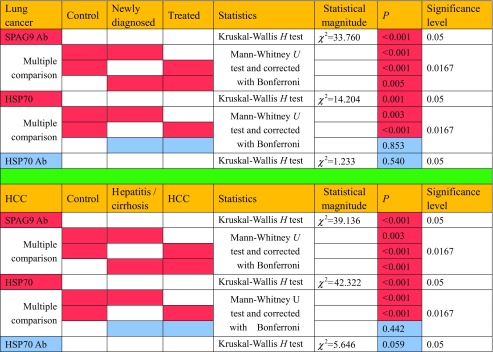
The means of the signal intensities in the SPAG9 ELISA for lung cancer patients (0.579 ± 0.472) and HCC patients (0.590 ± 0.274) were significantly higher than in healthy subjects (0.187 ± 0.117) (P < 0.001), and there were statistically significant differences between the two types of tumors. The concentrations of serum HSP70 in sera from lung cancer patients (13.26 ± 9.37) and HCC patients (23.23 ± 13.64) were significantly higher than in healthy subjects (P < 0.001) irrespective of the disease phase. The result that the concentration of SPAG9 in lung cancer patient sera was lower than that in HCC patient sera was statistically significant (P < 0.05). There were no differences in the levels of serum HSP70 antibody among the patients with HCC or lung cancer and healthy controls (Fig. 1 and Table 2).
Fig. 1.
The means of (a) the signal intensities in the SPAG9 ELISA, (b) the concentrations of serum HSP70, and (c) the serum HSP70 antibody based on ELISA in lung cancer and HCC patients and controls,
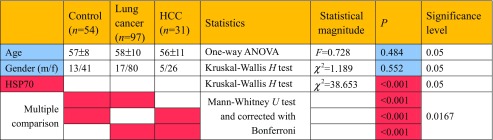
Table 2.
Comparison of serum HSP70 levels between HCC and lung cancer groups
Levels of serum SPAG9 antibody, HSP70, and HSP70 antibody in patients newly diagnosed with lung cancer and treated for lung cancer
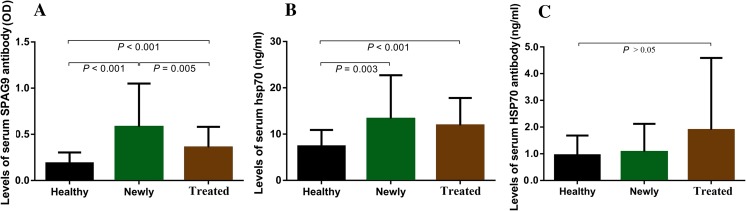
The means of the signal intensity in the SPAG9 ELISA for newly diagnosed lung cancer patients (0.579 ± 0.472) and treated lung cancer patients (0.357 ± 0.225) were significantly higher than in healthy subjects (0.187 ± 0.117) (P < 0.001). The mean of the signal intensity in the SPAG9 ELISA for newly diagnosed lung cancer patients was also significantly higher than in treated lung cancer patients (P = 0.005). Levels of serum HSP70 in newly diagnosed lung cancer patients (13.26 ± 9.37) and treated lung cancer patients (11.88 ± 5.92) were significantly higher than in healthy subjects (8.55 ± 3.53) (P = 0.003, P < 0.001), but there was no statistical difference between newly diagnosed lung cancer patients and treated lung cancer patients. There were no differences in the levels of serum HSP70 antibody among the patients newly diagnosed with lung cancer and treated for lung cancer and healthy controls (Fig. 2 and Table 3).
Fig. 2.
Levels of (a) serum SPAG9 antibody, (b) serum HSP70, and (c) serum HSP70 antibody in newly diagnosed lung cancer patients, treated lung cancer patients, and controls
Table 3.
Comparison of serum HSP70 and SPAG9 antibody levels in different groups
Levels of serum SPAG9 antibody, HSP70, and HSP70 antibody in HCC and hepatitis/cirrhosis patients
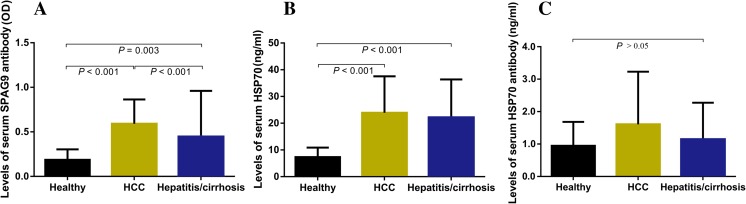
The means of the signal intensities in the SPAG9 ELISA for HCC patients of (0.590 ± 0.274) and hepatitis/cirrhosis patients (0.445 ± 0.515) were significantly higher than that in healthy subjects (0.187 ± 0.117) (P < 0.001 and P = 0.003, respectively). The mean of the signal intensity in the SPAG9 ELISA for HCC patients was significantly higher than that for hepatitis/cirrhosis patients (P < 0.001). Levels of serum HSP70 in HCC patients (23.23 ± 13.64) and in hepatitis/cirrhosis patients (22.14 ± 14.10) were significantly higher than in healthy subjects (8.55 ± 3.53) (P < 0.001) but there was no statistical difference between HCC and hepatitis/cirrhosis patients. There were no differences in the levels of serum HSP70 antibody among the patients with HCC and hepatitis/cirrhosis and healthy controls. (Fig. 3 and Table 3).
Fig. 3.
Levels of (a) serum SPAG9 antibody, (b) serum HSP70, and (c) serum HSP70 antibody in HCC patients, hepatitis/cirrhosis patients, and healthy controls
Discussion
The self/non-self discrimination theory maintains that the body distinguishes between self and non-self components (Bretscher and Cohn 1970; Burnet 1959). In 1994, Matzinger (1994) put forward the danger theory that holds that dangerous situations rather simply non-self antigen can activate the immune response. During infection, stress, injury, necrosis, or tumor formation, for example, certain molecules can trigger host defenses and upregulate costimulatory molecules that induce dendritic cell maturation, leading to effective antigen presentation to T and B cells.
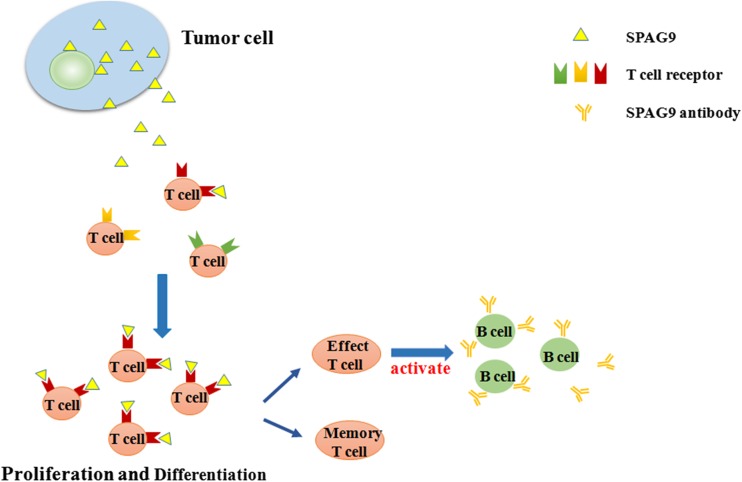
Endogenous proteins such as SPAG9 can induce strong humoral immune responses (Wang et al. 2013; Yu et al. 2012; Agarwal et al. 2014b) when present on the surface of the tumor cells or released from tumor cells to the outside of the cell (Sinha et al. 2013b). In this research, we found that the levels of serum SPAG9 antibody in lung cancer patients and HCC patients were significantly higher than in healthy subjects. The immune response to SPAG9 can be categorized as non-self antigen activation of the immune response because in these cancer patients, SPAG9 is present in peripheral blood, a location where it is not normally found, because of the characteristics of CTA (Fig. 4).
Fig. 4.
Mechanism of SPAG9 antibody production that, based on SNSD theory, activates the Th1 arm of the immune system
In support of the danger theory, however, HSP70 and SPAG9 are DAMPs and serve as danger signals to initiate the humoral immune response (Fig. 5). Interestingly, our data showed that unlike SPAG9, which stimulated the immune system to produce antibodies both in HCC and lung cancer patients, the elevated levels of HSP70 in the serum from cancer patients were not accompanied by increases in levels of antibody to this protein.
Fig. 5.
Tumor cells release DAMPs, such as HSP70 and SPAG9, which induce the Th2 humoral immune response as predicted by the danger theory
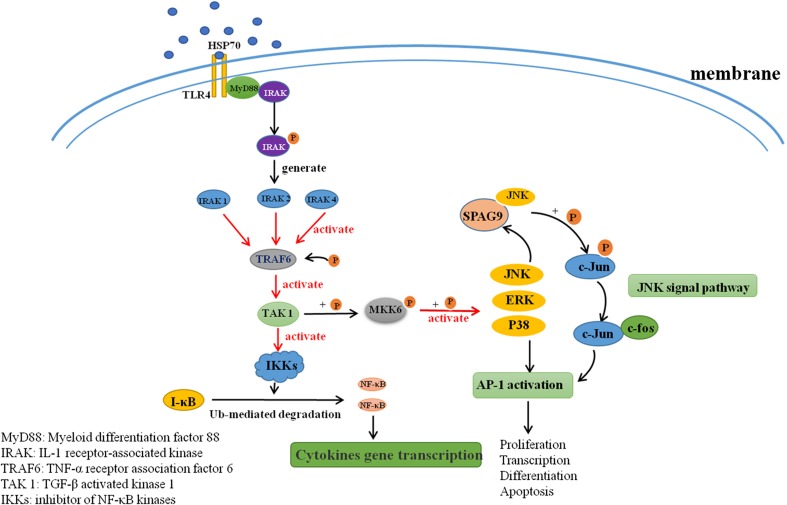
TLRs, especially TLR4, recognize distinct PAMPs and DAMPs that play critical roles in both innate and adaptive immunity. Studies have found that TLRs are expressed on the surfaces of a variety of immune cells and on the surfaces of tumor cells (Huang et al. 2008). The TLR signaling pathways activate ERK, JNK, and p38 (Takeda and Akira 2004), which in turn regulate many immunologically relevant proteins that can trigger tumor self-protection mechanisms (Huang et al. 2005). In this study, we found that on average the serum levels of HSP70 and of SPAG9 antibody were significantly higher in patients with lung cancer and HCC than in healthy controls. No differences in HSP70 antibody levels were observed. It may be that HSP70 released by tumor cells binds to receptors (such as TLR4) on tumor cell surfaces to promote the expression of tumor-specific proteins (such as SPAG9) that in turn promote the growth and metastasis of tumor cells (Fig. 6).
Fig. 6.
HSP70 binds to TLR4 triggering tumor self-protection mechanisms leading to tumor cell secretion of SPAG9
The level of serum SPAG9 antibody in newly diagnosed lung cancer patients was significantly higher than in treated lung cancer patients, but there were no differences in levels of HSP70 or HSP70 antibody. Similarly, levels of serum SPAG9 antibody in HCC patients were significantly higher than in hepatitis/cirrhosis patients but there were no differences in levels of HSP70 or HSP70 antibody. This indicated that HSP70, unlike SPAG9, stimulates the body’s immune system to produce specific antibodies. Concentrations of HSP70 and of SPAG9 antibody in patients with HCC were significantly higher than in patients with lung cancer; the reason is not clear but is worthy of further research. Each of the two theories of how the immune system is activated, the SNSD theory and the danger theory, has some support from this study. The immune stimulatory mechanisms of the human body are very complicated, and we have a long way to go to understand the truth.
Acknowledgements
This work was supported by a research grant from the Health Department of Hunan Province Foundation (B2011-099) and Second People’s Hospital of Hunan Province Key Specialized Foundation.
Abbreviations
- DAMPs
Damage-associated molecular patterns
- HSP
Heat shock protein
- PAMPs
Pathogen-associated molecular patterns
- TLR
Toll-like receptor
- APC
Antigen presenting cell
- SNSD
Self/non-self discrimination
- SPAG9
Sperm-associated antigen 9
- HCC
Hepatocellular carcinoma
- CTA
Cancer-testis antigen
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The study was discussed by the ethics committee of our institute and did not involve the interests of patients; approval was given by the committee. All subjects signed informed consent agreements.
Footnotes
In this paper, we discuss the how our findings relate to the self/non-self discrimination and danger theories of the involvement of the immune system in cancer development. We first put the DAMP protein and CTA combined research.
References
- Agarwal S, Parashar D, Gupta N, et al. (2014a) Sperm associated antigen 9 (SPAG9) expression and humoral response in benign and malignant salivary gland tumors. Oncoimmunology 3(12) [DOI] [PMC free article] [PubMed]
- Agarwal S, Parashar D, Gupta N, et al. (2014b) Sperm associated antigen 9 (SPAG9) expression and humoral response in benign and malignant salivary gland tumors. Oncoimmunology 3(12) [DOI] [PMC free article] [PubMed]
- Alaiya AA, Oppermann M, Langridge J, Roblick U, Egevad L, Brindstedt S, et al. Identification of proteins in human prostate tumor material by two-dimensional gel electrophoresis and mass spectrometry. Cell Mol Life Sci. 2001;58:307–311. doi: 10.1007/PL00000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169(3950):1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- Burnet M (1959) The clonal selection theory of acquired immunity. Cambridge University Press, London
- Chen T, Cao X. Stress for maintaining memory: HSP70 as a mobile messenger for innate and adaptive immunity. Eur J Immunol. 2010;40(6):1541–1544. doi: 10.1002/eji.201040616. [DOI] [PubMed] [Google Scholar]
- Garg M, Chaurasiya D, Rana R, et al. Sperm-associated antigen 9, a novel cancer testis antigen, is a potential target for immunotherapy in epithelial ovarian cancer. Clin Cancer Res. 2007;13(5):1421–1428. doi: 10.1158/1078-0432.CCR-06-2340. [DOI] [PubMed] [Google Scholar]
- Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, Xiong H. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65(12):5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- Huang B, Zhao J, Unkeless JC, et al. TLR signaling by tumor and immune cells: a double-edged sword. Oncogene. 2008;27(2):218–224. doi: 10.1038/sj.onc.1210904. [DOI] [PubMed] [Google Scholar]
- Jagadish N, Rana R, Selvi R, et al. Molecular cloning and characterization of the macaque sperm associated antigen 9 (SPAG9): an orthologue of human SPAG9 gene. Mol Reprod Dev. 2005;71(1):58–66. doi: 10.1002/mrd.20245. [DOI] [PubMed] [Google Scholar]
- Jagadish N, Rana R, Mishra D, et al. Immunogenicity and contraceptive potential of recombinant human sperm associated antigen (SPAG9) J Reprod Immunol. 2005;67(1–2):69–76. doi: 10.1016/j.jri.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Kanojia D, Garg M, Gupta S, Gupta A, Suri A. Sperm-associated antigen 9, a novel biomarker for early detection of breast cancer. Cancer Epidemiol Biomark Prev. 2009;18:630–639. doi: 10.1158/1055-9965.EPI-08-0629. [DOI] [PubMed] [Google Scholar]
- Kanojia D, Garg M, Saini S, et al. Sperm associated antigen 9 plays an important role in bladder transitional cell carcinoma. PLoS One. 2013;8(12):e81348–e81348. doi: 10.1371/journal.pone.0081348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Rd SJ, Singh UP, et al. Targeting Hsp70: a possible therapy for cancer. Cancer Lett. 2016;374(1):156–166. doi: 10.1016/j.canlet.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanneau D, De TA, Maurel S, et al. Apoptosis versus cell differentiation: role of heat shock proteins HSP90, HSP70 and HSP27. Prion. 2007;1(1):53–60. doi: 10.4161/pri.1.1.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li Y, Liu D, et al. Extracellular HSP70/HSP70-PCs promote epithelial-mesenchymal transition of hepatocarcinoma cells. PLoS One. 2013;8(12):e84759. doi: 10.1371/journal.pone.0084759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk JM, Lam CT, Siu AFM, et al. Proteomic profiling of hepatocellular carcinoma in Chinese cohort reveals heat-shock proteins (Hsp27, Hsp70, GRP78) up-regulation and their associated prognostic values. Proteomics. 2006;6(3):1049–1057. doi: 10.1002/pmic.200500306. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12(1):991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Meng L, Hunt C, Yaglom JA, et al. Heat shock protein Hsp72 plays an essential role in Her2-induced mammary tumorigenesis. Oncogene. 2011;30(25):2836–2845. doi: 10.1038/onc.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaji EN, Carvalho E, Oliveira ML, et al. Trends in adjuvant development for vaccines: DAMPs and PAMPs as potential new adjuvants. Braz J Med Biol Res. 2011;44(6):500–513. doi: 10.1590/S0100-879X2011000600003. [DOI] [PubMed] [Google Scholar]
- Murphy ME. The HSP70 family and cancer. Carcinogenesis. 2013;34(6):1181–1188. doi: 10.1093/carcin/bgt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeu T, Cooper EL. The danger theory: 20 years later. Front Immunol. 2012;3:287–287. doi: 10.3389/fimmu.2012.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radons J. The human HSP70 family of chaperones: where do we stand? Cell Stress Chaperones. 2016;21(3):379–404. doi: 10.1007/s12192-016-0676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B, Zou G, Huang Y, Xu G, Xu F, He J, Zhu H, Yu P. Serum levels of HSP70 and other DAMP proteins can aid in patient diagnosis after traumatic injury. Cell Stress Chaperones. 2016;21(4):677–686. doi: 10.1007/s12192-016-0694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B, Wei X, Zou G, He JXG, Xu F, Huang Y, Zhu H, Li Y, Ma G, Yu P. Cancer testis antigen SPAG9 is a promising marker for the diagnosis and treatment of lung cancer. Oncol Rep. 2016;35(5):2599–2605. doi: 10.3892/or.2016.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A, Agarwal S, Parashar D, et al. Down regulation of SPAG9 reduces growth and invasive potential of triple-negative breast cancer cells: possible implications in targeted therapy. J Exp Clin Cancer Res. 2013;32(1):69–69. doi: 10.1186/1756-9966-32-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A, Agarwal S, Parashar D, et al. Down regulation of SPAG9 reduces growth and invasive potential of triple-negative breast cancer cells: possible implications in targeted therapy. J Exp Clin Cancer Res. 2013;32(1):69–69. doi: 10.1186/1756-9966-32-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Gao J, Inagaki Y, Kokudo N, Hasegawa K, Sugawara Y, Tang W. Biomarkers: evaluation of screening for and early diagnosis of hepatocellular carcinoma in Japan and China. Liver Cancer .2 (1):31–39, 2013. [DOI] [PMC free article] [PubMed]
- Srikrishna G, Freeze HH. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer 1. Neoplasia. 2009;11(7):615–628. doi: 10.1593/neo.09284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16(1):3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Wang Y, Dong Q, Yuan M, et al. Clinical significance and biological roles of SPAG9 overexpression in non-small cell lung cancer. Lung Cancer. 2013;81(2):266–272. doi: 10.1016/j.lungcan.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Xie C, Fu L, Liu N, et al. Overexpression of SPAG9 correlates with poor prognosis and tumor progression in hepatocellular carcinoma. Tumor Biol. 2014;35(8):7685–7691. doi: 10.1007/s13277-014-2030-x. [DOI] [PubMed] [Google Scholar]
- Yu P, Yan L, Zhang H, et al. Expression and clinical significance of sperm-associated antigen 9 in patients with endometrial carcinoma. Int J Gynecol Cancer: Off J Int Gynecol Cancer Soc. 2012;22(1):87–93. doi: 10.1097/IGC.0b013e3182370f2e. [DOI] [PubMed] [Google Scholar]