Abstract
Alcohol abuse is a risk factor for a distinct form of congestive heart failure, known as alcoholic cardiomyopathy (ACM). Here, we investigate how microRNAs may participate in the induction of cardiomyocyte apoptosis associated with ethanol exposure in vitro. Increasing the concentrations of ethanol to primary rat cardiomyocytes resulted in elevated apoptosis assessed by annexin V and propidium iodide staining, and reduced expression of an enzyme for alcohol detoxification aldehyde dehydrogenase 2 (ALDH2). These ethanol effects were accompanied by a substantial elevation of miR-378a-5p. Driving miR-378a-5p overexpression in cardiomyocytes decreased ALDH2. The specific interaction of miR-378a-5p with the 3’UTR of ALDH2 was examined by luciferase reporter assays, and we found that miR-378a-5p activity depends on a complementary base pairing at the 3′-UTR region of ALDH2 mRNA. Finally, ethanol-induced apoptosis in cardiomyocytes was attenuated in the presence of anti-miR378a-5p. Collectively, these data implicate a likely involvement of miR-378a-5p in the stimulation of cardiomyocyte apoptosis through ALDH2 gene suppression, which might play a potential role in the pathogenesis of ACM.
Keywords: Alcoholism, microRNA, Apoptosis, Cardiomyocyte, Aldehyde dehydrogenase
Introduction
Alcoholic cardiomyopathy (ACM), which is characterized by cardiac hypertrophy and compromised myocardial contractility associated with chronic alcohol consumption, has long been recognized as one of the major toxicological effects of ethanol in the cardiovascular system (Guzzo-Merello et al. 2015; Piano and Phillips 2014). The link between alcoholism and left ventricle dysfunction has been reported in heart disease (Guzzo-Merello et al. 2014; Guzzo-Merello et al. 2015; Piano and Phillips 2014). For instance, the incidence of congestive cardiomyopathy is greatly enhanced in populations of patients involved with ethanol addiction (Fernandez-Sola 2015). Some of the cardiotoxicity of alcohol have been attributed to acetaldehyde, an oxidized product of ethanol in the human body, which can impair cardiac excitation-contraction coupling and inhibit sarco/endoplasmic-reticulum calcium release (O’Brien et al. 2005; Ren and Wold 2008). Additionally, acetaldehyde is far more toxic and reactive than ethanol in terms of induced tissue and cell injury. By directly interacting with DNA, protein, or lipid, acetaldehyde elicits its cell-damaging effects including mutagenesis, oxidative stress, pro-inflammatory reactions, and cell death (Dey and Cederbaum 2006; Setshedi et al. 2010). Under normal conditions, acetaldehyde is rapidly metabolized in the liver; whereas the levels of acetaldehyde such as in the blood can increase significantly following alcohol intake (Balbo and Brooks 2015; Brooks and Theruvathu 2005). Thus, the research for the mechanisms underlying alcohol-induced myocardial damage has been mainly focused on the toxicity of acetaldehyde (Ren 2007; Ren and Wold 2008). The main enzyme removing acetaldehyde is a family of proteins known as aldehyde dehydrogenase (ALDH) (Edenberg 2007), which catalyze the oxidation of acetaldehyde in the presence of nicotinamide-adenine dinucleotide (NAD). Perhaps the most promising data to support the significance of the acetaldehyde hypothesis come from the animal studies in which the manipulations of acetaldehyde-metabolizing enzymes including ALDH can attenuate or exacerbate cardiac hypertrophy and contractile defect after alcohol exposure (Doser et al. 2009; Li and Ren 2008; Ma et al. 2010).
Aldehyde dehydrogenase 2 (ALDH2) is a crucial ALDH family member in the mitochondria, capable of detoxifying mitochondrial reactive aldehydes that are stimulated by oxidative stress, such as the lipid peroxidation by-product (4-hydroxinonenal). The cardioprotective role of ALDH2 has been demonstrated in various models, including acute ischemia/reperfusion injury (Gong et al. 2012), heart failure (Gomes et al. 2014), and particularly in alcoholic cardiomyopathy (Doser et al. 2009; Ma et al. 2010). In fetal human cardiac myocytes in vitro, ALDH2 overexpression results in an alleviation of acetaldehyde-elicited cell apoptosis (Li et al. 2006). Global transgenic overexpression of ALDH2 in mice effectively decreases chronic alcohol intake-elicited myocardial hypertrophy and contractile defect (Doser et al. 2009). On the contrary, knockout of ALDH2 in mice aggravates the acute ethanol exposure-induced cardiomyocyte dysfunction with elevation in cardiac acetaldehyde levels (Ma et al. 2010). It seems that a potential therapeutic approach for ACM might benefit from ALDH2 upregulation. In fact, physiologic signaling cascades or pharmacological chemicals that activate ALDH2 have been suggested to contribute cardioprotection in reperfusion arrhythmias (Koda et al. 2010), ischemia injury (Robador et al. 2012), or postmyocardial infarction (Gomes et al. 2014). Studying the regulatory pathways for ALDH2 expression in cardiomyocyte following ethanol exposure thus should provide important insights to the molecular targets of ACM treatment.
In the present study, we designed in vitro experiments to evaluate the potential impact of microRNAs (miRNA) on alcohol-induced apoptosis in primary cardiomyocytes. MicroRNAs are a major class of molecular modulators in biology whose functions are mainly through gene repression. The role of microRNAs in alcohol-induced multi-organ injury have been implicated, such as in alcoholic liver disease (Gao and Bataller 2011; Miranda et al. 2010; Natarajan et al. 2015). The exploration for the possible involvement of microRNAs in ALDH2 gene modulation under ethanol exposure may be useful to treat patients with ACM.
Methods and Materials
Rat primary cardiomyocyte culturing
We isolated primary cardiomyocytes from adult rat hearts by a modified method as described (Xu and Colecraft 2009). In brief, the hearts dissected from rat were perfused with collagenase and protease. Ca2+-tolerant cells were obtained by stepwise increases in extracellular Ca2+ concentration in three subsequent wash steps. Cells were filtered, resuspended in DMEM/M-199 containing 4% horse serum at 37 °C. The procedure regarding animal handling and dissection was performed in accordance with the animal protocol approved by the Committee on the Ethics of Animal Experiments of Changhai Hospital. Alcohol (up to 0.5% ethanol in media) was used to treat cells for 4 days.
Apoptosis assay
Apoptosis of primary rat cardiomyocyte following ethanol treatment was assessed by FACS analysis of annexin V/propidium iodide (PI) staining. After treatments, the cells were trypsinized and counted. The cells at 1 × 106 cells/ml in 1 × binding buffer (Sigma-Aldrich) were incubated with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (Sigma-Aldrich) for 15 min in the dark at room temperature. The levels of stain were then analyzed by flow cytometry (FACSCalibur; BD Biosciences), and apoptosis was determined by calculating the percentages of PI-negative and annexin V-positive cells in CellQuest Pro software (BD Biosciences).
Quantitative real-time PCR
We assessed the mRNA level of ALDH2 gene by real-time PCR. Total isolated RNA from cell cultures was reverse transcribed to complementary cDNAs using Superscript II according to manufacturer’s instructions (Biorad). SYBR Green dye-based detection method was used by using the SYBR Green PCR Master Mix assay (Applied Biosystems). A series of dilutions of control cDNA were used to generate the standard curves and validate the melting curves for each primer set. Specific primers used included ALDH2, forward 5′- CAGCTACACCCGCCACGAGC-3′, reverse 5′- GCGGTAGGGCCGAATCCAGG-3′; GAPDH, forward 5′- ATGTGCCGGACCTTGGAAG -3′, reverse: 5′- CCTCGGGTTAGCTGAGAGATCA-3′. Triplicated PCR reactions were carried out for each sample. Minus reverse transcriptase and no template samples were used as negative controls, and GAPDH was used as a housekeeping gene for normalization.
Western blotting
Western blotting was performed in cultured cells following as indicated. The cells were lysed in buffer containing 1% NP40, 50 mM Tris, 5 mM EDTA, 1% SDS, 1% sodium deoxycholate, 1% Triton X-100, 1 mM PMSF, 10 mg/ml aprotinin, 1 mg/ml leupeptin, and pH = 7.5. Protein amounts were measured by Bradford assay and 50 micrograms total proteins was resolved on SDS-PAGE. Following an electric transfer to a PVDF membrane, proteins on the blots were blocked by 5% nonfat milk and incubated with primary antibodies, including ALDH2 (1:1000, Abcam) or GAPDH (1:1000, Abcam) at 4 °C overnight. The membranes were then incubated by HRP conjugated secondary antibody, and signals were visualized by an enhanced ECL-based imaging system.
MicroRNA profiling
Total RNA including miRNA was isolated by using Trizol (Invitrogen) and was labeled as the first strand biotin-cDNA. The potential regulatory miRNAs were selected by TargetScanHuman (Lewis et al. 2005) based on 3′-UTRs of rat ALDH2 genes. The resultant miRNAs were analyzed with a customized probe set containing designed oligos (IDT) based on the miRNA registry database (Liu et al. 2008). We then used this customized miRNA microarray in the primary cardiomyocyte treated with ethanol. We performed the hybridization step by a hybridization station (Tecan) followed by an indirect detection of streptavidin-Alexa647 conjugate (Invitrogen). The images of microarray were analyzed by GenePix Pro (Molecular Devices).
MicroRNA assays
Expression of rat miR-378a-5p was determined with TaqMan Advanced miRNA Assays (rno481145_mir), purchased from ThermoFisher Scientific, according to manufacturer’s instructions. The miRNA mimic (MC10049) and inhibitor (MH10049) for rat miR-378a-5p were purchased from ThermoFisher Scientific, and transfected into cells using Nucleofector system (Lonza).
Luciferase assay
We used reporter assay to evaluate the miR-378a binding sites in the ALDH2 3′ UTR. The wild type or mutant miR-378a-5p target sequences at the 3′ UTR of ALDH2 were cloned into the pGL3 plasmid at the downstream of luciferase gene. All transfections were performed by Nucleofector system (Lonza), and the activities of firefly luciferase and Renilla luciferase in the cell lysates were measured with the Dual-Luciferase Assay System (Promega).
Results
Ethanol exposure induces apoptosis in rat primary cardiomyocytes
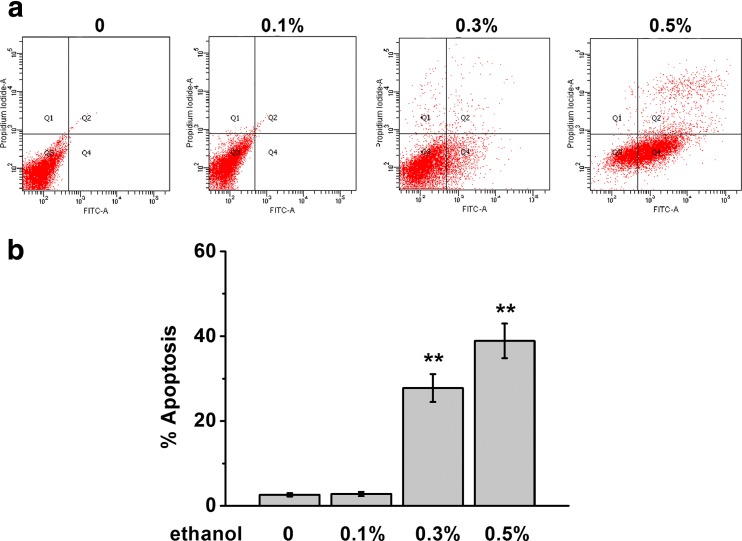
Effect of alcohol was studied in vitro by treating primary rat cardiomyocyte with various concentrations of ethanol (Fig. 1). Four days later, cell apoptosis was evaluated by double staining of annexin V/PI (Fig. 1a). Gating at the early cell death/apoptosis population with specific surface markers (PI-negative and annexin V-positive), we observed a dose response of ethanol exposure in cardiomyocyte apoptosis (Fig. 1a, b). Comparing to vehicle-treated control cells, the percentage of apoptotic cells was greatly increased starting at 0.3% ethanol treatments in cardiomyocytes.
Fig. 1.
Ethanol exposure induces apoptosis in rat primary cardiomyocytes. The cells were exposed to different concentrations of ethanol (0.1, 0.3, and 0.5%) for 4 days and subjected to detection. a, b Annexin V-FITC/PI staining was employed to detect the apoptosis. Apoptosis percentage in each group was analyzed by flow cytometry. Data were presented as mean ± S.D. **p < 0.01 compared to control (no ethanol treatment)
Ethanol exposure decreases ALDH2 expression in rat primary cardiomyocytes
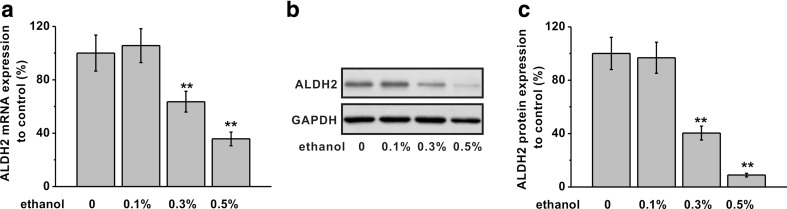
As an important enzyme of ethanol metabolism, ALDH2 gene expression was measured in primary cardiomyocytes following ethanol treatment (the same conditions as Fig. 1). As Fig. 2a shows, the mRNA levels of ALDH2 were significantly reduced in cells under 0.3 or 0.5% ethanol. Importantly, this change in transcript also corresponded to a decrease of ALDH2 protein (Fig. 2b, c).
Fig. 2.
Effects of ethanol exposure on ALDH2 mRNA and protein expressions in rat primary cardiomyocytes. The cells were exposed to different concentrations of ethanol (0.1, 0.3, and 0.5%) for 4 days and subjected to detection. a ALDH2 mRNA expression was analyzed by RT-PCR. GAPDH was used as an internal control. b ALDH2 protein expression was analyzed by western blot. GAPDH was employed as a loading control. Relative ALDH2 protein expression to control in each group was shown in c. Data were presented as mean ± S.D. **p < 0.01 compared to control (no ethanol treatment)
MiR-378a-5p mediates the ALDH2 reduction in cardiomyocytes stimulated by ethanol
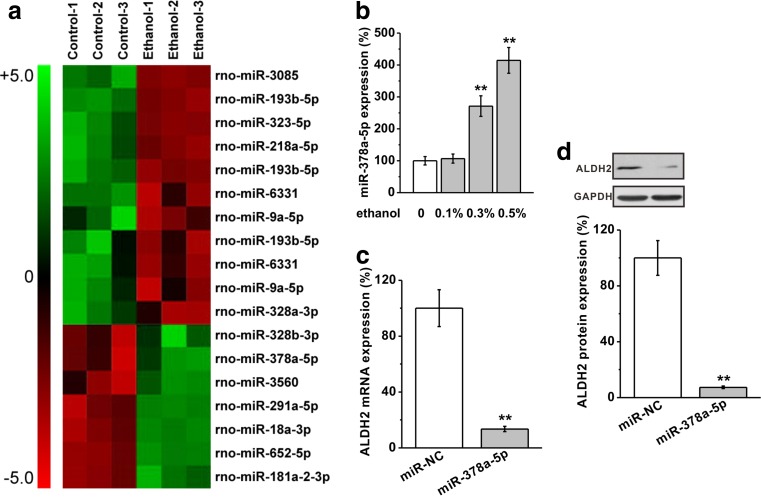
In order to search for putative microRNAs that may mediate the ethanol effect on ALDH2 suppression, we have analyzed the sequence at the 3′ untranslated region (UTR) of rat ALDH2 gene for the complementary sequence of microRNAs by using TargetScanHuman (Lewis et al. 2005). Eighteen candidate miRNAs were selected and studied in a microarray-based customized screen to examine their expression levels in cardiomyocytes following ethanol exposure (Fig. 3a). As shown in Fig. 3a and confirmed by Fig. 3b, 0.3 and 0.5% ethanol treatment on cardiomyocytes for 4 days induced a significant increase in miR-378a-5p. We then directly tested its role on ALDH2 expression by overexpressing miR-378a-5p in cardiomyocyte, which resulted in a significant reduction of ALDH2 expression (Fig. 3c). In agreement with the findings on gene modulation, we further demonstrated that the ALDH2 protein was also downregulated by miR-378a-5p overexpression in rat cardiomyocytes (Fig. 3d). Taken together, these data suggest that miR-378a-5p induced by ethanol exposure could mediate the suppression of ALDH2 in cardiomyocytes.
Fig. 3.
Ethanol (0.5%) inhibits ALDH2 expressions through miR-378a-5p in rat primary cardiomyocytes. a 18 μ-RNAs predicted to target ALDH2 were found to be upregulated or downregulated by ethanol treatment by microarray analysis. b Level of miR-378a-5p was analyzed by mature miRNA assay in rat primary cardiomyocytes exposed to ethanol. c ALDH2 mRNA level in the cells transfected with either negative control miR (miR-NC) or miR-378a-5p. mRNA levels were analyzed by RT-PCR. GAPDH was used as an internal control. d ALDH2 protein expression level was examined by western blot analysis. GAPDH was employed as a loading control. Data were presented as mean ± S.D. **p < 0.01 compared to control (no ethanol treatment)
MiR-378a-5p targets on 3’UTR of ALDH2 to repress gene expression
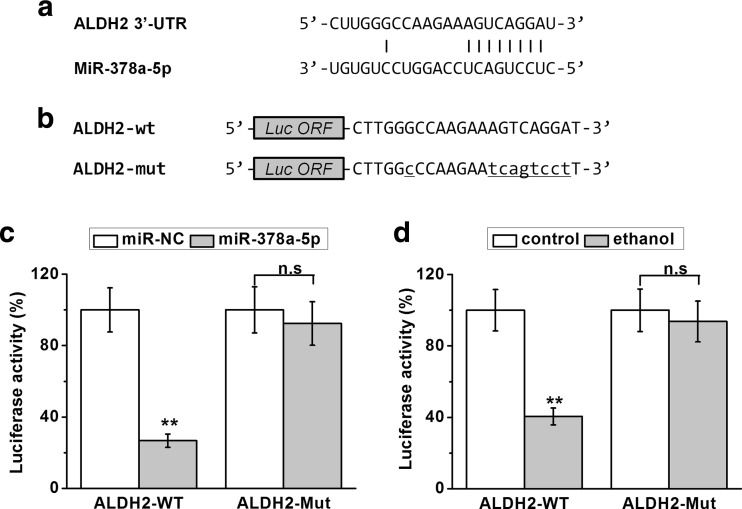
To test the specific interaction of miR-378a-5p with the 3’UTR of ALDH2, we then performed a luciferase reporter assay. The potential base pairing of miR-378a-5p at the 3′-UTRs of ALDH2 was highlighted in Fig. 4a. In order to examine the activity of 3′-UTR of ALDH2, the wild type or mutant miR-378a-5p target sequences at the 3′ UTR of ALDH2 were cloned into the pGL3 plasmid at the downstream of luciferase gene as indicated (Fig. 4b). As shown in Fig. 4c, co-transfection of miR-378a-5p greatly decreased the reporter activity of wild type 3′-UTR but not on the mutated 3-UTR of ALDH2. Similar modulation was found in ethanol-treated cells (Fig. 4d). The ethanol treatment also decreased the luciferase activity of wild type but not the mutant 3′-UTR of ALDH2. These results confirm that ethanol-stimulated miR-378a-5p could repress ALDH2 gene expression in primary cardiomyocytes, possibly through a direct base pairing with the 3′-UTR region of ALDH2 mRNA.
Fig. 4.
MiR-378a-5p directly targets the 3′-UTR on the mRNA of ALDH2. a Sequences of the predicted miR-378a-5p targeting sites on the 3′-UTR of ALDH2 mRNA. b Wild type (-WT) or mutated (-Mut) sequences from ALDH2 mRNA 3′-UTR were cloned downstream of luciferase reporter gene (LUF). Luciferase activities of -WT and -Mut constructs for ALDH2 were determined in rat primary cardiomyocytes with miR-NC/miR-378a-5p transfection (c), or with ethanol treatment (d). Data were presented as mean ± S.D. **p < 0.01 and n.s (p > 0.05) compared to control (no ethanol treatment)
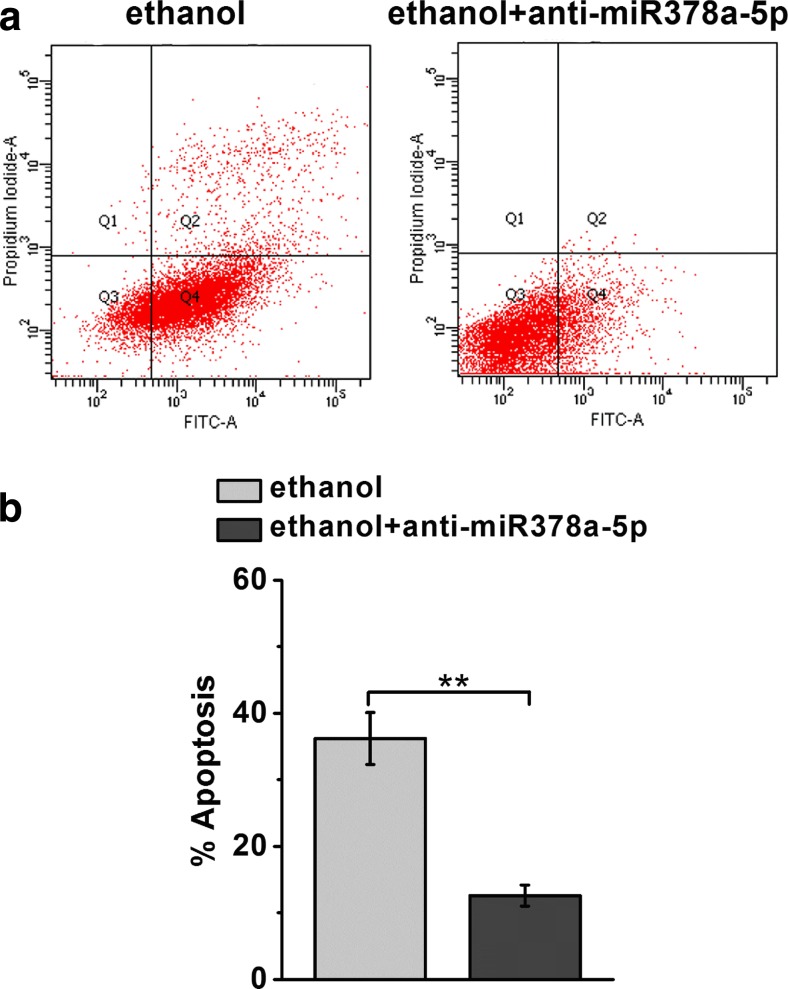
Blocking miR-378a-5p attenuates cell apoptosis of cardiomyocytes stimulated by ethanol
To further study the potential role of miR-378a-5p in ethanol-stimulated apoptosis in cardiomyocytes, we used miR-378a-5p inhibitor (anti- miR378a-5p). Comparing to controls, the induction of apoptosis by ethanol was substantially decreased in cardiomyocytes treated with anti-miR378a-5p (Fig. 5a, b). This data implies that miR378a-5p is indeed required for the induction of cell apoptosis by ethanol in primary cardiomyocytes.
Fig. 5.
MiR-378a-5p inhibitor (anti-miR-378a-5p) significantly reduces ethanol-induced apoptosis in rat primary cardiomyocytes. The cells were treated with ethanol (0.5%) in addition with or without anti-miR-378a-5p for 4 days. a, b Annexin V-FITC/PI staining and flow cytometry was employed to detect the apoptosis. Data were presented as mean ± S.D. **p < 0.01 compared to ethanol-treated group
Discussion
The hallmark of alcoholic cardiomyopathy is cardiac hypertrophy (cardiomegaly) and myocardial dysfunction (compromised contractility), although the precise explanations remain elusive. As one contributor to the onset of alcoholic cardiomyopathy, cell death is directly implicated in long-term alcohol effects, particularly including ethanol-stimulated apoptosis in the cardiac muscle. It has been observed that in the hearts of individuals with history of alcoholism the structural heart damage is associated with higher apoptotic indexes compared with control subject, a similar change as the damaged hearts of long-standing hypertensive origin (Fernandez-Sola et al. 2006). The resulting increase in cellular apoptosis has been linked to oxidative stress and may ultimately contribute to the adverse regulation of cardiac remodeling in chronic alcoholism (Jing et al. 2012).
Here, our study in primary cardiomyocyte presents a novel mechanism underlying such apoptotic response following ethanol exposure for 4 days in culture. Specifically in this setting, the acute effects of ethanol on cardiomyocyte are the main focus of the study. A direct consequence of acute ethanol treatment was examined. In general, the findings support the acetaldehyde theory in which the first oxidized metabolite of ethanol is believed to mediate the major toxic effects of alcoholic injury. In agreement with the notion of acetaldehyde as a primary cause for the loss of cardiomyocytes from alcohol-induced apoptosis, we found that the expression of ALDH2, the key acetaldehyde-metabolizing enzyme, is dramatically suppressed in primary cardiomyocyte upon an extended culture in the presence of 0.3–0.5% ethanol. Alcohol intake can increase the acetaldehyde level significantly (Balbo and Brooks 2015; Brooks and Theruvathu 2005). In principle, the observed ALDH2 reduction may exacerbate cell death stimulated by ethanol, as an anti-apoptotic action of ALDH2 associated with p47 (phox) NADPH oxidase has been implied by the studies using ALDH2 knockout mice (Liao et al. 2012). Reversely, it has been demonstrated that the increase of ALDH2 gene expression could confer protection to acetaldehyde- or ethanol-stimulated cardiomyocyte injury in vitro (Li et al. 2006). As a response to enhance ethanol catabolism, the upregulation of ALDH2 expression has often been observed in several cases of ethanol exposure. For instance, ALDH2 mRNA of peripheral blood leukocytes increases following alcohol ingestion (0.4 g/kg body weight) in healthy young subjects (Kimura et al. 2009). In postmortem brain of alcohol-related disorders, elevated expression levels of ALDH2 were observed in the prefrontal cortex (Zhang et al. 2014). In addition, the activity of ALDH2 in the liver was significantly increased by acute ethanol exposure (6 g/kg intragastrically for 3 days) in mice (Ding et al. 2014). These results have suggested that ALDH2 gene modulation is likely presenting a compensatory regulation for ethanol metabolism, which is a potential therapeutic target for the prevention and treatment of alcohol-related disorders. In the current study, it is intriguing to find out that the expression of ALDH2 is an autonomous target of ethanol as well. However, acute ethanol exposure substantially reduced ALDH2 in the cardiomyocyte. The physiological relevance of such gene regulation is unclear at the moment. Rather than an evolutionary pathway to shape ethanol-related metabolism as previously discussed, the inhibition of ALDH2 by ethanol we found here may mediate an unknown effect to spread cell response to acute ethanol administration. As such, our data pinpoints ALDH2 gene suppression by ethanol as a possible pathological reaction that could amplify the deleterious effects of ethanol on cardiomyocyte. Presumably, this acetaldehyde-specific signaling appears to be critical for acute alcohol-induced myocardial damage, which may cause long-lasting in vivo effects such as the loss of cardiomyocytes due to apoptosis induction.
Alcohol ingestion can disrupt cardiovascular homeostasis through various intracellular pathways, including small regulatory RNAs. As master regulators of endogenous gene modulation in multiple diseases, the implication of microRNAs in mediating the ethanol effects has emerged as crucial mechanisms with therapeutic potential. It has been estimated in large-scale miRNA screens about 2–3% of miRNA expression can be altered by ethanol, with both up- and downregulated changes observed (Dolganiuc et al. 2009; Wang et al. 2009). Upregulation of miRNAs has been associated with alcoholism in the liver, brain, or circulation (Bala et al. 2011; Bala et al. 2012; Lewohl et al. 2011). Specifically, miR-378 expressions were found significantly higher in the serum of humans with alcohol use disorders (Ignacio et al. 2015). MiR-378 includes both miR-378-5p and miR-378-3p, which are mature microRNAs derived from a common hairpin RNA precursor expressed from a single non-coding gene (Nagalingam et al. 2013). Although neither miR-378-5p nor ALDH2 gene has been previously identified as a target of ethanol exposure, prior studies in ischemia conditions have shown a list of miRNAs that negatively regulate ALDH2 function could be associated with increased cardiomyocyte apoptosis. Overexpression of miR-34a, which increases in acute myocardial infarction patients, could reduce ALDH2 expression in neonatal rat cardiomyocyte (Fan et al. 2013). In a recent report, miR-28 was shown to possess the ability of ALDH2 repression as well (Li et al. 2015). Interestingly, miR-28 level is specifically increased in myocardial cells under hypoxic condition. These two microRNAs have not been examined in our experiments. Whether miR-34 or miR-28 expression could also be directly stimulated by ethanol will need to be determined by future studies in cardiomyocyte. Given the tremendous breadth of miRNA function that covers diverse gene modulations, it is reasonable to expect that more ethanol-targeted genes through miRNAs will be identified. Although the mechanism of miRNA regulation by alcohol still remains largely unexplored, the capacity of miRNAs to control large numbers of genes may be utilized as an evolutionary mechanism to develop alcohol-responsive changes as amplifiable modules at the cellular and system level.
The reason for increasing miR-378a-5p expression under ethanol exposure in the cardiomyocyte is intriguing. Previous studies have shown that miR-378 is expressed in cancer cells and involved in proliferation, angiogenesis, and tumor growth (Lee et al. 2007). In a recent report, Wang Z et al. (Wang et al. 2015) showed that expression of miR-378-5p was downregulated in colorectal cancer tissues and cell lines. Furthermore, they found that miR-378-5p can decrease proliferation in cancer cells as a potential tumor suppressor. On the other hand, miR-378 expression (including both miR-378-3p and miR-378-5p) is highly enriched in the heart and skeletal muscle tissues. Another study shows that postexercise increases miR-378-5p expression in human muscle biopsies, implicating its role in exercise adaptation (Camera et al. 2016). The cardiac function of miR-378-3p has been attributed to anti-hypertrophic signaling (Ganesan et al. 2013). Although our data suggest that the increased miR-378-5p is proapoptotic through repressing acetaldehyde metabolism, perhaps there could be other targets of miR-378-5p that provide benefits following cardiac stress. Alternatively, cardiac miR-378 expression may be used to adjust systemic homeostasis in response to alcohol uptake. For instance, miR-378 knockout mice lacking both miR-378-3p and miR-378-5p contain increased mitochondrial fatty acid metabolism and enhanced oxidative capacity of insulin-target tissues (Carrer et al. 2012). The knockout mice do not have a basal phenotype relating to heart. It would be important to evaluate the function of miR-378-5p in these mice after acute or chronic alcohol intake. The results of such study will help to further verify the role of microRNA in alcoholic cardiomyopathy, with the potential to clarify the acetaldehyde hypothesis by testing ALDH2 function in alcoholic complications.
Conclusion
In the current study, we found that expression of a specific microRNA, miR-378a-5p, is enhanced in cardiomyocytes treated with acute ethanol exposure. Overexpression of miR-378-5p suppresses ALDH2 through a complementary base pairing at its 3′-UTR. Functionally, inhibiting miR-378a-5p attenuates ethanol-stimulated apoptosis. Future studies are needed for studying the effects of chronic ethanol exposure on miR-378a-5p. These observations collectively suggest that ALDH2 repression through miR-378a-5p could potentially be a molecular target for alleviating ethanol-induced apoptosis in cardiomyocyte.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (NO. 81500210)
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Zhongkai Wang and Jingwen Song contributed equally.
Contributor Information
Feng Chen, Phone: +86-13761773484, Email: chenfengshanghai@163.com.
Xianxian Zhao, Phone: +86-02131161266, Email: zhaoxianxian168@126.com.
References
- Bala S, Marcos M, Kodys K, Csak T, Catalano D, Mandrekar P, Szabo G. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor α (TNFα) production via increased mRNA half-life in alcoholic liver disease. J Biol Chem. 2011;286:1436–1444. doi: 10.1074/jbc.M110.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala S, et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56:1946–1957. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbo S, Brooks PJ. Implications of acetaldehyde-derived DNA adducts for understanding alcohol-related carcinogenesis. Adv Exp Med Biol. 2015;815:71–88. doi: 10.1007/978-3-319-09614-8_5. [DOI] [PubMed] [Google Scholar]
- Brooks PJ, Theruvathu JA. DNA adducts from acetaldehyde: implications for alcohol-related carcinogenesis. Alcohol. 2005;35:187–193. doi: 10.1016/j.alcohol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Camera DM, Ong JN, Coffey VG, Hawley JA. Selective modulation of microRNA expression with protein ingestion following concurrent resistance and endurance exercise in human skeletal muscle. Front Physiol. 2016;7:87. doi: 10.3389/fphys.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrer M, et al. Control of mitochondrial metabolism and systemic energy homeostasis by microRNAs 378 and 378. Proc Natl Acad Sci U S A. 2012;109:15330–15335. doi: 10.1073/pnas.1207605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–S74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- Ding X, Beier JI, Baldauf KJ, Jokinen JD, Zhong H, Arteel GE. Acute ethanol preexposure promotes liver regeneration after partial hepatectomy in mice by activating ALDH2. American journal of physiology Gastrointestinal and liver physiology. 2014;306:G37–G47. doi: 10.1152/ajpgi.00085.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolganiuc A, Petrasek J, Kodys K, Catalano D, Mandrekar P, Velayudham A, Szabo G. MicroRNA expression profile in Lieber-DeCarli diet-induced alcoholic and methionine choline deficient diet-induced nonalcoholic steatohepatitis models in mice. Alcohol Clin Exp Res. 2009;33:1704–1710. doi: 10.1111/j.1530-0277.2009.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doser TA, Turdi S, Thomas DP, Epstein PN, Li SY, Ren J. Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake–induced myocardial hypertrophy and contractile dysfunction. Circulation. 2009;119:1941–1949. doi: 10.1161/CIRCULATIONAHA.108.823799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5–13. [PMC free article] [PubMed] [Google Scholar]
- Fan F, et al. MicroRNA-34a promotes cardiomyocyte apoptosis post myocardial infarction through down-regulating aldehyde dehydrogenase 2. Curr Pharm Des. 2013;19:4865–4873. doi: 10.2174/13816128113199990325. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sola J. Cardiovascular risks and benefits of moderate and heavy alcohol consumption. Nat Rev Cardiol. 2015;12:576–587. doi: 10.1038/nrcardio.2015.91. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sola J, Fatjo F, Sacanella E, Estruch R, Bosch X, Urbano-Marquez A, Nicolas JM. Evidence of apoptosis in alcoholic cardiomyopathy. Hum Pathol. 2006;37:1100–1110. doi: 10.1016/j.humpath.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Ganesan J, et al. MiR-378 controls cardiac hypertrophy by combined repression of mitogen-activated protein kinase pathway factors. Circulation. 2013;127:2097–2106. doi: 10.1161/CIRCULATIONAHA.112.000882. [DOI] [PubMed] [Google Scholar]
- Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes KM, et al. Aldehyde dehydrogenase 2 activation in heart failure restores mitochondrial function and improves ventricular function and remodelling. Cardiovasc Res. 2014;103:498–508. doi: 10.1093/cvr/cvu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Zhang Y, Zhang H, Gu H, Jiang Q, Hu S. Aldehyde dehydrogenase-2 activation during cardioplegic arrest enhances the cardioprotection against myocardial ischemia-reperfusion injury. Cardiovasc Toxicol. 2012;12:350–358. doi: 10.1007/s12012-012-9179-6. [DOI] [PubMed] [Google Scholar]
- Guzzo-Merello G, Cobo-Marcos M, Gallego-Delgado M, Garcia-Pavia P. Alcoholic cardiomyopathy. World J Cardiol. 2014;6:771–781. doi: 10.4330/wjc.v6.i8.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzo-Merello G, et al. Natural history and prognostic factors in alcoholic cardiomyopathy. JACC Heart Fail. 2015;3:78–86. doi: 10.1016/j.jchf.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Ignacio C, Hicks SD, Burke P, Lewis L, Szombathyne-Meszaros Z, Middleton FA. Alterations in serum microRNA in humans with alcohol use disorders impact cell proliferation and cell death pathways and predict structural and functional changes in brain. BMC Neurosci. 2015;16:55. doi: 10.1186/s12868-015-0195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L, et al. Chronic alcohol intake-induced oxidative stress and apoptosis: role of CYP2E1 and calpain-1 in alcoholic cardiomyopathy. Mol Cell Biochem. 2012;359:283–292. doi: 10.1007/s11010-011-1022-z. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Nishimura FT, Abe S, Fukunaga T, Tanii H, Saijoh K. A promoter polymorphism in the ALDH2 gene affects its basal and acetaldehyde/ethanol-induced gene expression in human peripheral blood leukocytes and HepG2 cells. Alcohol Alcohol. 2009;44:261–266. doi: 10.1093/alcalc/agn123. [DOI] [PubMed] [Google Scholar]
- Koda K, et al. Aldehyde dehydrogenase activation prevents reperfusion arrhythmias by inhibiting local renin release from cardiac mast cells. Circulation. 2010;122:771–781. doi: 10.1161/CIRCULATIONAHA.110.952481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Deng Z, Wang CH, Yang BB. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci U S A. 2007;104:20350–20355. doi: 10.1073/pnas.0706901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Nunez YO, Dodd PR, Tiwari GR, Harris RA, Mayfield RD (2011) Up-regulation of microRNAs in brain of human alcoholics Alcohol Clin Exp Res 35:1928–1937 doi:10.1111/j.1530-0277.2011.01544.x [DOI] [PMC free article] [PubMed]
- Li SP, Liu B, Song B, Wang CX, Zhou YC. miR-28 promotes cardiac ischemia by targeting mitochondrial aldehyde dehydrogenase 2 (ALDH2) in Mus musculus cardiac myocytes. Eur Rev Med Pharmacol Sci. 2015;19:752–758. [PubMed] [Google Scholar]
- Li SY, Li Q, Shen JJ, Dong F, Sigmon VK, Liu Y, Ren J. Attenuation of acetaldehyde-induced cell injury by overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene in human cardiac myocytes: role of MAP kinase signaling. J Mol Cell Cardiol. 2006;40:283–294. doi: 10.1016/j.yjmcc.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Li SY, Ren J. Cardiac overexpression of alcohol dehydrogenase exacerbates chronic ethanol ingestion-induced myocardial dysfunction and hypertrophy: role of insulin signaling and ER stress. J Mol Cell Cardiol. 2008;44:992–1001. doi: 10.1016/j.yjmcc.2008.02.276. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liao J, Sun A, Xie Y, Isse T, Kawamoto T, Zou Y, Ge J. Aldehyde dehydrogenase-2 deficiency aggravates cardiac dysfunction elicited by endoplasmic reticulum stress induction. Mol Med. 2012;18:785–793. doi: 10.2119/molmed.2011.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CG, Calin GA, Volinia S, Croce CM. MicroRNA expression profiling using microarrays. Nature protocols. 2008;3:563–578. doi: 10.1038/nprot.2008.14. [DOI] [PubMed] [Google Scholar]
- Ma H, et al. Aldehyde dehydrogenase 2 knockout accentuates ethanol-induced cardiac depression: role of protein phosphatases. J Mol Cell Cardiol. 2010;49:322–329. doi: 10.1016/j.yjmcc.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda RC, et al. MicroRNAs: master regulators of ethanol abuse and toxicity? Alcohol Clin Exp Res. 2010;34:575–587. doi: 10.1111/j.1530-0277.2009.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagalingam RS, Sundaresan NR, Gupta MP, Geenen DL, Solaro RJ, Gupta M. A cardiac-enriched microRNA, miR-378, blocks cardiac hypertrophy by targeting Ras signaling. J Biol Chem. 2013;288:11216–11232. doi: 10.1074/jbc.M112.442384. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Natarajan SK, Pachunka JM, Mott JL. Role of microRNAs in alcohol-induced multi-organ injury. Biomolecules. 2015;5:3309–3338. doi: 10.3390/biom5043309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol. 2005;35:609–662. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- Piano MR, Phillips SA. Alcoholic cardiomyopathy: pathophysiologic insights. Cardiovasc Toxicol. 2014;14:291–308. doi: 10.1007/s12012-014-9252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J (2007) Acetaldehyde and alcoholic cardiomyopathy: lessons from the ADH and ALDH2 transgenic models Novartis Found Symp 285:69–76; discussion 76–69, 198–199 [DOI] [PubMed]
- Ren J, Wold LE. Mechanisms of alcoholic heart disease. Ther Adv Cardiovasc Dis. 2008;2:497–506. doi: 10.1177/1753944708095137. [DOI] [PubMed] [Google Scholar]
- Robador PA, Seyedi N, Chan NY, Koda K, Levi R. Aldehyde dehydrogenase type 2 activation by adenosine and histamine inhibits ischemic norepinephrine release in cardiac sympathetic neurons: mediation by protein kinase Cepsilon. J Pharmacol Exp Ther. 2012;343:97–105. doi: 10.1124/jpet.112.196626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setshedi M, Wands JR, Monte SM. Acetaldehyde adducts in alcoholic liver disease. Oxidative Med Cell Longev. 2010;3:178–185. doi: 10.4161/oxim.3.3.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LL, et al. Ethanol exposure induces differential microRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation. Hum Reprod. 2009;24:562–579. doi: 10.1093/humrep/den439. [DOI] [PubMed] [Google Scholar]
- Wang Z, et al. MicroRNA-378-5p suppresses cell proliferation and induces apoptosis in colorectal cancer cells by targeting BRAF. Cancer Cell Int. 2015;15:40. doi: 10.1186/s12935-015-0192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Colecraft HM (2009) Primary culture of adult rat heart myocytes Journal of visualized experiments : JoVE doi:10.3791/1308 [DOI] [PMC free article] [PubMed]
- Zhang H, Wang F, Xu H, Liu Y, Liu J, Zhao H, Gelernter J. Differentially co-expressed genes in postmortem prefrontal cortex of individuals with alcohol use disorders: influence on alcohol metabolism-related pathways. Hum Genet. 2014;133:1383–1394. doi: 10.1007/s00439-014-1473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]