Abstract
Histone deacetylase 6 (HDAC6) plays an important role in stress responses such as misfolded protein-induced aggresomes, autophagy, and stress granules. However, precisely how HDAC6 manages response during and after cellular stress remains largely unknown. This study aimed to investigate the effect of HDAC6 on various stress and post-stress recovery responses. We showed that HIF-1α protein levels were reduced in HDAC6 knockout (KO) MEFs compared to wild-type (WT) MEFs in hypoxia. Furthermore, under hypoxia, HIF-1α levels were also reduced following rescue with either a catalytically inactive or a ubiqiutin-binding mutant HDAC6. HDAC6 deacetylated and upregulated the stability of HIF-1α, leading to activation of HIF-1α function under hypoxia. Notably, both the deacetylase and ubiquitin-binding activities of HDAC6 contributed to HIF-1α stabilization, but only deacetylase activity was required for HIF-1α transcriptional activity. Suppression of HDAC6 enhanced the interaction between HIF-1α and HSP70 under hypoxic conditions. In addition to hypoxia, depletion of HDAC6 caused hypersensitivity to cell death during oxidative stress and post-stress recovery. However, HDAC6 depletion had no effect on cell death in response to heat shock or ionizing radiation. Overall, our data suggest that HDAC6 may serve as a critical stress regulator in response to different cellular stresses.
Keywords: Histone deacetylase 6, Hypoxia, HIF-1α, Oxidative stress, Post-stress recovery, Apoptosis
Introduction
Cellular stress responses are an integral part of normal physiology to either ensure the cell’s survival or to eliminate damaged cells (Fulda et al. 2010). Cells have evolved multiple strategies to cope with various exogenous stresses, including the heat shock response, the unfolded protein response, the integrated stress response, the interferon response, and the endoplasmic reticulum (ER) stress response (DeGracia and Hu 2007). This group of stress response pathways is activated in response to intracellular protein damage. In general, cellular stress responses comprise two major parallel pathways (DeGracia and Hu 2007). The first is the transient suppression of protein synthesis as a means of dealing with the exogenous stressors (Brostrom and Brostrom 1998). This reversible translation arrest is recognized as a general response of eukaryotic cells to exogenous stressors. Examples of stresses that induce translation arrest are heat shock (Panniers 1994), heavy metal poisoning and oxidative stress (McEwen et al. 2005), ER stressors (Ron 2002), viral infection (Gale et al. 2000), nutrient deprivation (Halford et al. 2004), excessive free radical production (Kaufman 1999), and ethanol intoxication (Ron 2004), among others (DeGracia and Hu 2007). The second is transcription activation of stress protein-encoding genes, such as heat shock protein 70 (HSP70), ART4, and GADD34, by modulating transcriptional inducers. The selective synthesis of stress proteins provides a protective mechanism for cells to defend against and recover from stress-induced damage (DeGracia and Hu 2007). The balance between the intensity of stress-induced damage and the activity of translated stress proteins establishes a decision point, determining whether the consequence is cell survival or death (Beere 2005; Oyadomari and Mori 2004; Zhang and Kaufman 2006).
Hypoxia-inducible factor 1 (HIF-1) plays a key role in the cellular adaptive response to a lack of oxygen. HIF-1 is transcriptional regulator of angiogenesis, erythropoiesis, energy metabolism, and cell survival in mammals (Semenza 2003). HIF-1 is a heterodimer that consists of a constitutively expressed HIF-1β subunit and a HIF-1α subunit, the expression of which is highly regulated (Wang et al. 1995). The activity of HIF-1 is predominantly regulated by the stability of its α-subunit. HIF-1α is expressed but is rapidly degraded by the ubiquitin-proteasome system under normoxia, thereby maintaining an undetectable steady-state level. Under hypoxic conditions, the oxygen-dependent prolyl hydroxylase domains are inactive, and HIF-1α is not hydroxylated for the von Hippel-Lindau (VHL) interaction and degradation (Maxwell et al. 1999). Stable HIF-1α translocates to the nucleus, dimerizes with HIF-1β, and activates the transcription of target genes that contain a hypoxia response element (HRE). Several studies using cancer cell lines have described a role for HDACs in regulating the stability and activity of HIF-1α (Fath et al. 2006; Geng et al. 2011; Hutt et al. 2014; Kong et al. 2006; Qian et al. 2006), although the mechanisms of action vary widely according to cell type. For example, class II HDAC6 and HDAC4 are associated with HIF-1α, and inhibition of both HDACs reduces HIF-1α protein levels (Geng et al. 2011; Kong et al. 2006; Qian et al. 2006). Interestingly, treating cells with histone deacetylase inhibitor (HDACi) also reduces HIF-1α at the protein level under normoxic, hypoxic, and hypoxia mimic conditions (Kim et al. 2001; Kong et al. 2006; Lemoine et al. 2012; Qian et al. 2006). For example, TSA repressed HIF-1α levels in HCT116 cells (p53+/+) and in isogenic p53−/− HCT116-derived cells. The HDACi-mediated destabilization of HIF-1α is dependent on 26S proteasome degradation but is independent of VHL and p53 function (Bunz et al. 1998; Kong et al. 2006). HDAC6 siRNA-mediated HIF-1α inhibition is thought to be related to the acetylation of HSP90, which disrupts HSP90 chaperone function toward its client proteins, including HIF-1α (Kong et al. 2006; Qian et al. 2006). These results suggest that HDAC6 regulates HIF-1α protein acetylation and stability.
In addition to hypoxia, HDAC6 appears to mediate all three major cellular response pathways to cytotoxic accumulation of misfolded and aggregated proteins (Matthias et al. 2008). First, HDAC6 stimulates the formation of aggresomes by microtubule-dependent transport of misfolded ubiquitinated protein aggregates, resulting in reduced toxicity of scattered aggregates (Kawaguchi et al. 2003). The involvement of HDAC6 in this cytoprotective process provided the first indication that HDAC6 may act as a stress regulator. However, although aggresome formation reduces scattered aggregates, it does not remove all of the protein aggregates triggered by the proteasome machinery defect. Second, HDAC6 activates autophagy, one of the major degradation mechanisms, leading to further rescue of the cytotoxicity resulting from proteasome dysfunction (Iwata et al. 2005). Third, HDAC6 mediates the activation of heat shock protein accumulation. Proteasome dysfunction and the accumulation of protein aggregates cause activation of the major heat shock transcription factor HSF1 and accumulation of cellular heat shock proteins. HDAC6 senses the accumulation of ubiquitinated proteins via its ubiquitin-binding domain. The interaction between HDAC6 with ubiquitin then induces dissociation of the HSP90-HDAC6 complex, leading to dissociation of the HDAC6-p97/VCP complex and subsequently of the repressive HSP90-HSF1 complex (Boyault et al. 2007). These chaperones modulate protein folding and degradation of misfolded proteins and have an essential role in protecting cells from stress.
Taking all of these lines of evidence together, we hypothesized that HDAC6 modulates a cell’s capacity to respond to environmental challenges and its adaptive response against stress. However, the underlying molecular mechanisms of the role of HDAC6 in stress and post-stress recovery are still unclear. In this study, we addressed the mechanism by which HDAC6 discretely manages the cellular stress response during different stresses and after stress release.
Materials and methods
Cultivation of cell lines under normoxic and hypoxic conditions
All cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in medium with 10% fetal bovine serum and antibiotics under 5% CO2 and 95% air at 37 °C. Wild-type, HDAC6 KO MEFs, and MEFs expressing WT or a catalytically dead HDAC6 mutant were prepared as previously described (Boyault et al. 2007; Zhang et al. 2006; Zhang et al. 2008). All MEFs were kindly provided by Dr. Patrick Matthias (Friedrich Miescher Institute for Biomedical Research, Basel, Switzerland). Under normoxic conditions (21% O2), all cell lines were cultured at 37 °C under 5% CO2 and 98% humidity. Experiments under hypoxic conditions were performed at 37 °C under 5% CO2 and 1% O2 in an automated Xvivo system G300CL (BioSpherix, Lacona, NY, USA). A gas mixture of N2 and CO2 (Air Liquide, Paris, France) was connected to the system to maintain on a constant O2 of 1%, both in the working chamber and in the incubators. Prior to a specific treatment, cells were pre-incubated under normoxia or hypoxia for 24 h.
Cell death assays
Cells were treated with arsenite (0.5 mM), H2O2 (1 mM), or MG132 (5 μM) as a stressor. Annexin V-propidium iodide (PI) staining was performed as described by the manufacturer (FITC Annexin V Apoptosis Detection Kit; BD Pharmingen, San Diego, CA, USA). After treatment, cells were trypsinized and stained with 0.5 mg/ml Annexin V in binding buffer (10 mM HEPES free acid, 0.14 M NaCl, and 2.5 mM CaCl2) for 30 min. Afterwards, PI (5 mg/ml final concentration) was added and incubated for further 15 min. Cells were applied to a flow cytometer for analysis.
Western blot analysis
Western blot analysis was performed as previously described (Kwon et al. 2007). Source of primary antibodies is available upon the request.
Co-immunoprecipitation assay
For co-immunoprecipitation, ∼500 μg of cell extracts was incubated overnight with the primary antibody at 4 °C with gentle agitation. The protein G-agarose slurry (25 μl) was then added, and samples were incubated for 3 h at 4 °C with gentle agitation. Beads were washed three times with NP-40 lysis buffer and subsequently resuspended and boiled in 20 μl loading buffer for SDS–PAGE. Generally, input was loaded as 1/10 of the material used for the binding reaction.
Transient transfection and luciferase assay
MEFs were seeded onto 6-well plates and allowed to reach ∼50% confluency. Cells were cotransfected with 1 μg hypoxia response element (HRE) driving firefly-luciferase reporter and 50 ng Renilla vector as a control using Fugene 6 (Roche, Basel, Switzerland). Transfected cells were incubated for 24 h in 21% O2 then incubated for an additional 6 h with 500 μM CoCl2. Cell lysates were analyzed using a Dual Luciferase Reporter Assay kit (Promega, Fitchburg, WI, USA). Relative luciferase activity was determined by the ratio of firefly to Renilla.
Quantitative RT-PCR
A two-step approach was taken in which the initial reverse transcription was followed by the quantitative PCR amplification. Total RNA was extracted from cells using TRIzol Reagent (Invitrogen, USA), RNAsecure™ (Ambion, Austin, Tex, USA), and Turbo™ DNase (Ambion) according to the manufacturer’s instructions. cDNA derived from this RNA using SuperScript III Reverse Transcriptase (Invitrogen) was used as template for quantitative real-time PCR performed with the Applied Biosystems 7500 System (Applied Biosystems, Foster City, CA, USA). Relative mRNA expression data were analyzed using the 2−ΔΔCT method with actin as an endogenous control. The primer sequences for RT-PCR are available upon the request.
Statistical analysis
All data are presented as the mean ± SD from three independent experiments. Statistical significance was determined by Student’s t test of treated samples when compared to respective controls, *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
HDAC6 controls HIF-1α protein stability in non-transformed cells
HDAC6 and HDAC4 interact with HIF-1α and control the acetylation level and degradation of HIF-1α in a VHL-independent manner in cancer cell lines (Geng et al. 2011; Kong et al. 2006; Qian et al. 2006). To examine whether HDAC6 influences the stability of HIF-1α in non-transformed cells, we first determined the protein expression level of HIF-1α in wild-type (WT) and HDAC6 mutant mouse embryonic fibroblasts (MEFs) under normoxia, hypoxia, and hypoxic mimic, cobalt chloride (CoCl2). Under hypoxic conditions, hypoxic expression level of HIF-1α was lower in HDAC6 knockout (KO) cells than that in WT cells (Fig. 1b, c and lanes 1 and 2). Next, we performed a time course analysis of HIF-1α expression under hypoxic mimic stress conditions. Protein expression in WT MEFs reached a plateau after 6 h treatment of CoCl2 whereas in HDAC6 KO MEFs, the protein started to degrade at 6 h (Fig. 1d). To determine which functional domain of HDAC6 affects HIF-1α stability, we examined its stability in WT, HDAC6 KO, rescuant of WT HDAC6, and KO MEFs re-expressing either a catalytically inactive HDAC6 (HDm) or a ubiquitin-binding defective version of HDAC6 (Ubm) under hypoxic and hypoxic mimic conditions. WT and rescuant of WT HDAC6 cells expressed the highest levels of HIF-1α (Fig. 1b, c and lanes 1 and 3). Conversely, HDAC6 KO cells and both mutant cells (HDm and Ubm) showed that inhibition reduced HIF-1α protein expression in response to hypoxia (Fig. 1b, c). These results suggest that HDAC6 is involved in the stability of HIF-1α under hypoxic conditions.
Fig. 1.
Deacetylation of HIF-1α by HDAC6 regulates its degradation and transcriptional activity under hypoxia. MEFs were incubated for 24 h in 21% O2 (a), in 1% O2 (b), or for 6 h with 500 μM CoCl2 to mimic hypoxia (c). Whole cell lysates were immunoprecipitated with an anti-HIF-1α antibody. Immunocomplexes were either probed for anti-HIF-1α or anti-acetylated lysine antibody. Whole cell lysates of 10% were used as input control. d MEFs were incubated for 6, 12, or 24 h with 500 μM CoCl2 to mimic hypoxia or in 21% O2. Immunoblotting analysis was performed with indicated antibodies. α-Tubulin is used as a loading control. e MEFs were co-transfected with an HRE-firefly luciferase vector and a Renilla vector as a control. Transfected cells were incubated for 24 h in 21% O2 then incubated for an additional 6 h with 500 μM CoCl2. Dual luciferase activity was measured, and the ratio of firefly to Renilla was used as the relative luciferase activity. f MEFs were incubated for 6 h with 500 μM CoCl2 to mimic hypoxia. mRNA expression of HIF-1α target genes was analyzed by qRT-PCR. mRNA values were normalized to that of actin. *P < 0.05 based on two-tailed unpaired Student’s t test
Next, to investigate whether HIF-1α is directly deacetylated by HDAC6, we immunoprecipitated it using an anti-HIF-1α antibody and blotted with an anti-acetylated lysine antibody. HIF-1α was found to be acetylated in HDm mutant cells as well as in HDAC6 KO cells (Fig. 1b, c and lanes 2 and 4), even though the hypoxic expression and immunoprecipitation level of HIF-1α were much lower. Taken together, these data suggest that HDAC6 deacetylates HIF-1α and controls its stability through acetylation and ubiquitination.
HDAC6 controls HIF-1α transcriptional activity
To determine the functional consequences of HIF-1α, we transiently transfected MEFs with a hypoxic response element (HRE)-driven luciferase reporter gene vector, whose expression is dependent on the availability of HIF-1α. As a transfection control, the cells were co-transfected with Renilla luciferase vector. Cells were assayed for luciferase activity 24 h after transfection, with CoCl2 added to the culture medium for the last 6 h of culture. Interestingly, hypoxia induced a significant down-regulation in HIF-1/HRE activity in HDAC6 KO and HDm mutant cells transiently transfected with the HRE-driven luciferase reporter gene (Fig. 1e). Most importantly, the suppressive effect of HDAC6 depletion on HIF-1α resulted in significant inhibition of hypoxia-responsive genes such as vascular endothelial growth factor (VEGF), glucose transporter 1 (Glut1), and carbonic anhydrase IX (CAIX) (Fig. 1f). The increased acetylation level of HIF-1α protein was associated with a reduction in transcriptional activity as well as degradation of HIF-1α in HDAC6 KO and HDm mutant cells. Therefore, our results suggest that HIF-1α acetylation by HDAC6 suppression may compromise the transcriptional activity HIF-1α.
Interaction between HIF-1α and HSP70 is enhanced by inhibition of HIF-1α deacetylation
HDACi has been reported to induce HSP90 acetylation and causes the disassociation of client proteins (Kovacs et al. 2005). Furthermore, the oxygen-dependent degradation (ODD) domain of HIF-1α interacts with HSP70 (Zhou et al. 2004) and HIF-1α needs to the heat shock protein HSP70/90 chaperone complex to complete its maturation (Fath et al. 2006). These observations prompted us to investigate whether the HDAC6 affects association of HIF-1α with HSP90 and HSP70 under hypoxia. Immunoprecipitation studies have shown that the interaction between HIF-1α and HSP70 was enhanced in HDAC6 KO and HDm mutant cells under hypoxia (Fig. 2). It has also been reported that HDAC6 is involved in proteasome degradation of several proteins by inducing hyperacetylation and inhibiting chaperone function of HSP90 (Kovacs et al. 2005; Neckers et al. 2007). As HIF-1α is a client protein of HSP90, we addressed the effect of HDAC6 on the regulation of HIF-1α by HSP90 acetylation. As shown in Fig. 2b, coi-mmunoprecipitation experiments revealed that HDAC6 depletion did not alter HIF-1α/HSP90 association. These results imply that HDAC6 may directly affect the stability of HIF-1α.
Fig. 2.
Deacetylation of HIF-1α by HDAC6 reduces the interaction between HIF-1α and HSP70. MEFs were incubated for 24 h in 1% O2. a Immunoblotting analysis was performed with indicated antibodies. Whole cell lysates of 10% were used as input control. b Whole cell lysates were immunoprecipitated with an anti-HIF-1α antibody. The precipitate was immunoblotted with anti-HIF-1α, anti-HSP70, or anti-HSP90 antibodies. α-Tubulin is used as a loading control
Loss of HDAC6 causes hypersensitivity to cellular stresses
Mammalian cells have evolved a variety of mechanisms to facilitate cellular recovery from environmental stressors. Failure to respond stress results in cell death. Stress defense and apoptotic destruction tend to occur in a mutually exclusive manner. Thus, we investigated how HDAC6 functions in response to hypoxia and reoxygenation. Under hypoxic conditions, HDAC6 KO MEFs showed more hypersensitivity to apoptosis compared to under normoxia. HDAC6 HDm mutant cells showed no significant change in apoptosis as HDAC6 KO cells, but it was slightly affected in HDAC6 Ubm mutant cells. Reoxygenation of WT MEFs following hypoxia resulted in a complete return to the normoxic cells. However, HDAC6 KO, HDm, and Ubm cells did not fully return to normoxic cells following reoxygenation (Fig. 3). These findings suggest that HDAC6 may function as a stress regulator during hypoxia stress as well as during recovery.
Fig. 3.
Loss of HDAC6 causes hypersensitivity to apoptosis in response to hypoxia. MEFs were exposed to normoxia, hypoxia (1% O2, 24 h), or reoxygenation (1% O2, 24 h, and 21% O2, 48 h). The number of apoptotic cells was counted and graphed following an Annexin-V/PI assay. *P < 0.05, ***P < 0.001 based on two-tailed unpaired Student’s t test
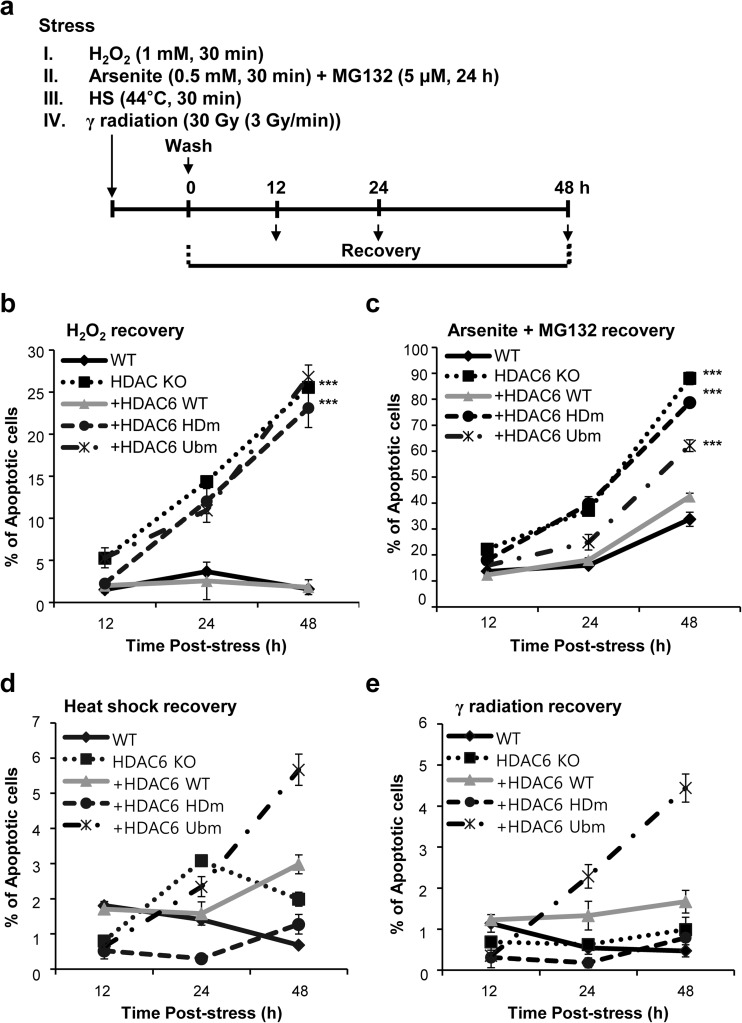
To test whether HDAC6 has a role in other oxidative stress responses in addition to hypoxia, we compared the recovery from stress of WT MEF cells with that of HDAC6 KO MEFs (Fig. 4a). To do this, we assessed the ability of the cells to recover from hydrogen peroxide (H2O2)-induced oxidative stress. As shown in Fig. 4b, Annexin-V/PI assays revealed that after recovery from stress, HDAC6 KO MEFs showed a significantly higher percentage of apoptosis (25–30%) compared to control WT MEFs (∼2%) over time. In contrast, even though HDAC6 KO cells had no a measurable effect on cell viability prior to the induction of stress, they recovered poorly after release. To further demonstrate that this poor recovery was due to a lack of HDAC6, we complemented HDAC6 KO MEFs with either WT HDAC6, HDm, or Ubm mutant forms. Importantly, the hypersensitivity of HDAC6 KO cells to stress was significantly alleviated by the reintroduction of WT, but not HDm or Ubm HDAC6. Next, to further confirm the role of HDAC6 in stress and recovery, MEFs were treated with the oxidative stress inducer arsenite and the proteasome inhibitor MG132 and apoptosis was analyzed after stress release. HDAC6 KO, HDm, and Ubm cells treated with MG132 for 24 h then arsenite for 30 min showed a significantly higher percentage (18–90%) of apoptosis compared to control WT and WT rescuant MEFs (13–43%) over time (Fig. 4c). In the absence of HDAC6 deacetylase activity or ubiquitin-binding activity, the cells’ ability to manage rapid and correct recovery after oxidative stress and misfolded protein-induced stress was significantly impaired. However, ionizing radiation and heat shock had no significant effect on cell death in cells lacking HDAC6 (Fig. 4d, e). These results suggest that the loss of HDAC6 render cells more sensitive to oxidative stress and post-stress recovery. Thus, we conclude that the role of HDAC6 in stress response is controlled by distinct mechanisms depending on the type of stress stimulus.
Fig. 4.
Depletion of HDAC6 results in reduced cell viability after stress release. a Schematic representation of the experimental procedure. MEFs of different genotypes were treated with 1 mM H2O2 for 30 min (b) or 5 μM MG 132 for 24 h then 0.5 mM arsenite for 30 min (c), heat shock at 44 °C for 30 min (d), or 30 Gy γ radiation (3 Gy/min) (e) and recovered at the indicated times after stress. The number of apoptotic cells was counted and graphed following an Annexin-V/PI assay. ***P < 0.001 based on two-tailed unpaired Student’s t test
Discussion
HIF-1α is one of the best-described regulatory gene and transcription factors that are activated under hypoxia, and it plays an important role in the activation of resistance mechanisms to anticancer drugs (Iida et al. 2012). It has been shown that HDAC6 and HDAC4 interact with HIF-1α directly to control the acetylation level and degradation of HIF-1α in a VHL-independent manner (VPA and LAQ824) (Qian et al. 2006). It has also been reported that HDAC6 controls HSP90 chaperone function and indirectly regulates HIF-1α stability in a VHL- and p53-independent manner. Inhibition by TSA or SAHA was equally effective under hypoxia as well as hypoxic mimics (Kong et al. 2006). The ARD1-mediated acetylation of HIF-1α increases the interaction between HIF-1 and pVHL and the degradation of HIF-1α in mammalian cells (Jeong et al. 2002). In contrast, two independent groups found that ARD1 could not acetylate Lys532 in HIF-1α in vitro (Arnesen et al. 2005; Murray-Rust et al. 2006). Moreover, ARD1 expression status has no effect on the expression of HIF-1α or on the function of HIF-regulated genes (Bilton et al. 2005; Fisher et al. 2005). Therefore, whether HDAC6 regulates HIF-1α directly or indirectly remains to be clarified.
In addition, because most studies on HIF-1α have been performed in transformed cancer cells, we investigated PTM, protein stability, and transcriptional activity of HIF-1α in non-transformed MEFs. Consistent with previous findings, we showed that HIF-1α protein expression levels were reduced in HDAC6 KO MEFs and in rescuants of HDAC6 HDm and Ubm under hypoxia and hypoxic mimic conditions. However, more importantly, we, for the first time, discovered that HDAC6 Ubm mutant cells have an impact on HIF-1α stability but no impact on its transcriptional activity. This indicates that HIF-1α is required for deacetylation to fully function in transcription. Thus, our results demonstrate that HDAC6 depletion acetylates HIF-1α and promotes HIF-1α degradation under hypoxia. In agreement with this finding, the HDAC6-selective inhibitor Tubastatin A (Schoepflin et al. 2016) and pan-HDACi SAHA (Hutt et al. 2014) blocked HIF-1α activity via a direct effect on HIF-1α stability via its acetylation and degradation. These data suggest that HDAC6 directly binds to and deacetylates HIF-1α. It may also protect its ubiquitin-binding sites and recruit deubiquitinating enzymes to these sites, thereby allowing HIF-1α survival under hypoxia.
Molecular chaperones contribute to HIF-1α transcriptional activity and stability under hypoxic and normoxic conditions (Bardos and Ashcroft 2005). HSP90 can interact with the Per-ARNT-Sim domain of HIF-1α (Katschinski et al. 2004). HSP70 interacts with the ODD domain of HIF-1α, which is hydroxylated at prolyl residues and required for its targeting to the proteasome (Zhou et al. 2004). The HSP70 protein complexes, acting upstream of HSP90, transiently hold nascent peptides, preventing irreversible aggregation and catalyzing refolding in an ATP- and chaperone-dependent process. HIF-1α needs HSP70/90 chaperone complex to completely mature (Fath et al. 2006). HSP90 inhibitors, such as geldanamycin, promote HIF-1α ubiquitination and proteasome-dependent degradation (Isaacs et al. 2002). HDAC6 functions as a HSP90 deacetylase: inactivation of HDAC6 leads to HSP90 hyperacetylation and loss of chaperone activity (Kovacs et al. 2005; Neckers et al. 2007). Our present studies show that interaction between HIF-1α and HSP70 is enhanced in HDAC6 KO and HDm mutant cells compared to WT cells under hypoxia but the hypoxic expression level of HIF-1α is reduced. This finding indicates that inactivation of HDAC6 causes hyperacetylation of HIF-1α and HSP90, enhanced interaction of HSP70 with HIF-1α and accumulation of immature HIF-1α/HSP70 complex but this interaction does not prevent HIF-1α degradation by the 20S proteasome. In contrast, the interaction between HIF-1α and HSP70 is dramatically decreased in HDAC6 Ubm mutant cells compared to WT cells under hypoxia, leading to rapid degradation of HIF-1α. Since HDAC6 Ubm mutant cells have no effect on transcriptional activity of HIF-1α, we hypothesized that the mutant is still able to bind to and deacetylate HIF-1α. It is therefore possible that a conformational change of mutant HDAC6 may inhibit to interact of HSP70 with HIF-1α and then expose the HIF-1α ubiquitin-binding sites, allowing it to be ubiquitinated and degraded by the 26S proteasome under hypoxia. However, how and why ubiquitin-binding domain of HDAC6 affects HIF-1α stability remains unknown. This possibility will also be further studied in the future. Intriguingly, loss of HDAC6 causes cells to become more sensitive to hypoxia and reoxygenation. Therefore, this implies that HDAC6 depletion causes a defect in cellular recovery from hypoxic stress. Taken together, these results support a key role for HDAC6 in quality control mechanism of HIF-1α during hypoxic stress and post-stress recovery.
It is well known that HDAC6 regulates the three major cellular stress response pathways induced by the cytotoxic accumulation of misfolded and aggregated proteins: aggresome, autophagy, and heat shock response (Matthias et al. 2008). Moreover, HDAC6 plays a critical role in translational arrest-induced stress. One of the most immediate responses to cellular stress, such as the oxidative stressor arsenite, is a reversible block in translation, and these translationally stalled mRNAs are sequestered in dynamic cytoplasmic structures called stress granules (SGs) (Anderson and Kedersha 2006). HDAC6 is a central component of this stress response, regulating SG formation and potentially contributing to the control of RNA metabolism and translation (Kwon et al. 2007). Previously, we reported that HDAC6 is required for cells to recover from oxidative stresses such as arsenite (Kwon et al. 2007). In agreement with previous findings, in the absence of intact HDAC6 function, cells that have been treated with another oxidative stress inducer, H2O2, undergo apoptosis and exhibit an impaired stress recovery. Moreover, following a concurrent block of two HDAC6-mediated stress response pathways, HDAC6-depleted cells showed a significantly impaired recovery, and cell death was increased compared to recovery from a single stress. In contrast to oxidative stress, we did not observe a clear role for HDAC6 in the cellular response to heat shock or ionizing radiation. These data suggest that HDAC6-deficient cells show severely impaired oxidative stress and cellular stress responses such as aggresomes, autophagy, and SGs and may ultimately cause cell death due to a defect in post-stress recovery. HDAC6 may play a pivotal role as a cellular stress manager, sensing different types of stress and sorting stress-related molecules into the appropriate stress response pathway. However, the exact mechanisms by which HDAC6 distinguishes and sorts different stressors are not yet fully understood. Taken together, our results suggest that HDAC6 serves as a master regulator of the cellular stress response to diverse stressors.
Acknowledgements
We wish to thank Dr. Patrick Matthias (Friedrich Miescher Institute for Biomedical Research, Basel, Switzerland) for providing HDAC6 KO and mutant MEFs and an anti-mHDAC6 antibody. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2014R1A1A2054026).
Abbreviations
- HDAC
Histone deacetylase
- HDACi
HDAC inhibitor
- HIF-1
Hypoxia-inducible factor 1
- H2O2
Hydrogen peroxide
- HRE
Hypoxic response element
- HSP
Heat shock protein
- KO
Knockout
- MEF
Mouse embryonic fibroblast
- ODD
Oxygen-dependent degradation
- PTM
Posttranslational modification
- SG
Stress granule
- VHL
von Hippel-Lindau
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Anderson P, Kedersha N. RNA granules. J Cell Biol. 2006;172:803–808. doi: 10.1083/jcb.200512082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnesen T, et al. Interaction between HIF-1 alpha (ODD) and hARD1 does not induce acetylation and destabilization of HIF-1 alpha. FEBS Lett. 2005;579:6428–6432. doi: 10.1016/j.febslet.2005.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardos JI, Ashcroft M. Negative and positive regulation of HIF-1: a complex network. Biochim Biophys Acta. 2005;1755:107–120. doi: 10.1016/j.bbcan.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Beere HM. Death versus survival: functional interaction between the apoptotic and stress-inducible heat shock protein pathways. J Clin Invest. 2005;115:2633–2639. doi: 10.1172/JCI26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilton R, et al. Arrest-defective-1 protein, an acetyltransferase, does not alter stability of hypoxia-inducible factor (HIF)-1alpha and is not induced by hypoxia or HIF. J Biol Chem. 2005;280:31132–31140. doi: 10.1074/jbc.M504482200. [DOI] [PubMed] [Google Scholar]
- Boyault C, et al. HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Genes Dev. 2007;21:2172–2181. doi: 10.1101/gad.436407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brostrom CO, Brostrom MA. Regulation of translational initiation during cellular responses to stress. Prog Nucleic Acid Res Mol Biol. 1998;58:79–125. doi: 10.1016/S0079-6603(08)60034-3. [DOI] [PubMed] [Google Scholar]
- Bunz F, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- DeGracia DJ, Hu BR. Irreversible translation arrest in the reperfused brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:875–893. doi: 10.1038/sj.jcbfm.9600388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath DM, et al. Histone deacetylase inhibitors repress the transactivation potential of hypoxia-inducible factors independently of direct acetylation of HIF-alpha. J Biol Chem. 2006;281:13612–13619. doi: 10.1074/jbc.M600456200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher TS, et al. Analysis of ARD1 function in hypoxia response using retroviral RNA interference. J Biol Chem. 2005;280:17749–17757. doi: 10.1074/jbc.M412055200. [DOI] [PubMed] [Google Scholar]
- Fulda S, Gorman AM, Hori O, Samali A. Cellular stress responses: cell survival and cell death. Int J Cell Biol. 2010;2010:214074. doi: 10.1155/2010/214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale M, Jr, Tan SL, Katze MG. Translational control of viral gene expression in eukaryotes. Microbiol Mol Biol Rev. 2000;64:239–280. doi: 10.1128/MMBR.64.2.239-280.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng H, et al. HDAC4 protein regulates HIF1alpha protein lysine acetylation and cancer cell response to hypoxia. J Biol Chem. 2011;286:38095–38102. doi: 10.1074/jbc.M111.257055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford NG, et al. Highly conserved protein kinases involved in the regulation of carbon and amino acid metabolism. J Exp Bot. 2004;55:35–42. doi: 10.1093/jxb/erh019. [DOI] [PubMed] [Google Scholar]
- Hutt DM, Roth DM, Vignaud H, Cullin C, Bouchecareilh M. The histone deacetylase inhibitor, Vorinostat, represses hypoxia inducible factor 1 alpha expression through translational inhibition. PLoS One. 2014;9:e106224. doi: 10.1371/journal.pone.0106224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida Y, et al. Hypoxia promotes glycogen synthesis and accumulation in human ovarian clear cell carcinoma. Int J Oncol. 2012;40:2122–2130. doi: 10.3892/ijo.2012.1406. [DOI] [PubMed] [Google Scholar]
- Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J Biol Chem. 2002;277:29936–29944. doi: 10.1074/jbc.M204733200. [DOI] [PubMed] [Google Scholar]
- Iwata A, Riley BE, Johnston JA, Kopito RR. HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin. J Biol Chem. 2005;280:40282–40292. doi: 10.1074/jbc.M508786200. [DOI] [PubMed] [Google Scholar]
- Jeong JW, et al. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell. 2002;111:709–720. doi: 10.1016/S0092-8674(02)01085-1. [DOI] [PubMed] [Google Scholar]
- Katschinski DM, Le L, Schindler SG, Thomas T, Voss AK, Wenger RH. Interaction of the PAS B domain with HSP90 accelerates hypoxia-inducible factor-1alpha stabilization. Cell Physiol Biochem. 2004;14:351–360. doi: 10.1159/000080345. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003;115:727–738. doi: 10.1016/S0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- Kim MS, et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001;7:437–443. doi: 10.1038/86507. [DOI] [PubMed] [Google Scholar]
- Kong X, Lin Z, Liang D, Fath D, Sang N, Caro J. Histone deacetylase inhibitors induce VHL and ubiquitin-independent proteasomal degradation of hypoxia-inducible factor 1alpha. Mol Cell Biol. 2006;26:2019–2028. doi: 10.1128/MCB.26.6.2019-2028.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JJ, et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18:601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Kwon S, Zhang Y, Matthias P. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes Dev. 2007;21:3381–3394. doi: 10.1101/gad.461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine M, et al. The pan-deacetylase inhibitor panobinostat induces cell death and synergizes with everolimus in Hodgkin lymphoma cell lines. Blood. 2012;119:4017–4025. doi: 10.1182/blood-2011-01-331421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias P, Yoshida M, Khochbin S. HDAC6 a new cellular stress surveillance factor. Cell Cycle. 2008;7:7–10. doi: 10.4161/cc.7.1.5186. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- McEwen E, et al. Heme-regulated inhibitor kinase-mediated phosphorylation of eukaryotic translation initiation factor 2 inhibits translation, induces stress granule formation, and mediates survival upon arsenite exposure. J Biol Chem. 2005;280:16925–16933. doi: 10.1074/jbc.M412882200. [DOI] [PubMed] [Google Scholar]
- Murray-Rust TA, Oldham NJ, Hewitson KS, Schofield CJ. Purified recombinant hARD1 does not catalyse acetylation of Lys532 of HIF-1alpha fragments in vitro. FEBS Lett. 2006;580:1911–1918. doi: 10.1016/j.febslet.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Neckers L, Kern A, Tsutsumi S. Hsp90 inhibitors disrupt mitochondrial homeostasis in cancer cells. Chem Biol. 2007;14:1204–1206. doi: 10.1016/j.chembiol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- Panniers R. Translational control during heat shock. Biochimie. 1994;76:737–747. doi: 10.1016/0300-9084(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Qian DZ, et al. Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1 alpha. Cancer Res. 2006;66:8814–8821. doi: 10.1158/0008-5472.CAN-05-4598. [DOI] [PubMed] [Google Scholar]
- Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002;110:1383–1388. doi: 10.1172/JCI0216784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D. Signaling cascades regulating NMDA receptor sensitivity to ethanol. Neuroscientist. 2004;10:325–336. doi: 10.1177/1073858404263516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepflin ZR, Shapiro IM, Risbud MV (2016) Class I and IIa HDACs Mediate HIF-1alpha Stability through PHD2-Dependent Mechanism while HDAC6, a Class IIb Member, Promotes HIF-1alpha Transcriptional Activity in Nucleus Pulposus Cells of the Intervertebral Disc. J Bone Miner Res. [DOI] [PMC free article] [PubMed]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006;66:S102–S109. doi: 10.1212/01.wnl.0000192306.98198.ec. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gilquin B, Khochbin S, Matthias P. Two catalytic domains are required for protein deacetylation. J Biol Chem. 2006;281:2401–2404. doi: 10.1074/jbc.C500241200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol. 2008;28:1688–1701. doi: 10.1128/MCB.01154-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Schmid T, Frank R, Brune B. PI3K/Akt is required for heat shock proteins to protect hypoxia-inducible factor 1alpha from pVHL-independent degradation. J Biol Chem. 2004;279:13506–13513. doi: 10.1074/jbc.M310164200. [DOI] [PubMed] [Google Scholar]